Novel Aryl-Imidazolium Ionic Liquids with Dual Brønsted/Lewis Acidity as Both Solvents and Catalysts for Friedel–Crafts Alkylation
Abstract
Featured Application
Abstract
1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Catalytic Activity Characterization in the Friedel–Crafts Alkylation Reaction
3. Results and Discussion
3.1. Structure and Properties Description of B-L MILs
3.2. Catalytic Application in the Solvent-Free Friedel–Crafts Alkylation Reaction
3.2.1. Effects of Different B-L MILs on the Friedel–Crafts Alkylation Reaction
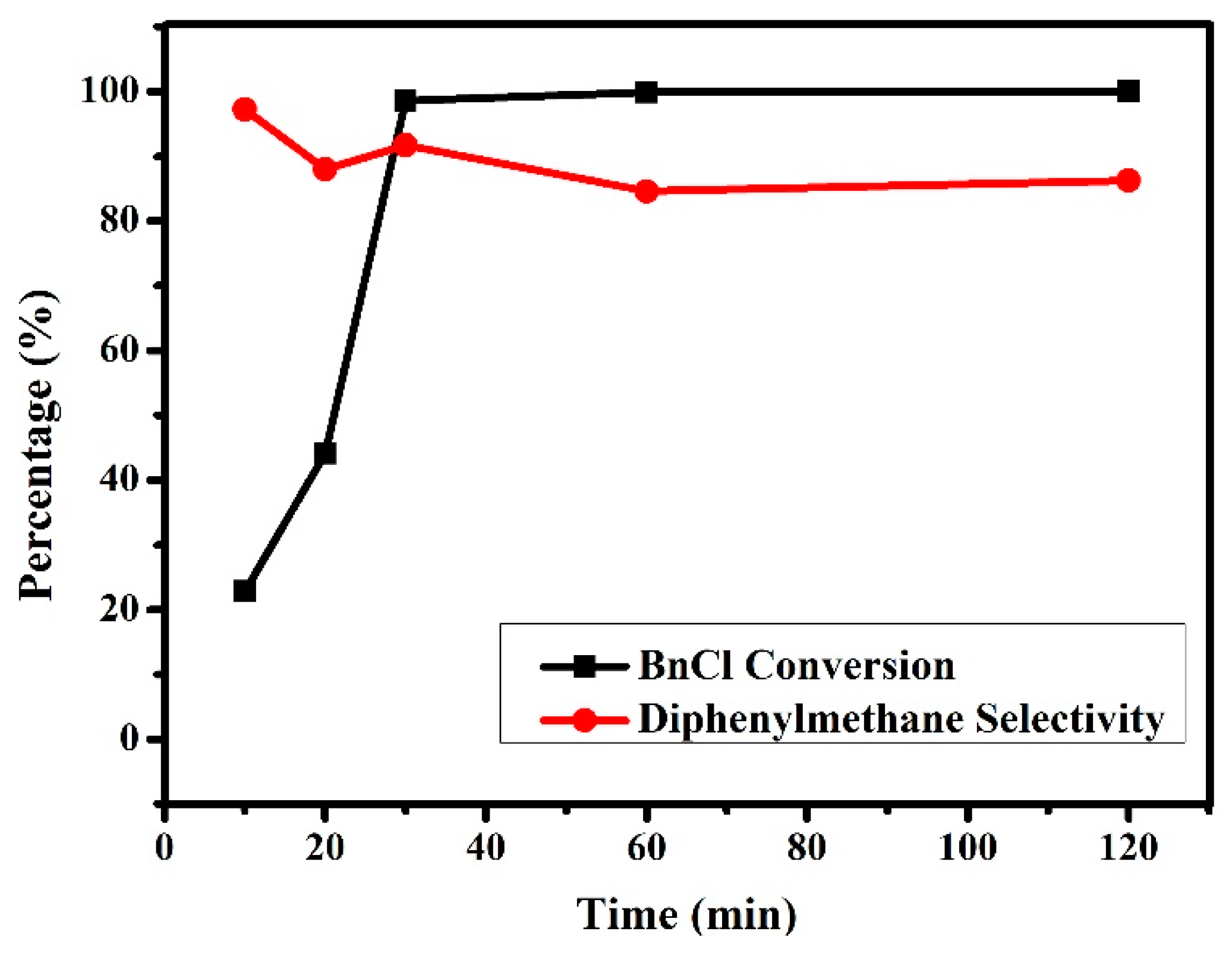
3.2.2. Effects of Reaction Time on the Friedel–Crafts Alkylation Reaction of p-Xylene with BnCl
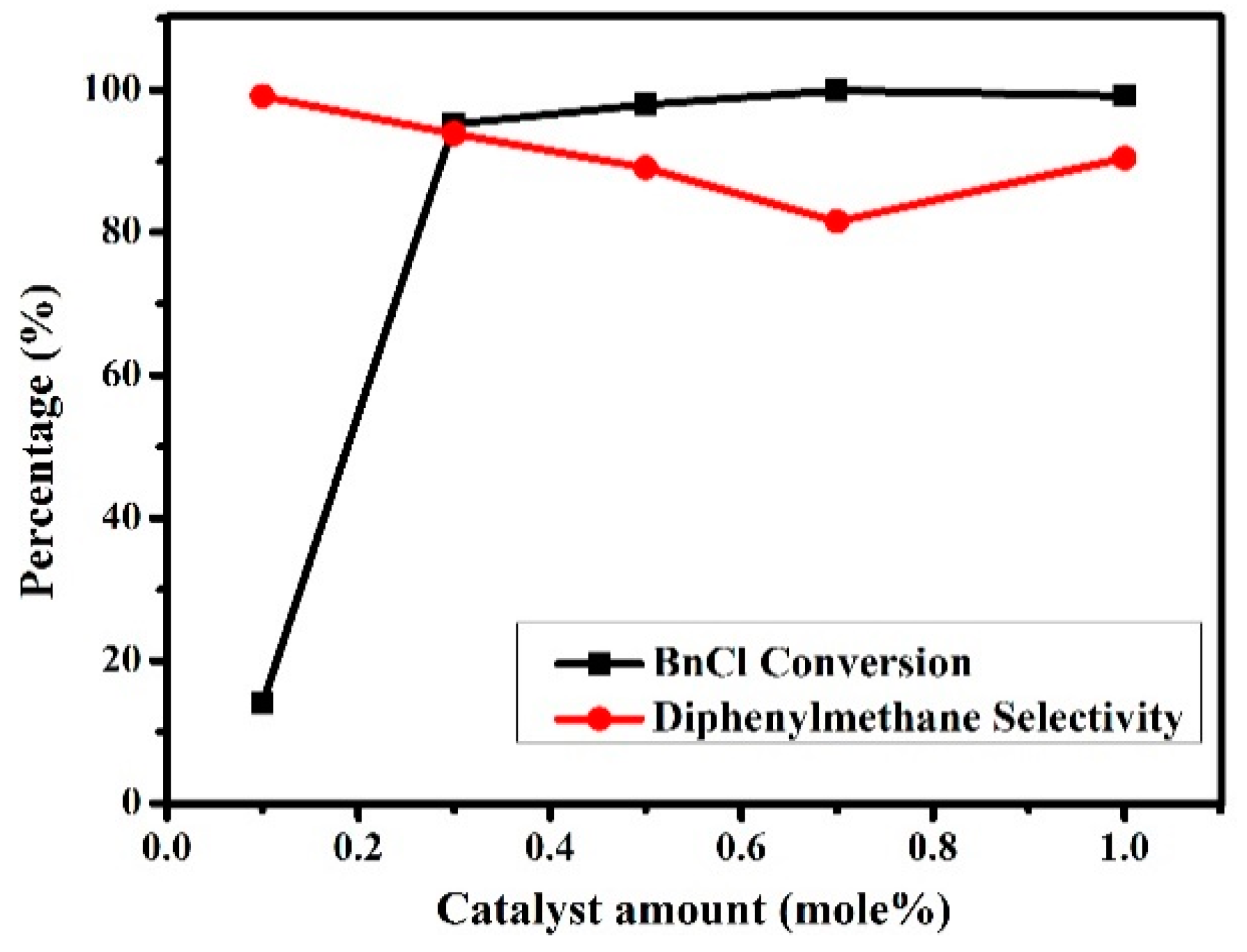
3.2.3. Effects of Catalyst Amounts on the Friedel–Crafts Alkylation Reaction of p-Xylene with BnCl
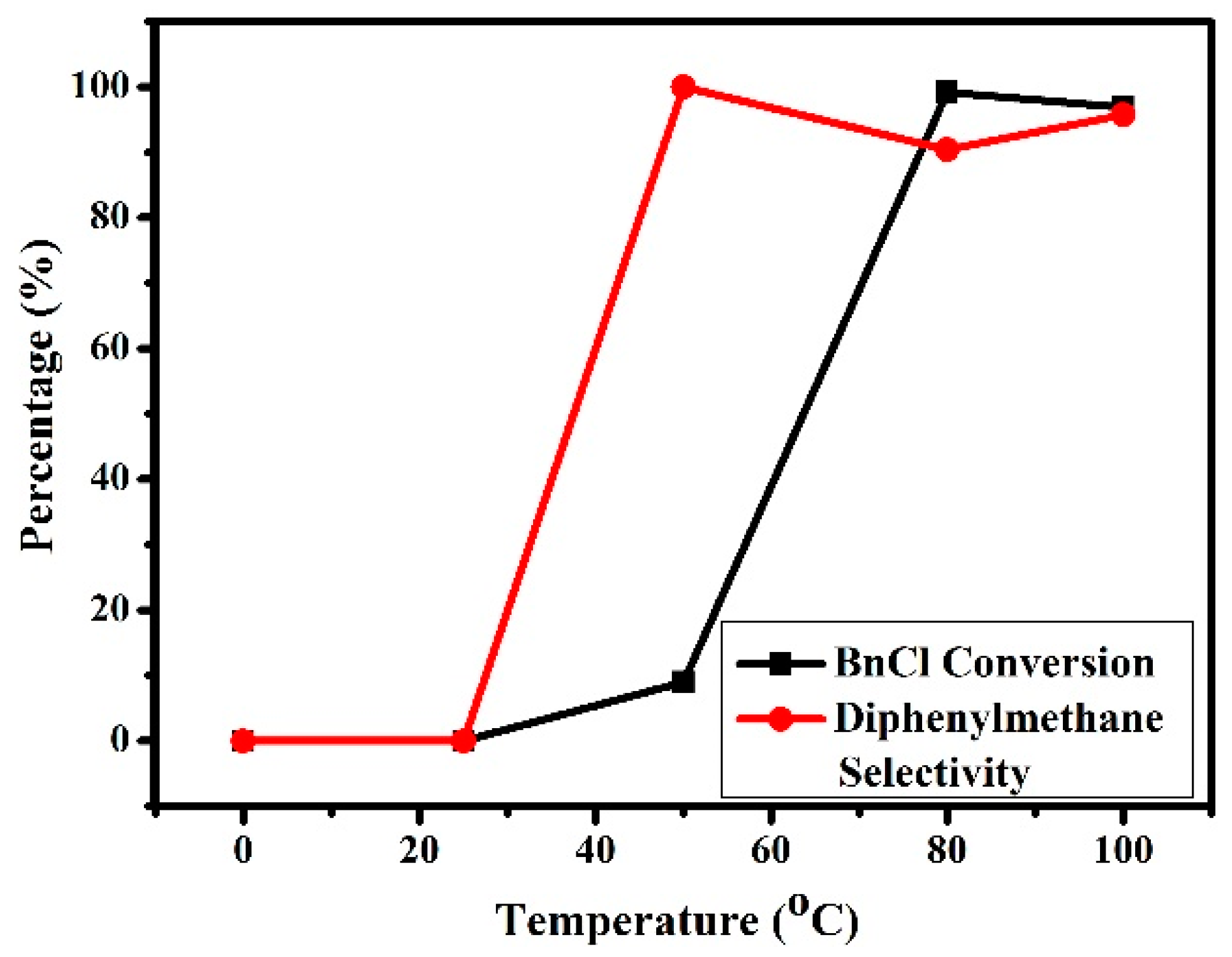
3.2.4. Effects of Reaction Temperature on the Friedel–Crafts Alkylation Reaction of p-Xylene with BnCl
3.2.5. Effects of the Molar Ratio of p-xylene to BnCl on the Friedel–Crafts Alkylation Reaction
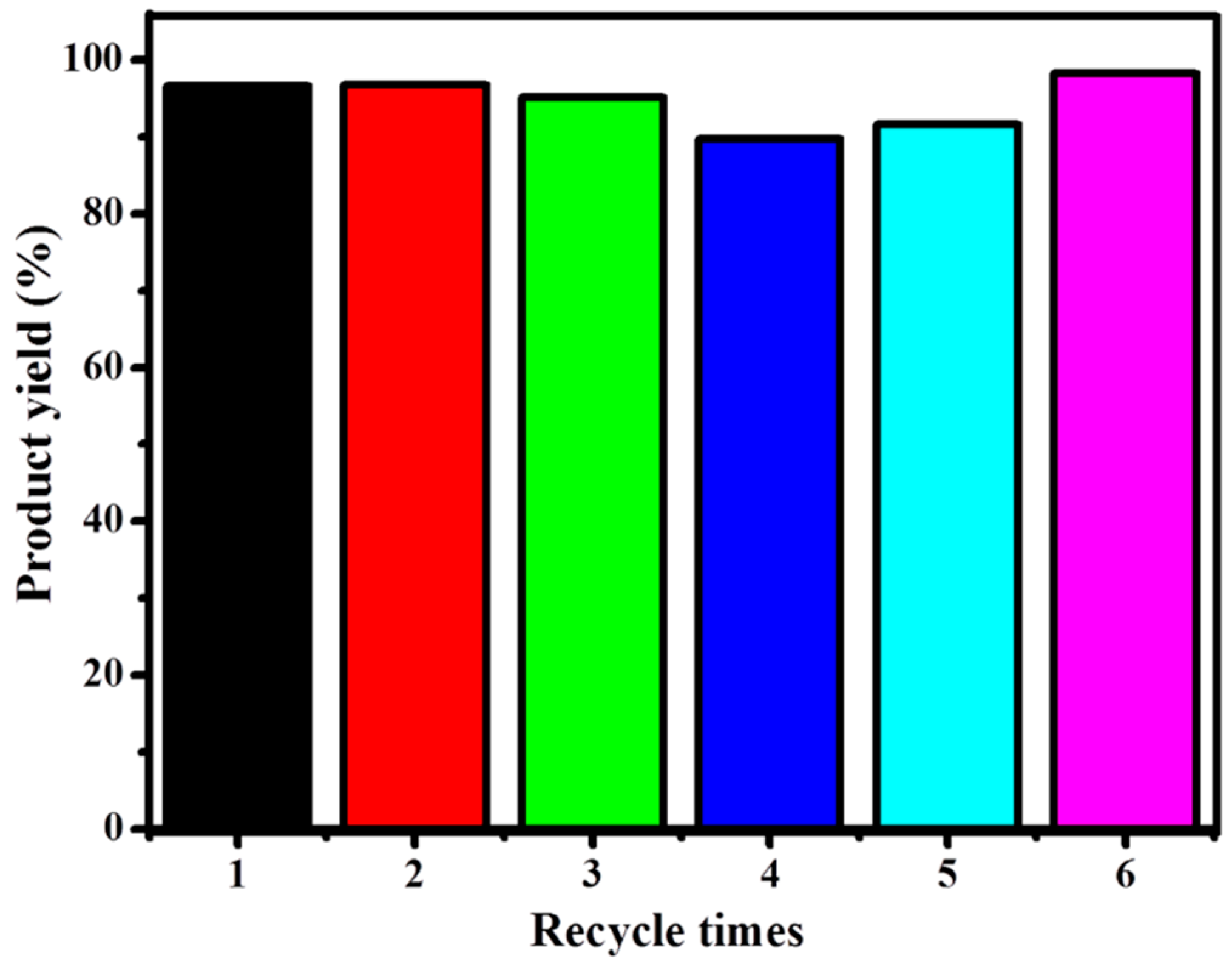
3.2.6. Catalyst Recycling and Stability
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhou, Q.; Srinivas, H.D.; Dasgupta, S.; Watson, M.P. Nickel-Catalyzed Cross-Couplings of Benzylic Pivalates with Arylboroxines: Stereospecific Formation of Diarylalkanes and Triarylmethanes. J. Am. Chem. Soc. 2013, 135, 3307–3310. [Google Scholar] [CrossRef]
- Lu, S.; Song, X.; Poh, S.B.; Yang, H.; Wong, M.W.; Zhao, Y. Access to Enantiopure Triarylmethanes and 1,1-Diarylalkanes by NHC-Catalyzed Acylative Desymmetrization. Chem.-Eur. J. 2017, 23, 2275–2281. [Google Scholar] [CrossRef] [PubMed]
- Jaratjaroonphong, J.; Sathalalai, S.; Techasauvapak, P.; Reutrakul, V. Iodine catalyzed Friedel-Crafts alkylation of electron-rich arenes with aldehydes: Efficient synthesis of triarylmethanes and diarylalkanes. Tetrahedron Lett. 2009, 50, 6012–6015. [Google Scholar] [CrossRef]
- Sunke, R.; Nallapati, S.B.; Kumar, J.S.; Kumar, K.S.; Pal, M. Use of AlCl3 in Friedel Crafts arylation type reactions and beyond: An overview on the development of unique methodologies leading to N-heteroarenes. Org. Biomol. Chem. 2017, 15, 4042–4057. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; He, T.; Wang, L. FeCl3 as Lewis acid catalyzed one-pot three-component aza-Friedel-Crafts reactions of indoles, aldehydes, and tertiary aromatic amines. Tetrahedron 2011, 67, 3420–3426. [Google Scholar] [CrossRef]
- Miller, J.M.; Goodchild, M.; Lakshmi, J.L.; Wails, D.; Hartman, J.S. Friedel-Crafts catalysis using supported reagents. Synthesis, characterization and catalytic application of ZnCl2 supported on fluoride-modified sol-gel-derived aluminosilicates. Catal. Lett. 1999, 63, 199–203. [Google Scholar] [CrossRef]
- Naganaboina, R.T.; Peddinti, R.K. BF3·Etherate-Mediated Friedel-Crafts Arylation of 2-Hydroxy-1,4- benzoxazines: Synthesis of 2-Aryl-1,4-benzoxazine Derivatives. J. Org. Chem. 2013, 78, 12819–12824. [Google Scholar] [CrossRef] [PubMed]
- Sabu, K.R.; Sukumar, R.; Rekha, R.; Lalithambika, M. A comparative study on H2SO4, HNO3 and HClO4 treated metakaolinite of a natural kaolinite as Friedel-Crafts alkylation catalyst. Catal. Today 1999, 49, 321–326. [Google Scholar] [CrossRef]
- Cheng, Y.; Ou, X.; Ma, J.; Sun, L.; Ma, Z.-H. A New Amphiphilic Brønsted Acid as Catalyst for the Friedel- Crafts Reactions of Indoles in Water. Eur. J. Org. Chem. 2019, 66–72. [Google Scholar] [CrossRef]
- Wilsdorf, M.; Leichnitz, D.; Reissig, H.-U. Trifluoromethanesulfonic Acid Catalyzed Friedel-Crafts Alkylations of 1,2,4-Trimethoxybenzene with Aldehydes or Benzylic Alcohols. Org. Lett. 2013, 15, 2494–2497. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Yu, N.; Peng, L.; Tan, R.; Zhao, H.; Yin, D.; Qiu, H.; Fu, Z.; Yin, D. Immobilized Chloroferrate Ionic Liquid: An Efficient and Reusable Catalyst for Synthesis of Diphenylmethane and its Derivatives. Catal. Lett. 2008, 123, 252–258. [Google Scholar] [CrossRef]
- Liu, S.; Obuchi, A.; Oi-Uchisawa, J.; Nanba, T.; Kushiyama, S. Synergistic catalysis of carbon black oxidation by Pt with MoO3 or V2O5. Appl. Catal. B Environ. 2001, 30, 259–265. [Google Scholar] [CrossRef]
- Welton, T. Room-Temperature Ionic Liquids. Solvents for Synthesis and Catalysis. Chem. Rev. 1999, 99, 2071–2084. [Google Scholar] [CrossRef]
- Wang, B.; Qin, L.; Mu, T.; Xue, Z.; Gao, G. Are Ionic Liquids Chemically Stable? Chem. Rev. 2017, 117, 7113–7131. [Google Scholar] [CrossRef]
- Adams, C.J.; Earle, M.J.; Roberts, G.; Seddon, K.R. Friedel-Crafts reactions in room temperature ionic liquids. Chem. Commun. 1998, 2097–2098. [Google Scholar]
- Wasserscheid, P.; Sesing, M.; Korth, W. Hydrogensulfate and tetrakis(hydrogensulfato)borate ionic liquids: Synthesis and catalytic application in highly Brønsted-acidic systems for Friedel-Crafts alkylation. Green Chem. 2002, 4, 134–138. [Google Scholar] [CrossRef]
- Gmouh, S.; Yang, H.; Vaultier, M. Activation of Bismuth(III) Derivatives in Ionic Liquids: Novel and Recyclable Catalytic Systems for Friedel-Crafts Acylation of Aromatic Compounds. Org. Lett. 2003, 5, 2219–2222. [Google Scholar] [CrossRef]
- Ross, J.; Xiao, J. Friedel-Crafts acylation reactions using metal triflates in ionic liquid. Green Chem. 2002, 4, 129–133. [Google Scholar] [CrossRef]
- Song, C.E.; Shim, W.H.; Roh, E.J.; Choi, J.H. Scandium(III) triflate immobilised in ionic liquids: A novel and recyclable catalytic system for Friedel-Crafts alkylation of aromatic compounds with alkenes. Chem. Commun. 2000, 1695–1696. [Google Scholar] [CrossRef]
- Liu, S.; Wang, K.; Yu, H.; Li, B.; Yu, S. Catalytic preparation of levulinic acid from cellobiose via Brønsted-Lewis acidic ionic liquids functional catalysts. Sci. Rep. 2019, 9, 1810. [Google Scholar] [CrossRef]
- Yadav, S.K.; Basaiahgari, A.; Gardas, R.L. Understanding the solvation behavior of SO3H functionalized Brønsted acidic ionic liquids in water and DMSO: Volumetric and acoustic approach. J. Mol. Liq. 2018, 266, 269–278. [Google Scholar] [CrossRef]
- Lunagariya, J.; Dhar, A.; Vekariya, R.L. Efficient esterification of n-butanol with acetic acid catalyzed by the Brønsted acidic ionic liquids: Influence of acidity. RSC Adv. 2017, 7, 5412–5420. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, J.; Ye, L.; Lin, H.; Yuan, Y. Effects of acidity and immiscibility of lactam-based Brønsted-acidic ionic liquids on their catalytic performance for esterification. Green Chem. 2010, 12, 661–665. [Google Scholar] [CrossRef]
- Xu, D.-Q.; Yang, W.-L.; Luo, S.-P.; Wang, B.-T.; Wu, J.; Xu, Z.-Y. Fischer Indole Synthesis in Brønsted Acidic Ionic Liquids: A Green, Mild, and Regiospecific Reaction System. Eur. J. Org. Chem. 2007, 1007–1012. [Google Scholar] [CrossRef]
- Xu, D.-Q.; Wu, J.; Luo, S.-P.; Zhang, J.-X.; Wu, J.-Y.; Du, X.-H.; Xu, Z.-Y. Fischer indole synthesis catalyzed by novel SO3H-functionalized ionic liquids in water. Green Chem. 2009, 11, 1239–1246. [Google Scholar] [CrossRef]
- Nagarajan, S.; Kandasamy, E. Reusable 1,2,4-Triazolium Based Brønsted Acidic Room Temperature Ionic Liquids as Catalyst for Mannich Base Reaction. Catal. Lett. 2014, 144, 1507–1514. [Google Scholar] [CrossRef]
- Tong, X.; Li, Y. Efficient and Selective Dehydration of Fructose to 5- Hydroxymethylfurfural Catalyzed by Brønsted-Acidic Ionic Liquids. ChemSusChem 2010, 3, 350–355. [Google Scholar] [CrossRef]
- Guo, S.; Du, Z.; Zhang, S.; Li, D.; Li, Z.; Deng, Y. Clean Beckmann rearrangement of cyclohexanone oxime in caprolactam-based Brønsted acidic ionic liquids. Green Chem. 2006, 8, 296–300. [Google Scholar] [CrossRef]
- Wang, A.; Zheng, X.; Zhao, Z.; Li, C.; Cui, Y.; Zheng, X.; Yin, J.; Yang, G. Brønsted acid ionic liquids catalyzed Friedel-Crafts Alkylations of electron-rich arenes with aldehydes. Appl. Catal. A Gen. 2014, 482, 198–204. [Google Scholar] [CrossRef]
- Lin, J.-H.; Zhang, C.-P.; Zhu, Z.-Q.; Chen, Q.-Y.; Xiao, J.-C. A novel pyrrolidinium ionic liquid with 1,1,2,2-tetrafluoro-2-(1,1,2,2- tetrafluoroethoxy)ethanesulfonate anion as a recyclable reaction medium and efficient catalyst for Friedel-Crafts alkylations of indoles with nitroalkenes. J. Fluorine Chem. 2009, 130, 394–398. [Google Scholar] [CrossRef]
- Taheri, A.; Lai, B.; Cheng, C.; Gu, Y. Brønsted acid ionic liquid-catalyzed reductive Friedel-Crafts alkylation of indoles and cyclic ketones without using external reductant. Green Chem. 2015, 17, 812–816. [Google Scholar] [CrossRef]
- Qiao, K.; Yokoyama, C. Novel Acidic Ionic Liquids Catalytic Systems for Friedel-Crafts Alkylation of Aromatic Compounds with Alkenes. Chem. Lett. 2004, 33, 472–473. [Google Scholar] [CrossRef]
- Zolfigol, M.A.; Moosavi-Zare, A.R.; Zarei, M. Friedel-Crafts alkylation of 4-hydroxycoumarin catalyzed by sulfonic-acid-functionalized pyridinium chloride as a new ionic liquid. C. R. Chim. 2014, 17, 1264–1267. [Google Scholar] [CrossRef]
- Wang, W.; He, Z.; Li, C.; You, Z.; Guo, H. Synthesis of Raspberry Ketone via Friedel-Crafts Alkylation Reaction Catalyzed by Sulfonic Acid-Functional Ionic Liquids. Pet. Chem. 2018, 58, 56–61. [Google Scholar] [CrossRef]
- Hayashi, S.; Hamaguchi, H. Discovery of a Magnetic Ionic Liquid [bmim]FeCl4. Chem. Lett. 2004, 33, 1590–1591. [Google Scholar] [CrossRef]
- Hayashi, S.; Saha, S.; Hamaguchi, H. A New Class of Magnetic Fluids: Bmim[FeCl4] and nbmim[FeCl4] Ionic Liquids. IEEE Trans. Magn. 2006, 42, 12–14. [Google Scholar] [CrossRef]
- Kumar, V.; Tomar, S.; Patel, R.; Yousaf, A.; Parmar, V.S.; Malhotra, S.V. FeCl3-Catalyzed Pechmann Synthesis of Coumarins in Ionic Liquids. Synth. Commun. 2008, 38, 2646–2654. [Google Scholar] [CrossRef]
- Saha, A.; Payra, S.; Dutta, D.; Banerjee, S. Acid-Functionalised Magnetic Ionic Liquid [AcMIm]FeCl4 as Catalyst for Oxidative Hydroxylation of Arylboronic Acids and Regioselective Friedel-Crafts Acylation. ChemPlusChem 2017, 82, 1129–1134. [Google Scholar]
- Bica, K.; Gaertner, P. An Iron-Containing Ionic Liquid as Recyclable Catalyst for Aryl Grignard Cross-Coupling of Alkyl Halides. Org. Lett. 2006, 8, 733–735. [Google Scholar] [CrossRef]
- Sayyahi, S.; Azin, A.; Saghanezhad, S.J. Synthesis and characterization of a novel paramagnetic functionalized ionic liquid as a highly efficient catalyst in one-pot synthesis of 1-amidoalkyl-2-naphtols. J. Mol. Liq. 2014, 198, 30–36. [Google Scholar] [CrossRef]
- Valkenberg, M.H.; deCastro, C.; Hölderich, W.F. Friedel-Crafts acylation of aromatics catalysed by supported ionic liquids. Appl. Catal. A Gen. 2001, 215, 185–190. [Google Scholar] [CrossRef]
- Panja, S.K.; Saha, S. Recyclable, magnetic ionic liquid bmim[FeCl4]- catalyzed, multicomponent, solvent-free, green synthesis of quinazolines. RSC Adv. 2013, 3, 14495–14500. [Google Scholar] [CrossRef]
- Nguyen, M.D.; Nguyen, L.V.; Jeon, E.H.; Kim, J.H.; Cheong, M.; Kim, H.S.; Lee, J.S. Fe-containing ionic liquids as catalysts for the dimerization of bicyclo[2.2.1]hepta-2,5-diene. J. Catal. 2008, 258, 5–13. [Google Scholar] [CrossRef]
- Solomons, G.; Fryhle, C.; Snyder, S. Organic Chemistry; Wiley: Hoboken, NJ, USA, 2013; ISBN 978-1118133576. [Google Scholar]
- Boon, J.A.; Levisky, J.A.; Pflug, J.L.; Wilkes, J.S. Friedel-Crafts Reactions in Ambient-Temperature Molten Salts. J. Org. Chem. 1986, 51, 480–483. [Google Scholar] [CrossRef]
- Valkenberg, M.H.; deCastro, C.; Hölderich, W.F. Immobilisation of chloroaluminate ionic liquids on silica materials. Top. Catal. 2001, 14, 139–144. [Google Scholar] [CrossRef]
- Yin, D.; Li, C.; Tao, L.; Yu, N.; Hu, S.; Yin, D. Synthesis of diphenylmethane derivatives in Lewis acidic ionic liquids. J. Mol. Catal. A Chem. 2006, 245, 260–265. [Google Scholar] [CrossRef]
- Li, C.; Liu, W.; Zhao, Z. Efficient synthesis of benzophenone derivatives in Lewis acid ionic liquids. Catal. Commun. 2007, 8, 1834–1837. [Google Scholar] [CrossRef]
- Sharma, R.; Mahajan, R.K. Influence of various additives on the physicochemical properties of imidazolium based ionic liquids: A comprehensive review. RSC Adv. 2014, 4, 748–774. [Google Scholar] [CrossRef]
- Wasserscheid, P.; Welton, T. Ionic Liquids in Synthesis; Wiley-VCH: Weinheim, Germany, 2008; ISBN 9783527621194. [Google Scholar]
- Chang, J.-C.; Yang, C.-H.; Sun, I.-W.; Ho, W.-Y.; Wu, T.-Y. Synthesis and Properties of Magnetic Aryl-Imidazolium Ionic Liquids with Dual Brønsted/Lewis Acidity. Materials 2018, 11, 2539. [Google Scholar] [CrossRef] [PubMed]
- Tareque, M.H.; Ismail, M.; Islam, S.T.A.; Kamruzzaman, M.; Saha, M. Reaction of Xylenes with Benzyl Chloride in the Presence of Anhydrous Aluminium Chloride. Bangladesh J. Sci. Ind. Res. 2006, 41, 251–256. [Google Scholar] [CrossRef]
- Kaneko, M.; Hayashi, R.; Cook, G.R. Intermolecular Friedel-Crafts reaction catalyzed by InCl3. Tetrahedron Lett. 2007, 48, 7085–7087. [Google Scholar] [CrossRef]
- Pal, N.; Paul, M.; Bhaumik, A. New mesoporous perovskite ZnTiO3 and its excellent catalytic activity in liquid. Appl. Catal. A Gen. 2011, 393, 153–160. [Google Scholar] [CrossRef]
- Yang, Y.-L.; Kou, Y. Determination of the Lewis acidity of ionic liquids by means of an IR spectroscopic probe. Chem. Commun. 2004, 226–227. [Google Scholar] [CrossRef] [PubMed]
- Atkins, P.; Paula, J.D. Physical Chemistry; W. H. Freeman: New York, US, 2009; ISBN 978-1429218122. [Google Scholar]
- Sun, I.-W.; Wu, S.-Y.; Su, C.-H.; Shu, Y.-L.; Wu, P.-L. Diels-Alder Reaction in Air- and Moisture-stable Zinc-containing Ionic Liquids. J. Chin. Chem. Soc. 2004, 51, 367–370. [Google Scholar] [CrossRef]
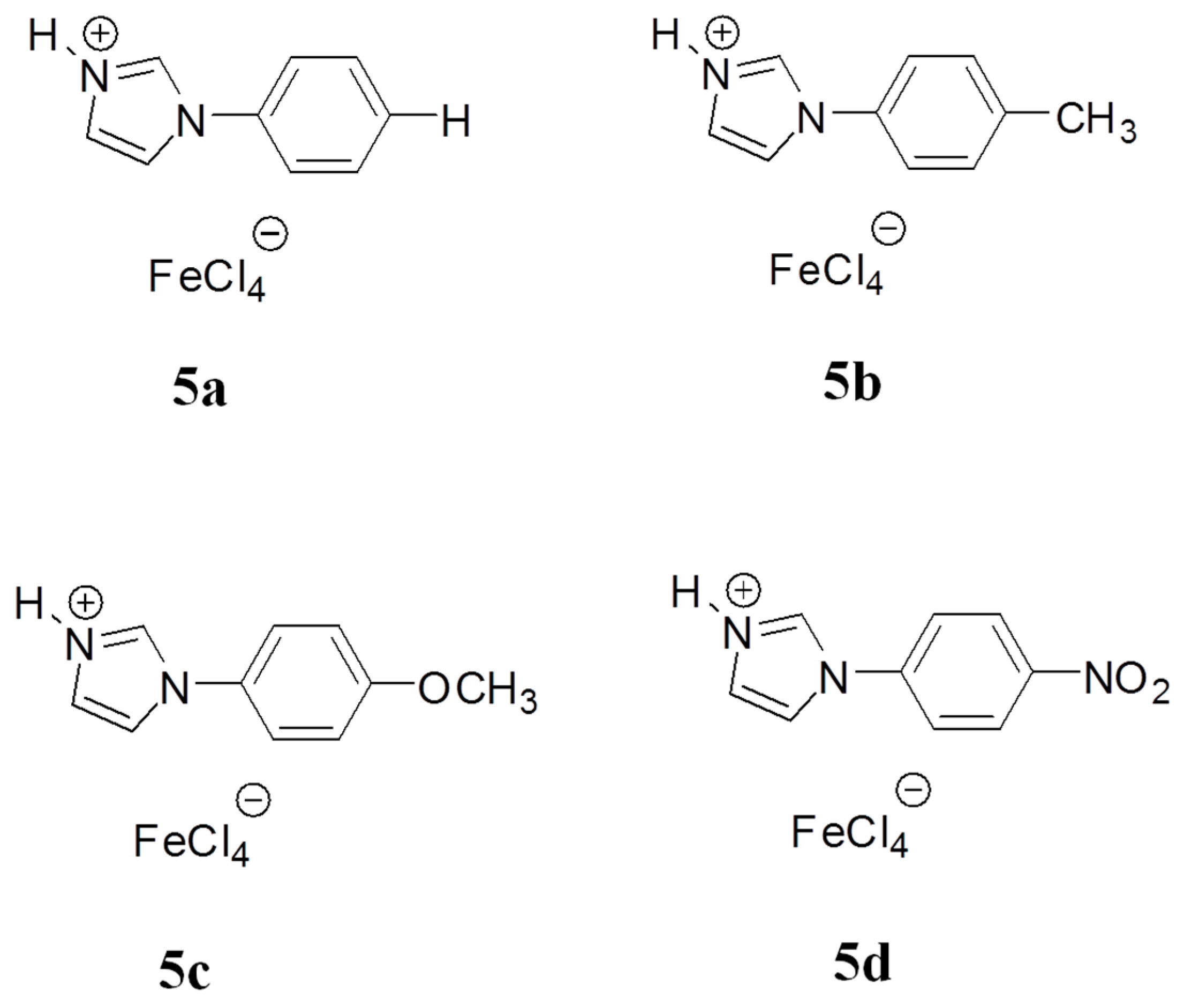
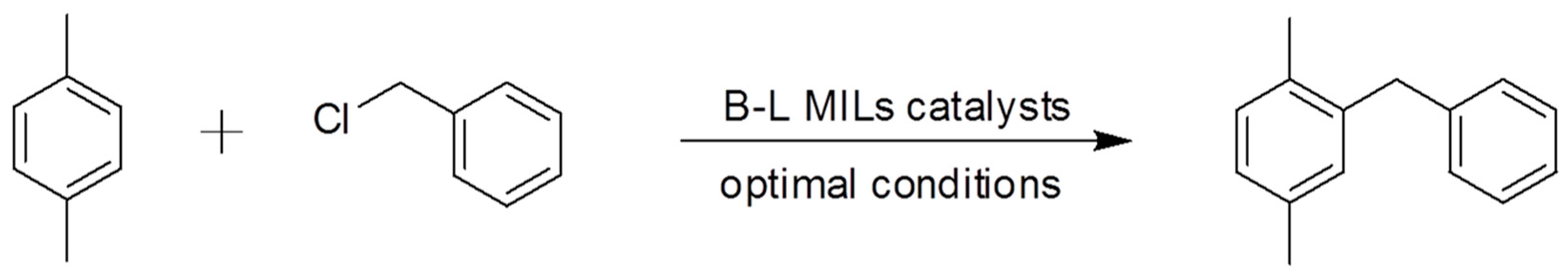

| Entry a | B-L MIL | Molar Ratio of IL/BnCl (mole %) | Molar Ratio of p-xylene: BnCl | BnCl Conversion (%) b | DPM Selectivity (%) b |
|---|---|---|---|---|---|
| 1 | 5a | 20.0 | 2:1 | 51.4 | 95.6 |
| 2 | 5b | 20.0 | 2:1 | 20.0 | 41.1 |
| 3 | 5c | 20.0 | 2:1 | 98.6 | 91.7 |
| 4 | 5d | 20.0 | 2:1 | 5.0 | 99.5 |
| 5 | 5c | 0.1 | 2:1 | 14.6 | 99.8 |
| 6 | 5c | 0.3 | 2:1 | 96.0 | 93.0 |
| 7 | 5c | 0.5 | 2:1 | 98.9 | 88.4 |
| 8 | 5c | 0.7 | 2:1 | 99.6 | 81.1 |
| 9 | 5c | 1.0 | 2:1 | 99.2 | 91.0 |
| 10 | 5c | 1.0 | 4:1 | 95.0 | 93.0 |
| 11 | 5c | 1.0 | 6:1 | 99.1 | 96.0 |
| 12 | 5c | 1.0 | 8:1 | 98.6 | 98.3 |
| 13 | 5c | 1.0 | 10:1 | 99.3 | 99.3 |
| 14 | 5c | 1.0 | 1:1 | 99.9 | 75.0 |
| Catalyst | Reaction Temperature (°C) | Reaction Time (h) | Catalyst Amount | Molar Ratio of Reactants | Re-Used Cycle Number | Product Yield (%) | Reference |
|---|---|---|---|---|---|---|---|
| 5c a | 80 | 0.5 | 1 mole % | 6:1 | 6 | 95.0 | This work |
| AlCl3 | 130 | 1 | 2 wt % | 6:1 | n.r.d | 96.5 | 52 |
| InCl3 | 50 | 16 | 10 mole % | 10:1 | n.r. | 75.0 | 53 |
| PmimCl-FeCl3-MCM-41 | 80 | 0.75 | 15 wt % | 10:1 | n.r. | 100.0 | 11 |
| MZT-11 | 75 | 24 | 1 wt % | 10:1 | n.r. | 92.3 | 54 |
| [HSO3-pmim][OTf] | 40 | 0.5 | 20 mole % | 2:1 | 5 | 93–97 | 29 |
| [Pyrro][H(CF2)4O(CF2)2SO3] | 50 | 24 | 2 mL | 2:1 | n.r. | 90.0 | 30 |
| [EMIM]Cl-AlCl3 b | 20 | 1 | n.r. | n.r. | n.r. | 89.0 | 15 |
| [BMIM][B(HSO4)4] | 50 | 1 | 5 mole % | 5:1 | n.r. | 51.0 | 16 |
| Bi(OTf)3/[EMIM][NTf2] | 150 | 3 | 5 mole % | 2:1 | 4 | 91.0 | 17 |
| Cu(OTf)3/[BMIM][BF4] | 80 | 24 | 5 mole % | 5:1 | n.r. | 81.0 | 18 |
| Sc(OTf)3/[BMIM][SbF6] | 20 | 12 | 20 mole % | 2:1 | n.r. | 92.0 | 19 |
| [BMIM]Cl-AlCl3 b, c | 80 | 2 | 1 mL | 10:1 | 1 | 100.0 | 47 |
| [BMIM]Cl-FeCl3 b, c | 80 | 2 | 1 mL | 10:1 | 3 | 97.1 | 47, 48 |
| [BMIM]Cl-FeCl2 b, c | 80 | 2 | 1 mL | 10:1 | 2 | 99.4 | 47 |
| [BMIM]Cl-ZnCl2 b | 80 | 2 | 1 mL | 10:1 | 8 | 98.6 | 47 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.-H.; Chang, J.-C.; Wu, T.-Y.; Sun, I.-W.; Wu, J.-H.; Ho, W.-Y. Novel Aryl-Imidazolium Ionic Liquids with Dual Brønsted/Lewis Acidity as Both Solvents and Catalysts for Friedel–Crafts Alkylation. Appl. Sci. 2019, 9, 4743. https://doi.org/10.3390/app9224743
Yang C-H, Chang J-C, Wu T-Y, Sun I-W, Wu J-H, Ho W-Y. Novel Aryl-Imidazolium Ionic Liquids with Dual Brønsted/Lewis Acidity as Both Solvents and Catalysts for Friedel–Crafts Alkylation. Applied Sciences. 2019; 9(22):4743. https://doi.org/10.3390/app9224743
Chicago/Turabian StyleYang, Che-Hsuan, Jui-Cheng Chang, Tzi-Yi Wu, I-Wen Sun, Jun-Hao Wu, and Wen-Yueh Ho. 2019. "Novel Aryl-Imidazolium Ionic Liquids with Dual Brønsted/Lewis Acidity as Both Solvents and Catalysts for Friedel–Crafts Alkylation" Applied Sciences 9, no. 22: 4743. https://doi.org/10.3390/app9224743
APA StyleYang, C.-H., Chang, J.-C., Wu, T.-Y., Sun, I.-W., Wu, J.-H., & Ho, W.-Y. (2019). Novel Aryl-Imidazolium Ionic Liquids with Dual Brønsted/Lewis Acidity as Both Solvents and Catalysts for Friedel–Crafts Alkylation. Applied Sciences, 9(22), 4743. https://doi.org/10.3390/app9224743