Featured Application
Reliable and rapid analysis of free fatty acid (FFA) in olive oil is possible from the relative intensity ratio of characteristic vibrational modes observed by Raman spectroscopy.
Abstract
Free fatty acid (FFA) is one of the most critical parameters for evaluating the quality of olive oil. In this paper, we present a simple and rapid Raman spectroscopy method for analyzing free fatty acid in olive oil. First, FFA degradation of carotenoids in olive oil is confirmed by analyzing the relative intensity of characteristic vibrational modes and introducing an intensity decrease factor. Second, it is demonstrated that the relative intensity ratio of the two characteristic vibrational modes at 1525 cm−1 and 1655 cm−1 presents a good and rapid analysis of FFA content in olive oil; the relative intensity ratio decreases linearly with FFA content. In addition, resonance Raman scattering of carotenoid is discussed, showing that a green laser should be utilized to study FFA in olive oil.
1. Introduction
Olive oil is extracted from olive fruit mechanically without any thermal or chemical treatment. It is highly prized for its health benefits, delicious taste and fine aroma [1,2,3,4,5]. Free fatty acid (FFA) content is one of the most critical parameters to evaluate the quality of olive oil, since FFA causes flavor deterioration and shelf life decrease of olive oil [6,7,8]. According to the International Olive Oil Council, the FFA content in extra virgin olive oil should be lower or equal to 0.8%, while low-quality olive oil with FFA content above 3.3% is considered unsuitable for human consumption and must be refined prior to consumption [9]. Therefore, rapid analysis of FFA content in olive oil deserves research attention.
Various methods have been applied for the analyses of FFA content in oil, such as titration method [10,11,12]; pH-metric, chromatographic, colorimetric techniques [13,14]; electrical impedance spectroscopy [15]; Fourier transform infrared absorption [16,17,18,19]; Fourier transform Raman scattering with infrared excitation at 1064 nm [1,20]; and Raman scattering with visible excitation [6,7]; and so forth. Practically, the titration method of the American Oil Chemists’ Society (AOCS) with NaOH is commonly applied for the determination of FFA content in olive oil. The Raman method has the advantages of short measurement time, nondestructive analyses, high sensitivity, no sample preparation, no consumption of chemicals, and so forth. Also, a visible excitation source can help to resonantly excite vibrational bands of many important constituents of oils [6,21]. In a previous visible Raman study of FFA content in extra virgin olive oil, it was reported that the spectral window of 945–1600 cm−1 is a useful fingerprint region for prediction of the FFA% [6]. In the study, Raman scattering signal obtained directly from experiment (i.e., absolute Raman intensity) were analyzed. For practical applications, the analysis of absolute Raman intensity would be difficult, since comparison of absolute intensity would require same experimental conditions.
In this paper, relative Raman intensity analysis — the study of relative intensity ratios of characteristic vibrational modes, is applied to investigate FFA in olive oils. Relative intensity ratios are more reliable parameters than absolute Raman intensities, thus would make practical application of Raman method possible. Our relative intensity analysis shows that in the spectral window of 945–1600 cm−1, the two characteristic vibrational modes at 1525 cm−1 and 1155 cm−1 are correlated with FFA content in olive oil. In addition, the relative intensity ratio of vibrational modes at 1525 cm−1 and 1655 cm−1 presents a good and rapid analysis of FFA content in extra virgin olive oil; the intensity ratio decreases linearly with FFA content. Portable Raman system operating at 785 nm had been applied for prediction of fatty acid composition in vegetable oils and identification of waste cooking oils [22,23]. The relative intensity analysis is not dependent on experimental conditions, thus applying it in portable Raman system operating with a visible laser would be very helpful for quick on-site quality evaluation of olive oils.
2. Experiment
Five extra virgin olive oil samples, one pomace olive oil sample, and their mixtures have been investigated. Sample A: Pago Baldios San Carlos Extra Virgin Olive Oil (originally from Spain) and sample B: Lorenzo No. 1 Extra Virgin Olive Oil (originally from Italy) were purchased from a Shinsegae Department Store, in Seoul, Korea. Sample C is Huilerie Loued Extra Virgin Olive Oil (originally from Tunisia), which was purchased from a duty-free store in Wuhan, China. Sample D: Luhua Extra Virgin Olive Oil (originally from Spain) and sample E: Olivoila Extra Virgin Olive Oil (originally from Italy) were purchased from a Carrefour store in Wuhan, China. Sample F is Costa d’Oro Pomace Olive Oil (originally from Italy), which was purchased from Taobao in China. The FFA contents of Sample A, B, C, D, E, and F were 0.1%, 0.15%, 0.4%, 0.6%, 0.8%, and 3%, respectively; which were declared by the producers of these samples. Also, the mixtures of C and F, C and D, D and E, and C and E were prepared by mixing two samples with a desired volume ratio under stirring, then waiting one day for equilibrium.
Raman spectra of the samples were obtained in backscattering configuration with two different micro-Raman systems, XperRam200 system from NANOBASE company and DXR system from ThermoFisher company (Waltham, MA, USA). For the XperRam200 system, the excitation source was a 532 nm green laser. For the DXR system, two excitation sources were used: a 633 nm red laser and a 785 nm near infrared laser. The scattered signal was detected by air-cooled charge-coupled device (CCD) detector. The samples were placed on glass flasks. The laser power on sample was varied up to 5 mW to check the laser power influences on the Raman spectral features of the samples, which indicated that laser heating or damage of the samples was negligible in our experiment. All the spectra were recorded at room temperature about 25 °C with 15 s acquisition time and 2-scan average. All the spectra were baseline corrected with Origin software, and wavenumber calibrated using the 521 cm−1 vibrational mode of a Si substrate. Also, the Si spectrum was measured after experiments to ensure the calibration was not changed during the measurements. For each sample, 5 random spectra were measured and the average spectrum was used for the analyses of relative intensity ratios.
3. Results and Discussion
Figure 1 presents the Raman scattering spectra of the six olive oil samples of A, B, C, D, E, and F with 532 nm green laser excitation. All these samples show characteristic vibrational modes at 1080 cm−1, 1265 cm−1, 1300 cm−1, 1440 cm−1, 1655 cm−1, and 1750 cm−1. The mode at 1080 cm−1 can be assigned to (C–C) stretching of the (CH2)n group; the mode at 1265 cm−1 can be assigned to (=C–H) deformation of cis (R–HC=CH–R); the mode at 1300 cm−1 can be assigned to (C–H) bending twist of the CH2 group; the mode at 1440 cm−1 can be assigned to (C–H) scissoring of CH2; the mode at 1655 cm−1 can be assigned to (C=C) of cis (RHC=CHR); the mode at 1750 cm−1 can be assigned to (C=O) stretching of RC=OOR [24,25,26,27]. These are the common characteristic vibrational modes of almost all edible vegetable oils.
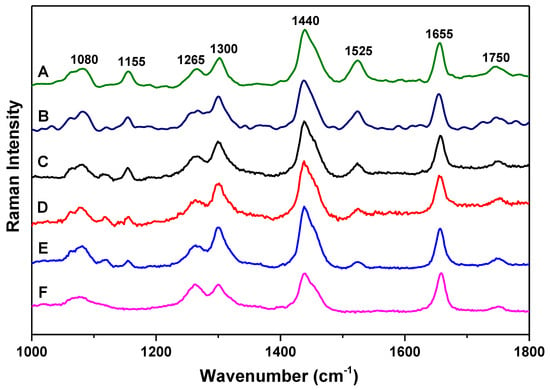
Figure 1.
Raman spectra of olive oil samples A, B, C, D, E, and F of different origin. Each spectrum is y-shifted for clarity.
The major difference between low-quality pomace olive oil and extra virgin olive oil samples is the appearance of the two vibrational modes at 1155 cm−1 and 1525 cm−1. In the extra virgin olive oil samples, these two modes can be observed, while in the low-quality pomace olive oil sample, these two modes are not detectable. Also, the intensity of these two modes varies with the FFA content in extra virgin olive oil samples. The mode at 1155 cm−1 can be assigned to C–C stretching of carotenoids, and the mode at 1525 cm−1 can be assigned to C=C stretching of carotenoids [26,28,29]. The carotenoids play an important role as natural antioxidants in olive oils. The observation of these two modes would be very helpful for the analyses of different quality olive oils.
As can be seen in Figure 1, the intensity of the two vibrational modes at 1155 cm−1 and 1525 cm−1 decreases systematically with increasing FFA content in olive oil, which is consistent with reported results [6]. This intensity decrease indicates that FFA would degrade carotenoids in olive oil, thus higher FFA content is associated with lower intensity of carotenoids vibrational modes. Due to the FFA degradation of carotenoids, when an extra virgin olive oil is mixed with a lower-quality pomace olive oil, the intensity decrease of carotenoids Raman modes due to volume increase would be more significant than that of other modes in extra virgin olive oil. Below, we present a simple Raman method to confirm the FFA degradation of carotenoids by analyzing the relative intensity of characteristic vibrational modes and introducing an intensity decrease factor. This method would be helpful also to study possible degradation process in other materials.
Figure 2 presents the Raman spectra of the mixtures of sample C and F with volume ratios of 2:1, 3:2, 1:1, 2:3, and 1:2. For comparison, the Raman spectra of sample C and F are also included in the figure, and the intensities of all the spectra are normalized using the intensity of the vibrational mode at 1655 cm−1 as a reference. In Figure 2, the spectra were normalized only for better observation of the difference of relative intensity ratios. The mode at 1655 cm−1 mode has relatively good line-shape and is not much affected by the neighboring modes, thus was chosen for normalization. As can be seen in Figure 2, the intensity decrease of the two vibrational modes at 1155 cm−1 and 1525 cm−1 is much faster than other modes of extra virgin olive oil. Also, the intensity decrease of the two modes is faster than the volume increase when sample F is added to sample C. Furthermore, the intensity of these two modes has more significant intensity decrease when the volume ratio of sample F increases in the mixture. These observations indicate that when FFA content increases in the mixture, FFA would have more significant degradation of carotenoid, whose characteristic vibrational modes are 1155 cm−1 and 1525 cm−1.
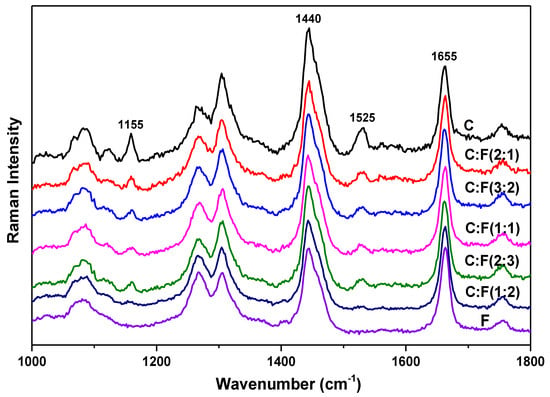
Figure 2.
Raman spectra of the mixtures of sample C and F with volume ratios of 2:1, 3:2, 1:1, 2:3, and 1:2. For comparison, the spectra of sample C and F are included in the figure. The intensities of all the spectra are normalized using the intensity of the vibrational mode at 1655 cm−1 as a reference. Each spectrum is y-shifted for clarity.
For a clear representation of the intensity decrease of the two vibrational modes at 1155 cm−1 and 1525 cm−1 in the mixture, an intensity decrease factor x is introduced as follows. When samples C and F are mixed, for example with a volume ratio of 1:1, we assume that the intensities of 1155 cm−1 and 1525 cm−1 modes in the mixture would be x/2 (x should be smaller than 1) of their intensities in sample C, and the intensities of all the other modes in samples C and F would be half of their intensities. Then in the mixture (M), the relative intensity ratio of IM,1440/IM,1525 and IM,1655/IM,1525 can be estimated by (IC,1440/xIC,1525 + IF,1440/xIF,1525) and (IC,1655/xIC,1525 + IF,1655/xIF,1525), respectively. Therefore, through the analysis of relative intensity ratios, the intensity decrease factor x of 1525 cm−1 mode can be calculated with the ratio of {(IC,1440/IC,1525) − (IC,1655/IC,1525)·(IF,1440/IF,1655)} to {(IM,1440/IM,1525) − (IM,1655/IM,1525)·(IF,1440/IF,1655)}. The calculated values for intensity decrease factor x of 1525 cm−1 mode are presented in Table 1. Similarly, the intensity decrease factor x of 1155 cm−1 mode can be calculated; the values are also included in Table 1. The intensity decrease factor for 1155 cm−1 and 1525 cm−1 modes should be same, the small difference between the two x values would be mainly due to analysis error from measuring the intensities of the vibrational modes. The mode at 1525 cm−1 is less affected by its surrounding peaks than that of 1155 cm−1, thus the intensity decrease factor x of 1525 cm−1 mode would be more accurate than that of 1155 cm−1. As can be seen in Table 1, the intensity decrease factor x is indeed smaller than 1 and it decreases systematically with increasing FFA content in the mixture. This further confirms the FFA degradation of carotenoid in olive oil.

Table 1.
The experimental relative intensity ratios: I1440/I1525, I1440/I1655, I1655/I1525; and the calculated intensity decrease factors: x1525, x1155.
The above results show that relative Raman intensity analysis is very helpful for investigating FFA content in extra virgin olive oil. The analysis confirmed that the two vibrational modes at 1155 cm−1 and 1525 cm−1 are characteristic Raman modes for analyzing FFA content in extra virgin olive oil, and their intensities decrease systematically with increasing FFA content in extra virgin olive oil. This is consistent with the reported result that the spectral region of 945–1600 cm−1, which includes the carotenoid Raman modes, is important for predicting the FFA content in olive oil [6]. In the study, absolute Raman intensities of various FFA content extra virgin olive oil samples were analyzed, and it was found that the absolute Raman intensity of the spectral window 945–1600 cm−1 increases systematically with decreasing FFA content in extra virgin olive oils. In the Raman study, the analysis of relative intensity ratios would be easier and more accurate than absolute intensity, since absolute Raman intensity depends on various factors such as alignment of the Raman system, laser power stability, focus of the laser, absorption coefficient of the sample, and so forth. We show below that the study of relative intensity ratio of vibrational modes at 1525 cm−1 and 1655 cm−1 presents a good and rapid analysis of FFA content in extra virgin olive oil, which would be helpful for quick quality evaluation of extra virgin olive oil.
Figure 3 presents the relative intensity ratios of I1525/I1655 and I1155/I1655 as a function of FFA content in extra virgin olive oil samples; for comparison, the relative intensity ratio of I1440/I1655 is also presented in the figure. Seven different FFA contents from 0.1% to 0.8% are included in Figure 3. Samples A, B, C, D, and E had FFA content of 0.1%, 0.15%, 0.4%, 0.6%, and 0.8%, respectively. Mixtures of C and D, and D and E with volume ratio 1:1 were prepared to obtain FFA content of 0.5% and 0.7%, respectively. Also, a mixture of C and E with volume ratio 1:1 was prepared to obtain FFA content of 0.6%, for comparison with sample D. As can be seen in Figure 3, the relative intensity ratios of I1525/I1655 and I1155/I1655 decrease linearly with increasing FFA contentin extra virgin olive oils. The intensity ratio of I1525/I1655 shows a better linear correlation with FFA content in extra virgin olive oil than that of I1155/I1655. This would be due to the vibrational mode at 1525 cm−1 being less affected by its surrounding peaks than that of 1155 cm−1, consistent with the result in Table 1. Therefore, the relative intensity ratio of I1525/I1655 would be a good parameter for rapid analyses of FFA content in extra virgin olive oil.
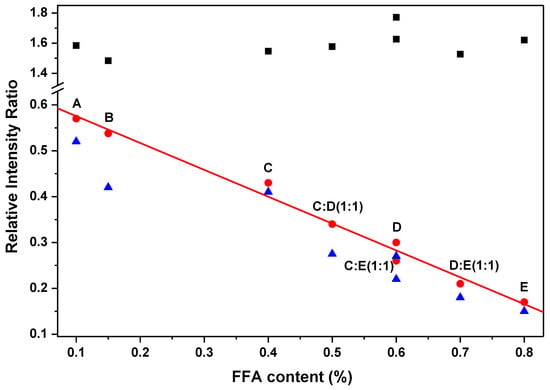
Figure 3.
Relative intensity ratios of I1525/I1655 (bullet), I1155/I1655 (triangle), and I1440/I1655 (square) as a function of free fatty acid (FFA) content in extra virgin olive oil. The straight line is a linear fitting of I1525/I1655.
In the above analysis, the mode at 1655 cm−1 was chosen as a reference for calculation of relative intensity ratios. This would be valid for the Raman study of same type of vegetable oils. For comparison of different types of vegetable oils, the mode at 1440 cm−1 could be chosen as a reference [30]. In our recent study, the relative intensity ratio of 1655 cm−1 to 1440 cm−1 has been systematically investigated in various types of vegetable oils and three different types of pure unsaturated fatty acids (oleic acid, linoleic acid, and α-linolenic acid) [31]. This study showed that the mode at 1440 cm−1 can be chosen as a reference for first order differentiation of different types of vegetable oils; and for more accurate analysis of same type of vegetable oils, the mode at 1655 cm−1 can be chosen as a reference. In addition, a systematic density functional theory (DFT) calculation of the Raman spectra of unsaturated fatty acids up to six carbon–carbon double bonds, is currently underway, which also supports the above method for choosing the reference mode. The DFT result will be presented elsewhere.
The extra virgin olive oil samples were purchased from standard supermarkets in China and Korea; the samples and their mixtures were investigated without other treatment. Through relative intensity analysis, we first confirmed that the intensities of characteristic vibrational modes at 1155 cm−1 and 1525 cm−1 of extra virgin olive oil decrease systematically with increasing FFA content; then showed that the intensity ratio of I1525/I1655 decreases linearly with FFA content. This linear correlation presents a good and rapid analysis of FFA content in extra virgin olive oil. In recent years, portable and handheld Raman systems have been developed. One advantage of our method is that it is not depending on experimental conditions. Thus, when our method is combined with portable and handheld Raman systems, it would be powerful for quick on-site quality evaluation of extra virgin olive oil in supermarkets.
The main purpose of this paper is to propose that relative Raman intensity analysis is very useful for quick quality evaluation of extra virgin olive oils. For practical application of our method, a standard correlation between FFA content and relative intensity ratio I1525/I1655 should be established, which would require accurate measurements of FFA% and I1525/I1655. With 532 nm laser excitation, the intensity of 1525 cm−1 mode is quite weak; a significant enhancement of this mode would be very helpful to improve the accuracy of I1525/I1655. Thus, a resonance investigation would be necessary, as discussed below.
To achieve significant enhancement of 1525 cm−1 mode, the wavelength of excitation laser should be close to the absorption edge of carotenoids. The absorption study showed that the absorption edge of carotenoids is about 510 nm [32]. Therefore, the intensity of the 1525 cm−1 mode would be strongly enhanced with excitation about 510 nm, and decrease with higher excitation wavelength. This is consistent with a reported study with 514.5 nm laser [6]; and our study with 532 nm, 633 nm, and 785 nm lasers, which is presented in Figure 4. It was shown that with 514.5 nm laser excitation, 1525 cm−1 mode have very strong intensity, which is even higher than that of 1655 cm−1 mode [6]. While 1525 cm−1 mode is much weaker than 1655 cm−1 mode with 532 nm laser excitation, as can be seen in Figure 4, 1525 cm−1 mode is too weak to be observed with 785 nm laser excitation, and with 633 nm laser excitation, a strong luminescence peak at about 670 nm is observed, and no vibrational modes can be observed due to the strong luminescence background. Therefore, a green laser of 514.5 nm would be ideal for practical application of relative intensity analysis of FFA in olive oils.
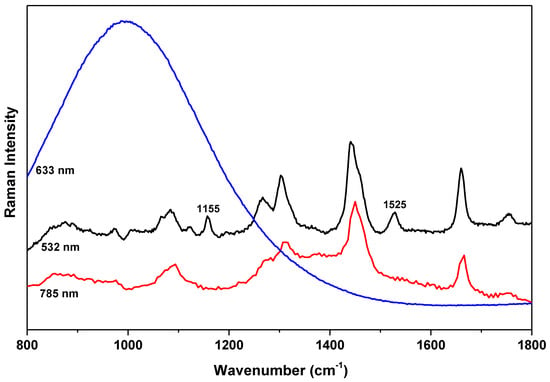
Figure 4.
Raman spectra of olive oil sample C by three different wavelength lasers of 532 nm, 633 nm, and 785 nm. Each spectrum is y-shifted for clarity.
Our above results indicate that the study of relative intensity ratio of the two characteristic vibrational modes at 1525 cm−1 and 1655 cm−1 with 514.5 nm green laser excitation would be of great interest for quick on-site quality evaluation of olive oils. This simple relative intensity analysis of 1525 cm−1 and 1655 cm−1 modes would be powerful only for quick evaluation of similar types of high-quality olive oils, such as extra virgin olive oils. For quick on-site detection of olive oil adulteration, further systematic studies would be needed. For instance, if olive oils are adulterated with other types of vegetables oils, then the mode at 1655 cm−1 would not be a good reference, thus the intensity ratio of I1525/I1655 cannot be simply applied for detecting olive oil adulteration. In a recent study, Li et al. [33] showed that Raman spectroscopy combined with iPLS and SiPLS is helpful for detection of olive oil adulteration with waste cooking oil. In our recent study, it was shown that Raman spectroscopy combined with two-dimensional correlation spectroscopy (2DCOS) is helpful for differentiating different types of vegetable oils [31]. These studies suggest that Raman spectroscopy combined with 2DCOS and chemometrics method would be helpful for practical application of on-site quality evaluation of olive oil adulteration.
4. Conclusions
In conclusion, Raman spectroscopy has been applied for rapid analysis of FFA content in olive oil. Our results show that the intensities of the two characteristic vibrational modes at 1525 cm−1 and 1155 cm−1 decrease systematically with increasing FFA content in olive oil, due to carotenoid degradation caused by FFA. In addition, the relative intensity ratio of the vibrational modes at 1525 cm−1 and 1655 cm−1 decreases linearly with FFA content, thus it can be applied for rapid analysis of the quality of olive oil. Furthermore, it has been discussed that a 514.5 nm green laser would be ideal for Raman study of FFA in olive oil.
Author Contributions
J.Q. performed the experiments, analyzed the data, and wrote the original draft; H.-Y.H. analyzed the data and participated in discussions of the research; I.-S.Y. and X.-B.C. supervised the project and revised the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (Grant No. 11574241) and the National Research Foundation of Korea (NRF) grant funded by the Korean Ministry of Science and ICT (No. 2017R1A2B2009309).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yang, H.; Irudayaraj, J. Comparison of Near-Infrared, Fourier Transform-Infrared, and Fourier Transform-Raman Methods for Determining Olive Pomace Oil Adulteration in Extra Virgin Olive Oil. J. Am. Oil Chem. Soc. 2001, 78, 889–895. [Google Scholar] [CrossRef]
- Kiritsakis, A.K.; Lenart, E.B.; Willet, W.C. Olive Oil: From the Tree to the Table, 2nd ed.; Food & Nutrition Press: Trumbull, CT, USA, 1998; pp. 1–25. [Google Scholar]
- Baeten, V.; Meurens, M.; Morales, M.T.; Aparicio, R. Detection of virgin olive oil adulteration by Fourier transform Raman spectroscopy. J. Agric. Food Chem. 1996, 44, 2225–2230. [Google Scholar] [CrossRef]
- Morales, M.T.; Alonso, M.V.; Rios, J.J.; Aparicio, R. Virgin olive oil aroma: Relationship between volatile compounds and sensory attributes by chemometrics. J. Agric. Food Chem. 1995, 43, 2925–2931. [Google Scholar] [CrossRef]
- Overton, S.V.; Manura, J.J. Analysis of volatile organic in cooking oils by thermal desorption gas chromatography mass spectrometry. J. Agric. Food Chem. 1995, 43, 1314–1320. [Google Scholar] [CrossRef]
- I-Abassy, R.M.E.; Donfack, P.; Materny, A. Rapid Determination of Free Fatty Acid in Extra Virgin Olive Oil by Raman Spectroscopy and Multivariate Analysis. J. Am. Oil Chem. Soc. 2009, 86, 507–511. [Google Scholar] [CrossRef]
- Abassy, R.M.E.; Donfack, P.; Materny, A. Visible Raman spectroscopy for the discrimination of olive oils from different vegetable oils and the detection of adulteration. J. Raman Spectrosc. 2009, 40, 1284–1289. [Google Scholar] [CrossRef]
- Perretti, G.; Finotti, E.; Adamuccio, S.; Sera, R.D.; Montanari, L. Composition of organic and conventionally produced sunflower seed oil. J. Am. Oil Chem. Soc. 2004, 81, 1119–1123. [Google Scholar] [CrossRef]
- O’Brien, R.D. Fats and Oils, Formulating and Processing for Applications, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2004; pp. 34–35. [Google Scholar]
- Coteron, A.; Martinez, M.; Aracil, J. Reactions of Olive Oil and Glycerol over Immobilized Lipases. J. Am. Oil Chem. Soc. 1998, 75, 657–660. [Google Scholar] [CrossRef]
- Mariotti, E.; Mascini, M. Determination of extra virgin olive oil acidity by FIA-Titration. Food Chem. 2001, 73, 235–238. [Google Scholar] [CrossRef]
- Sun-Waterhouse, D.; Zhou, J.; Miskelly, G.M.; Wibisono, R.; Wadhwa, S.S. Stability of encapsulated olive oil in the presence of caffeic acid. Food Chem. 2011, 126, 1049. [Google Scholar] [CrossRef]
- Ackman, R.G. Gas-Liquid chromatographic analysis of fatty acids and glycerides in foods. Food Sci. Technol. 1992, 53, 47–63. [Google Scholar]
- Tuŕyan, Y.I.; Berezin, O.Y.; Kuselman, I.; Shenhar, A. pH-Metric determination of acid values in vegetable oils without titration. J. Am. Oil Chem. Soc. 1996, 73, 295–301. [Google Scholar] [CrossRef]
- Grossi, M.; di Lecce, G.; GallinaToschi, T.; Riccò, B. A novel electrochemical method for olive oil acidity determination. Microelectron. J. 2014, 45, 1701–1707. [Google Scholar] [CrossRef]
- Al-Alawi, A.; van de Voort, F.R.; Sedman, J. New FTIR method for the determination of FFA in oils. J. Am. Oil Chem. Soc. 2004, 81, 441–446. [Google Scholar] [CrossRef]
- Bertran, E.; Blanco, M.; Coello, J.; Iturriaga, H.; Maspoch, S.; Montoliu, I. Determination of olive oil free fatty acid by Fourier transform infrared spectroscopy. J. Am. Oil Chem. Soc. 1999, 76, 611–616. [Google Scholar] [CrossRef]
- Man, Y.B.C.; Setiowaty, G. Application of Fourier transform infrared spectroscopy to determine free fatty acid contents in palm olein. Food Chem. 1999, 66, 109–114. [Google Scholar] [CrossRef]
- Ng, C.L.; Wehling, R.L.; Cuppett, S.L. Method for determining frying oil degradation by Near-Infrared spectroscopy. J. Agric. Food Chem. 2007, 55, 593–597. [Google Scholar] [CrossRef]
- Muik, B.; Lendl, B.; Molina-Díaz, A.; Ayora-Canada, M.J. Direct, reagent-free determination of free fatty acid content in olive oil and olives by Fourier transform Raman spectrometry. J. Anal. Chim. Acta 2003, 487, 211–220. [Google Scholar] [CrossRef]
- Cannizzaro, C.; Rhiel, M.; Marison, I.; von Stockar, U. On-Line monitoring of Phaffiarhodozyma Fed-Batch process with in situ dispersive Raman spectroscopy. Biotechnol. Bioeng. 2003, 83, 668–680. [Google Scholar] [CrossRef]
- Dong, W.; Zhang, Y.; Zhang, B.; Wang, X. Rapid prediction of fatty acid composition of vegetable oil by Raman spectroscopy coupled with least squares support vector machines. J. Raman Spectrosc. 2013, 44, 1739–1745. [Google Scholar] [CrossRef]
- Huang, F.; Li, Y.; Guo, H.; Xu, J.; Chen, Z.; Zhang, J.; Wang, Y. Identification of waste cooking oil and vegetable oil via Raman spectroscopy. J. Raman Spectrosc. 2016, 47, 860–864. [Google Scholar] [CrossRef]
- Baeten, V.; Dardenne, P.; Aparicio, R. Interpretation of Fourier transform Raman spectra of the unsaponifiable matter in a selection of edible oils. J. Agric. Food Chem. 2001, 49, 5098–5107. [Google Scholar] [CrossRef] [PubMed]
- Osawaa, C.C.; Gonc-alvesa, L.A.G.; Ragazzib, S. Correlation between free fatty acids of vegetable oils evaluated by rapid tests and by the official method. J. Food Compost Anal. 2007, 20, 523–528. [Google Scholar] [CrossRef]
- Bernstein, P.S. New insights into the role of the macular carotenoids in age-related macular degeneration. Resonance Raman studies. J. Pure Appl. Chem. 2002, 74, 1419–1425. [Google Scholar] [CrossRef]
- Fan, Y.; Li, S.; Xu, D.P. Raman spectra of oleic acid and linoleic acid. Spectrosc. Spect. Anal. 2013, 33, 3240–3324. [Google Scholar]
- Withnall, R.; Chowdhry, B.Z.; Silver, J.; Edwards, H.G.M.; de Oliveira, L.F.C. Raman spectra of carotenoids in natural products. Spectrochim. Acta A 2003, 59, 2207–2212. [Google Scholar] [CrossRef]
- Macernis, M.; Sulskus, J.; Malickaja, S. Resonance Raman spectra and electronic transitions in carotenoids: A density functional theory study. J. Phys. Chem. A 2014, 118, 1817–1825. [Google Scholar] [CrossRef]
- López-Díez, E.C.; Bianchi, G.; Goodacre, R. Rapid Quantitative Assessment of the Adulteration of Virgin Olive Oils with Hazelnut Oils Using Raman Spectroscopy and Chemometrics. J. Agric. Food Chem. 2003, 51, 6145–6150. [Google Scholar] [CrossRef]
- Qiu, J.; Hou, H.Y.; Huyen, N.T.; Yang, I.S.; Chen, X.B. Raman spectroscopy and 2DCOS analysis of unsaturated fatty acid in edible vegetable oils. Appl. Sci. 2019, 9, 2807. [Google Scholar] [CrossRef]
- Silva, V.D.; Conceição, J.N.; Oliveira, I.P.; Lescano, C.H.; Muzzi, R.M.; Filho, O.P.S.; Conceição, E.C.; Casagrande, G.A.; Caires, A.R.L. Oxidative Stability of Baru (Dipteryxalata Vogel) Oil Monitored by Fluorescence and Absorption Spectroscopy. J. Spectrosc. 2015, 2015, 803705. [Google Scholar]
- Li, Y.; Fang, T.; Zhu, S.; Huang, F.; Chen, Z.; Wang, Y. Detection of olive oil adulteration with waste cooking oil via Raman spectroscopy combined with iPLS and SiPLS. Spectrochim. Acta A 2018, 189, 37–43. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).