Frequency and Intensity of Electrical Stimulation of Human Sympathetic Ganglia Affect Heart Rate Variability and Pain Threshold
Abstract
Featured Application
Abstract
1. Introduction
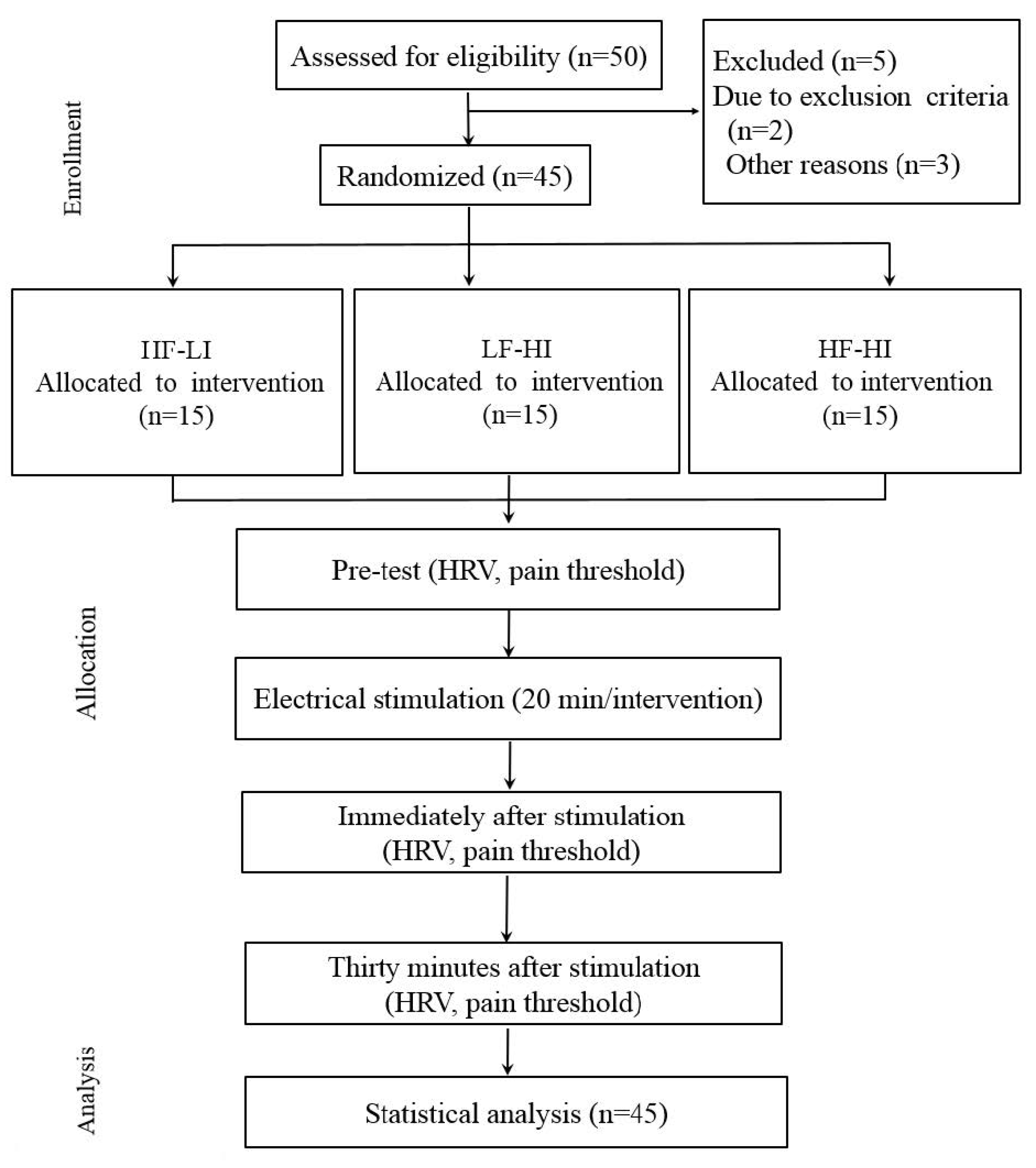
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bogduk, N. Spinal manipulation for neck pain does not work. J. Pain 2003, 4, 427–428. [Google Scholar] [CrossRef]
- Fuentes, J.P.; Armijo Olivo, S.; Magee, D.J.; Gross, D.P. Effectiveness of interferential current therapy in the management of musculoskeletal pain: A systematic review and meta-analysis. Phys. Ther. 2010, 90, 1219–1238. [Google Scholar] [CrossRef] [PubMed]
- Desantana, J.M.; Sluka, K.A.; Lauretti, G.R. High and low frequency TENS reduce postoperative pain intensity after laparoscopic tubal ligation: A randomized controlled trial. Clin. J. Pain 2009, 25, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. Purinergic receptors and pain. Curr. Pharm. Des. 2009, 15, 1717–1735. [Google Scholar] [CrossRef]
- Casellini, C.M.; Parson, H.K.; Richardson, M.S.; Nevoret, M.L.; Vinik, A.I. Sudoscan, a noninvasive tool for detecting diabetic small fiber neuropathy and autonomic dysfunction. Diabetes Technol. Ther. 2013, 15, 948–953. [Google Scholar] [CrossRef] [PubMed]
- Monroe, T.; Carter, M.; Feldt, K.; Tolley, B.; Cowan, R.L. Assessing advanced cancer pain in older adults with dementia at the end-of-life. J. Adv. Nurs. 2012, 68, 2070–2078. [Google Scholar] [CrossRef]
- Maeda, K.; Yasuda, H. Symptomatic treatment of painful diabetic neuropathy. Nihon. Rinsho. 2005, 63, 609–613. (In Japanese) [Google Scholar]
- Liang, F.; Chen, R.; Nakagawa, A.; Nishizawa, M.; Tsuda, S.; Wang, H.; Koya, D. Low-frequency electroacupuncture improves insulin sensitivity in obese diabetic mice through activation of SIRT1/PGC-1α in skeletal muscle. Evid. Based Complement. Alternat. Med. 2011, 2011, 735297. [Google Scholar] [CrossRef]
- Picelli, A.; Tamburin, S.; Bonetti, P.; Fontana, C.; Barausse, M.; Dambruoso, F.; Gajofatto, F.; Santilli, V.; Smania, N. Botulinum toxin type A injection into the gastrocnemius muscle for spastic equinus in adults with stroke: A randomized controlled trial comparing manual needle placement, electrical stimulation and ultrasonography-guided injection techniques. Am. J. Phys. Med. Rehabil. 2012, 91, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Kajbafzadeh, A.M.; Sharifi-Rad, L.; Nejat, F.; Kajbafzadeh, M.; Talaei, H.R. Transcutaneous interferential electrical stimulation for management of neurogenic bowel dysfunction in children with myelomeningocele. Int. J. Colorectal. Dis. 2012, 27, 453–458. [Google Scholar] [CrossRef]
- Sabut, S.K.; Sikdar, C.; Kumar, R.; Mahadevappa, M. Functional electrical stimulation of dorsiflexor muscle: Effects on dorsiflexor strength, plantarflexor spasticity, and motor recovery in stroke patients. NeuroRehabilitation 2011, 29, 393–400; [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Hui-Chan, C.W. Transcutaneous electrical stimulation on acupuncture points improves muscle function in subjects after acute stroke: A randomized controlled trial. J. Rehabil. Med. 2009, 41, 312–316. [Google Scholar] [CrossRef]
- Cheing, G.L.; Chan, W.W. Influence of choice of electrical stimulation site on peripheral neurophysiological and hypoalgesic effects. J. Rehabil. Med. 2009, 41, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Lymperopoulos, A.; Rengo, G.; Koch, W.J. Adrenergic nervous system in heart failure: Pathophysiology and therapy. Circ. Res. 2013, 113, 739–753. [Google Scholar] [CrossRef] [PubMed]
- Capote, L.A.; Mendez Perez, R.; Lymperopoulos, A. GPCR signaling and cardiac function. Eur. J. Pharmacol. 2015, 763, 143–148. [Google Scholar] [CrossRef]
- Ge, H.Y.; Fernández-de-las-Peñas, C.; Arendt-Nielsen, L. Sympathetic facilitation of hyperalgesia evoked from myofascial tender and trigger points in patients with unilateral shoulder pain. Clin. Neurophysiol. 2006, 117, 1545–1550. [Google Scholar] [CrossRef]
- Fisher, J.P.; Young, C.N.; Fadel, P.J. Central sympathetic overactivity: Maladies and mechanisms. Auton. Neurosci. 2009, 148, 5–15. [Google Scholar] [CrossRef]
- Kim, D.K.; Rhee, J.H.; Kang, S.W. Reorganization of the brain and heart rhythm during autogenic meditation. Front. Integr. Neurosci. 2014, 7, 109. [Google Scholar] [CrossRef]
- Dong, S.; Jacob, T.J. Combined non-adaptive light and smell stimuli lowered blood pressure, reduced heart rate and reduced negative affect. Physiol. Behav. 2016, 15, 94–105. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, J.; Ward, A.R.; Robertson, V.J. A comparison of true and premodulated interferential currents. Arch. Phys. Med. Rehabil. 2004, 85, 409–415. [Google Scholar] [CrossRef]
- Lombardi, F. Clinical implications of present physiological understanding of HRV components. Card Electrophysiol. Rev. 2002, 6, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Toledo, M.A.; Junqueira, L.F., Jr. Cardiac autonomic modulation and cognitive status in Alzheimer’s disease. Clin. Auton. Res. 2010, 20, 11–17. [Google Scholar] [CrossRef] [PubMed]
- De Vilhena Toledo, M.A.; Junqueira, L.F., Jr. Cardiac sympathovagal modulation evaluated by short-term heart interval variability is subtly impaired in Alzheimer’s disease. Geriatr. Gerontol. Int. 2008, 8, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Kurono, Y.; Minagawa, M.; Ishigami, T.; Yamada, A.; Kakamu, T.; Hayano, J. Acupuncture to Danzhong but not to Zhongting increases the cardiac vagal component of heart rate variability. Auton. Neurosci. 2011, 161, 116–120. [Google Scholar] [CrossRef]
- Neddermeyer, T.J.; Flühr, K.; Lötsch, J. Principle components analysis of pain thresholds to thermal, electrical, and mechanical stimuli suggests a predominant common source of variance. Pain 2008, 138, 286–291. [Google Scholar] [CrossRef]
- Stuart, F. Human Physiology, 8th ed.; McGraw-Hill Companies: Boston, MA, USA, 2004. [Google Scholar]
- Wang, J.D.; Kuo, T.B.; Yang, C.C. An alternative method to enhance vagal activities and suppress sympathetic activities in humans. Auton. Neurosci. 2002, 30, 90–95. [Google Scholar] [CrossRef]
- Léonard, G.; Cloutier, C.; Serge, M. Reduced analgesic effect of acupuncture-like TENS but not conventional TENS in opioid-treated patients. Pain 2011, 12, 213–221. [Google Scholar] [CrossRef]
- Parati, G.; Mancia, G.; Di Rienzo, M.; Castiglioni, P. Point: Cardiovascular variability is/is not an index of autonomic control of circulation. J. Appl. Physiol. 2006, 101, 676–678. [Google Scholar] [CrossRef]
- Kakigi, R.; Inui, K.; Tamura, Y. Electrophysiological studies on human pain perception. Clin. Neurophysiol. 2005, 116, 743–763. [Google Scholar] [CrossRef] [PubMed]
- Martins, H.R.; Zanetti, R.; dos Santos, C.C.; Manzano, G.M.; Tierra-criollo, C.J. Current perception threshold and reaction time in the assessment of sensory peripheral nerve fibers through sinusoidal electrical stimulation at different frequencies. Rev. Bras. Eng. Biomed. 2013, 29, 278–285. [Google Scholar] [CrossRef][Green Version]
- Olive, J.L.; Slade, J.M.; Dudley, G.A.; McCully, L.K. Blood flow and muscle fatigue in SCI individuals during electrical stimulation. J. Appl. Physiol. 2003, 94, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Ikoma, A.; Handwerker, H.; Miyachi, Y.; Schmelz, M. Electrically evoked itch in humans. Pain 2005, 113, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Abrahams, M.J. Neuropathic pain in soft tissue complaints. Best Pract. Res. Clin. Rheumatol. 2007, 21, 223–244. [Google Scholar] [CrossRef]
- Platon, B.; Andréll, P.; Raner, C.; Rudolph, M.; Dvoretsky, A.; Mannheimer, C. High-frequency, high-intensity transcutaneous electrical nerve stimulation as treatment of pain after surgical abortion. Pain 2010, 148, 114–119. [Google Scholar] [CrossRef]
- Pietrosimone, B.G.; Saliba, S.A.; Hart, J.M.; Hertel, J.; Kerrigan, D.C.; Ingersoll, C.D. Effects of transcutaneous electrical nerve stimulation and therapeutic exercise on quadriceps activation in people with tibiofemoral osteoarthritis. J. Orthop. Sports Phys. Ther. 2011, 41, 4–12. [Google Scholar] [CrossRef]
- Ebadi, S.; Ansari, N.N.; Henschke, N.; Naghdi, S.; van Tulder, M.W. The effect of continuous ultrasound on chronic low back pain: Protocol of a randomized controlled trial. BMC Musculoskelet. Disord. 2011, 12, 59. [Google Scholar] [CrossRef]
- Chang, F.C.; Tsai, H.Y.; Yu, M.C.; Yi, P.L.; Lin, J.G. The central serotonergic system mediates the analgesic effect of electroacupuncture on ZUSANLI (ST36) acupoints. J. Biomed. Sci. 2004, 11, 179–185. [Google Scholar] [CrossRef]
- Bergadano, A.; Andersen, O.K.; Arendt-Nielsen, L.; Spadavecchia, C. Modulation of nociceptive withdrawal reflexes evoked by single and repeated nociceptive stimuli in conscious dogs by low-dose acepromazine. Vet. Anaesth. Analg. 2009, 36, 261–272. [Google Scholar] [CrossRef]
- Chen, C.C.; Johnson, M.I. An investigation into the hypoalgesic effects of high- and low-frequency transcutaneous electrical nerve stimulation (TENS) on experimentally-induced blunt pressure pain in healthy human participants. J. Pain 2010, 11, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Claydon, L.S.; Chesterton, L.S.; Barlas, P.; Sim, J. Effects of simultaneous dual-site TENS stimulation on experimental pain. Eur. J. Pain 2008, 12, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.; Martinson, M. Efficacy of electrical nerve stimulation for chronic musculoskeletal pain: A meta-analysis of randomized controlled trials. Pain 2007, 130, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Kosek, E.; Hansson, P. The influence of experimental pain intensity in the local and referred pain area on somatosensory perception in the area of referred pain. Eur. J. Pain 2002, 6, 413–425. [Google Scholar] [CrossRef]
- Johnson, M.I.; Tabasam, G. An investigation into the analgesic effects of interferential currents and transcutaneous electrical nerve stimulation on experimentally induced ischemic pain in otherwise pain-free volunteers. Phys. Ther. 2007, 83, 208–223. [Google Scholar]
- Chen, C.C.; Johnson, M.I. Differential frequency effects of strong nonpainful transcutaneous electrical nerve stimulation on experimentally induced ischemic pain in healthy human participants. Clin. J. Pain 2011, 27, 434–441. [Google Scholar] [CrossRef]
| Characteristics | HF-LI | LF-HI | HF-HI | F | P | |
|---|---|---|---|---|---|---|
| Sex | Men | 7 | 8 | 9 | - | |
| Women | 8 | 7 | 6 | |||
| Age (years) | 22.00 ± 1.41 | 22.45 ± 1.44 | 22.64 ± 1.63 | 0.527 | 0.596 | |
| Height (cm) | 166.27 ± 7.46 | 167.09 ± 8.50 | 170.64 ± 8.08 | 0.615 | 0.547 | |
| Weight (kg) | 62.55 ± 4.55 | 60.82 ± 4.08 | 65.09 ± 5.93 | 0.512 | 0.640 | |
| Variable | Group (n = 15 Per Group) | Time From Electrical Stimulation | F (p) | ||||
|---|---|---|---|---|---|---|---|
| Before | Immediately After | Thirty Minutes After | Group | Period | Group × Period | ||
| HRV (Hz) | HF-LI | 69.94 ± 6.88 | 72.54 ± 6.10 | 70.99 ± 6.28 | 4.10 (0.02 *) | 8.57 (0.00 *) | 2.94 (0.02 *) |
| LF-HI | 65.88 ± 2.90 | 68.42 ± 3.31 | 65.69 ± 3.22 | ||||
| HF-HI | 66.46 ± 3.29 | 66.43 ± 3.19 | 66.72 ± 4.92 | ||||
| Pain threshold (mA) | HF-LI | 0.92 ± 0.24 | 1.07 ± 0.32 | 1.09 ± 0.24 | 6.81 (0.04 *) | 37.82 (0.00 *) | 4.99 (0.00 *) |
| LF-HI | 0.90 ± 0.27 | 1.23 ± 0.34 | 1.31 ± 0.24 | ||||
| HF-HI | 1.09 ± 0.28 | 1.73 ± 0.59 | 1.68 ± 0.40 | ||||
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, S.-H. Frequency and Intensity of Electrical Stimulation of Human Sympathetic Ganglia Affect Heart Rate Variability and Pain Threshold. Appl. Sci. 2019, 9, 4490. https://doi.org/10.3390/app9214490
Cho S-H. Frequency and Intensity of Electrical Stimulation of Human Sympathetic Ganglia Affect Heart Rate Variability and Pain Threshold. Applied Sciences. 2019; 9(21):4490. https://doi.org/10.3390/app9214490
Chicago/Turabian StyleCho, Sung-Hyoun. 2019. "Frequency and Intensity of Electrical Stimulation of Human Sympathetic Ganglia Affect Heart Rate Variability and Pain Threshold" Applied Sciences 9, no. 21: 4490. https://doi.org/10.3390/app9214490
APA StyleCho, S.-H. (2019). Frequency and Intensity of Electrical Stimulation of Human Sympathetic Ganglia Affect Heart Rate Variability and Pain Threshold. Applied Sciences, 9(21), 4490. https://doi.org/10.3390/app9214490





