Characterization of Residual Biomasses and Its Application for the Removal of Lead Ions from Aqueous Solution
Abstract
1. Introduction
2. Aims and Objectives
3. Materials and Methods
3.1. Experimental Materials
3.2. Biomass Preparation
3.3. Biomass Characterization
3.4. Adsorption Study
3.5. Kinetic Study and Isotherm Modeling
4. Results
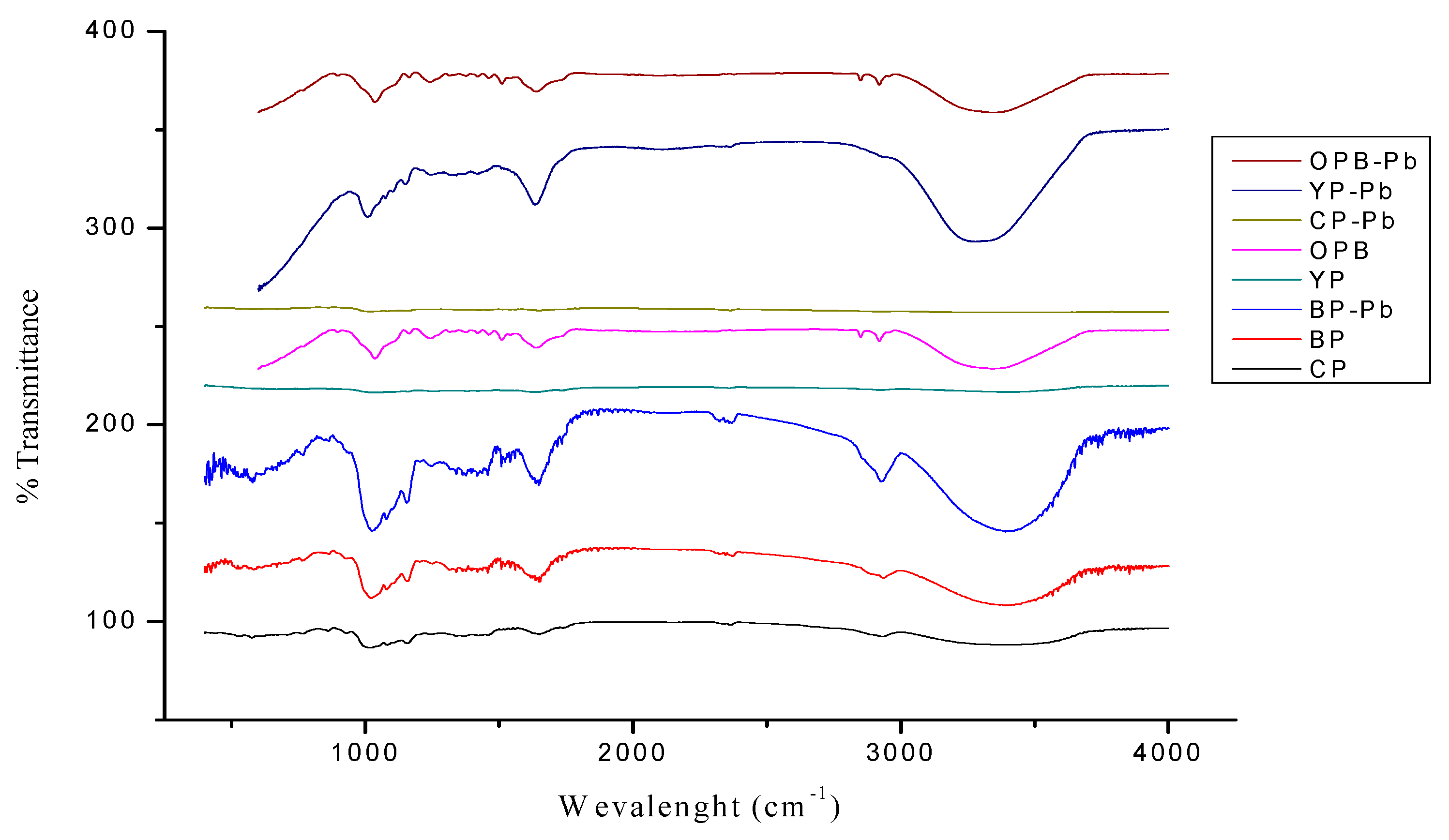
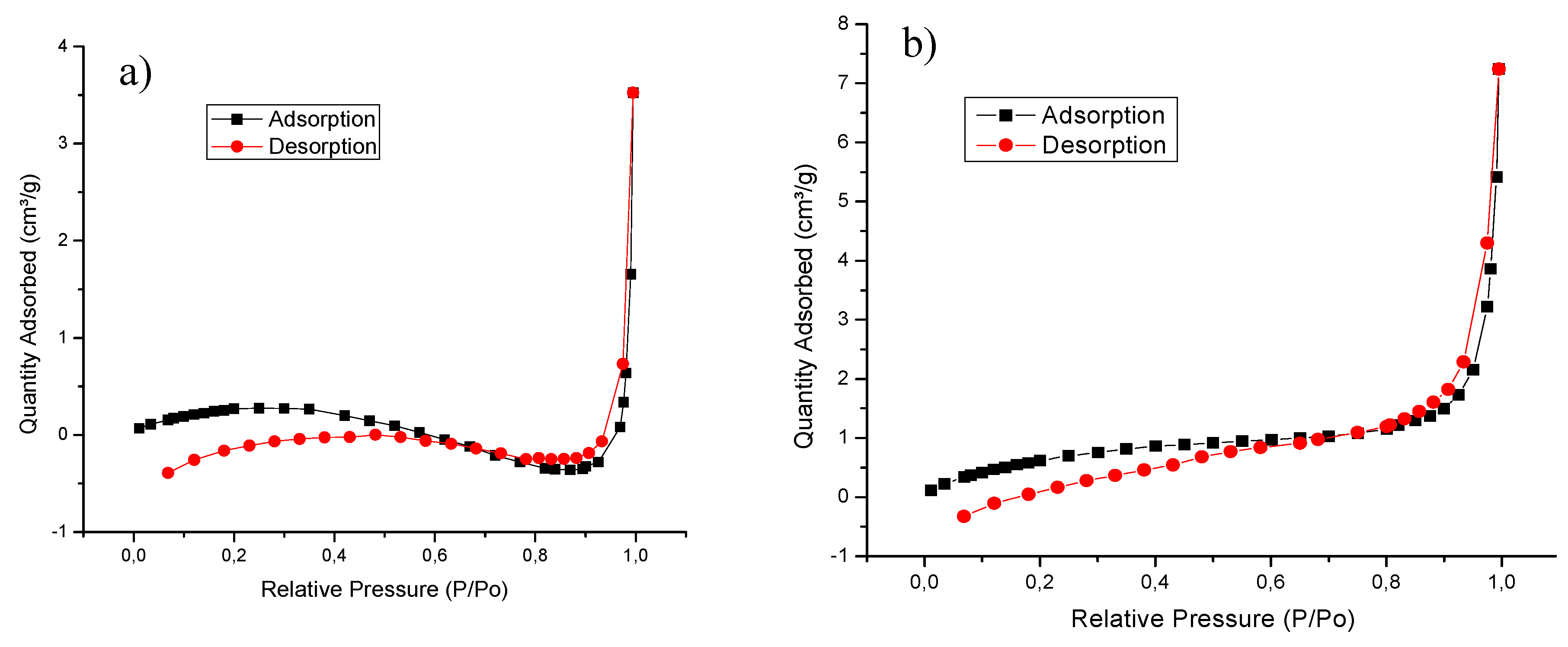
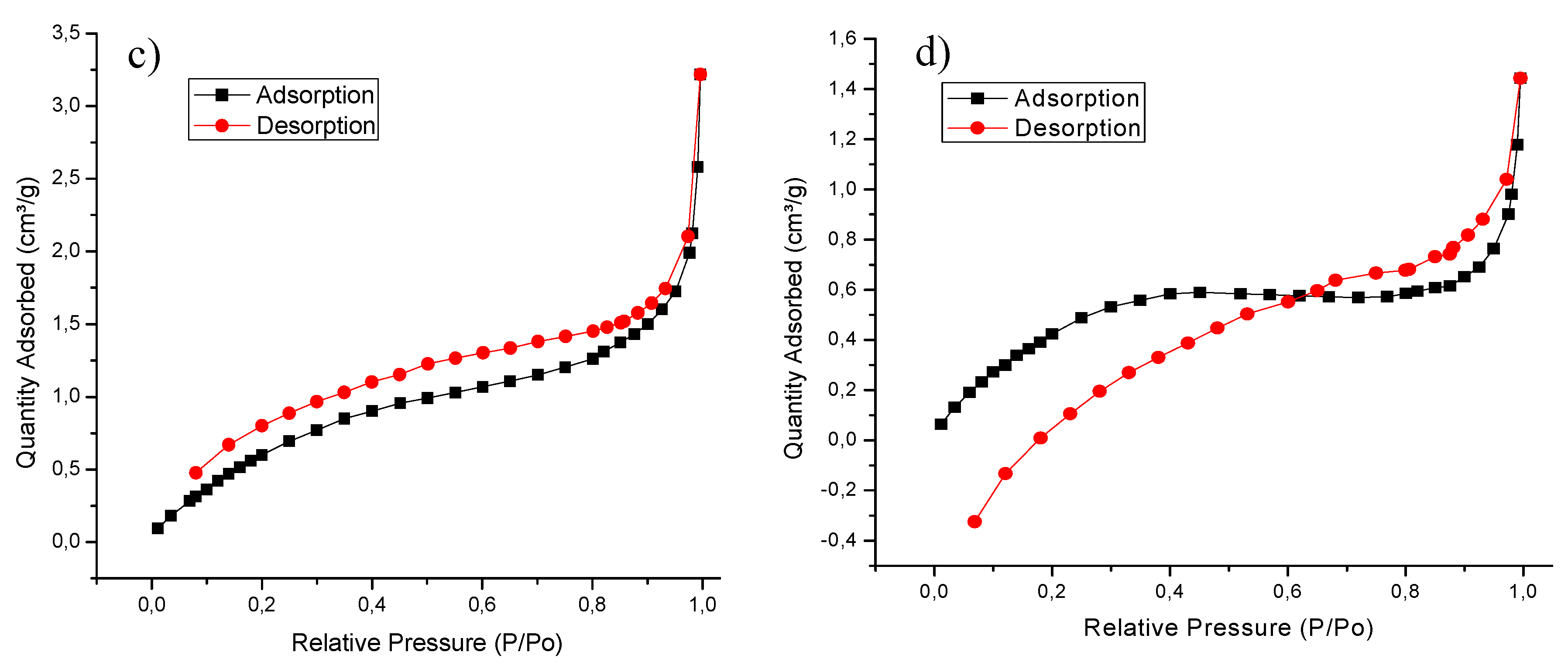
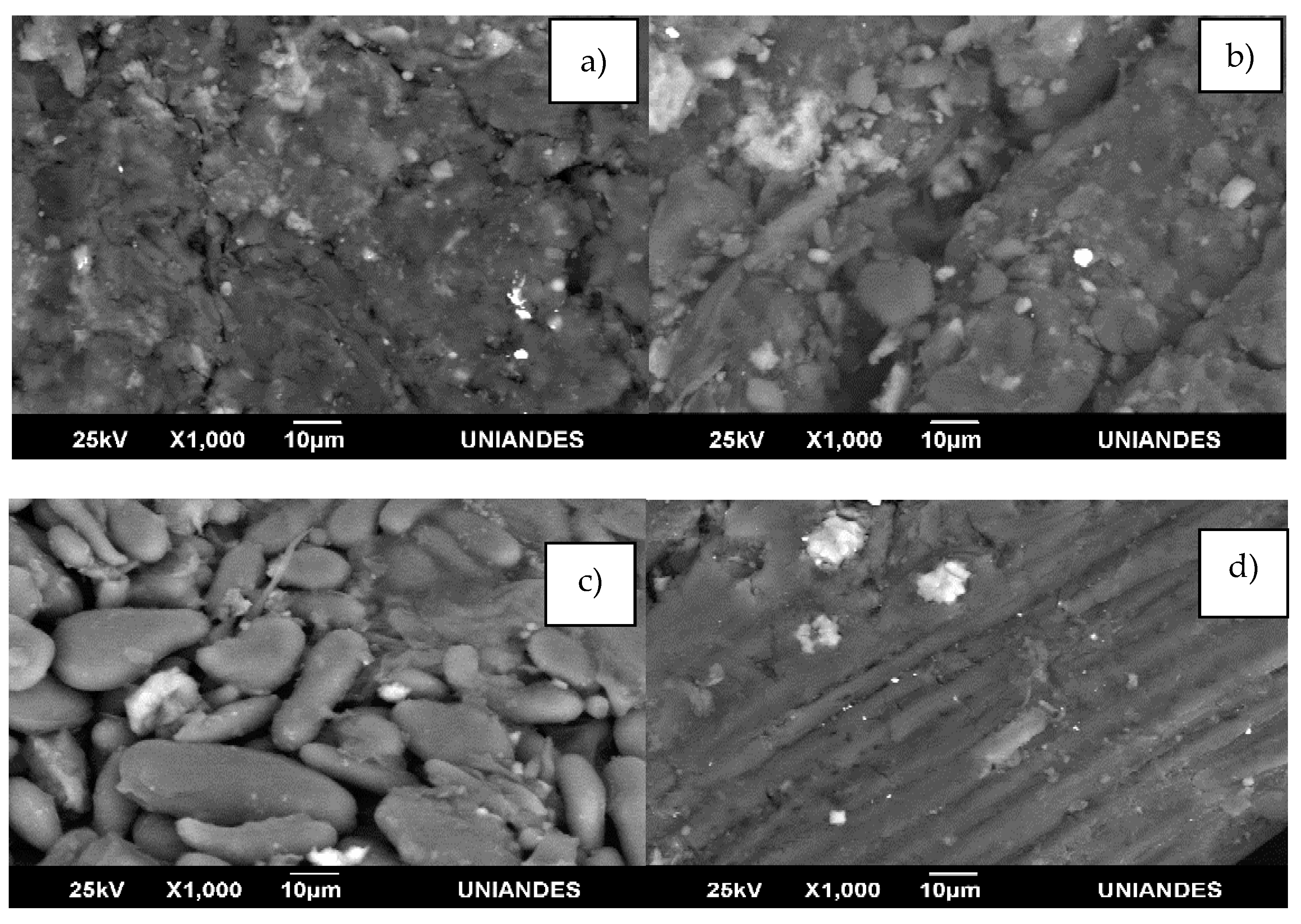
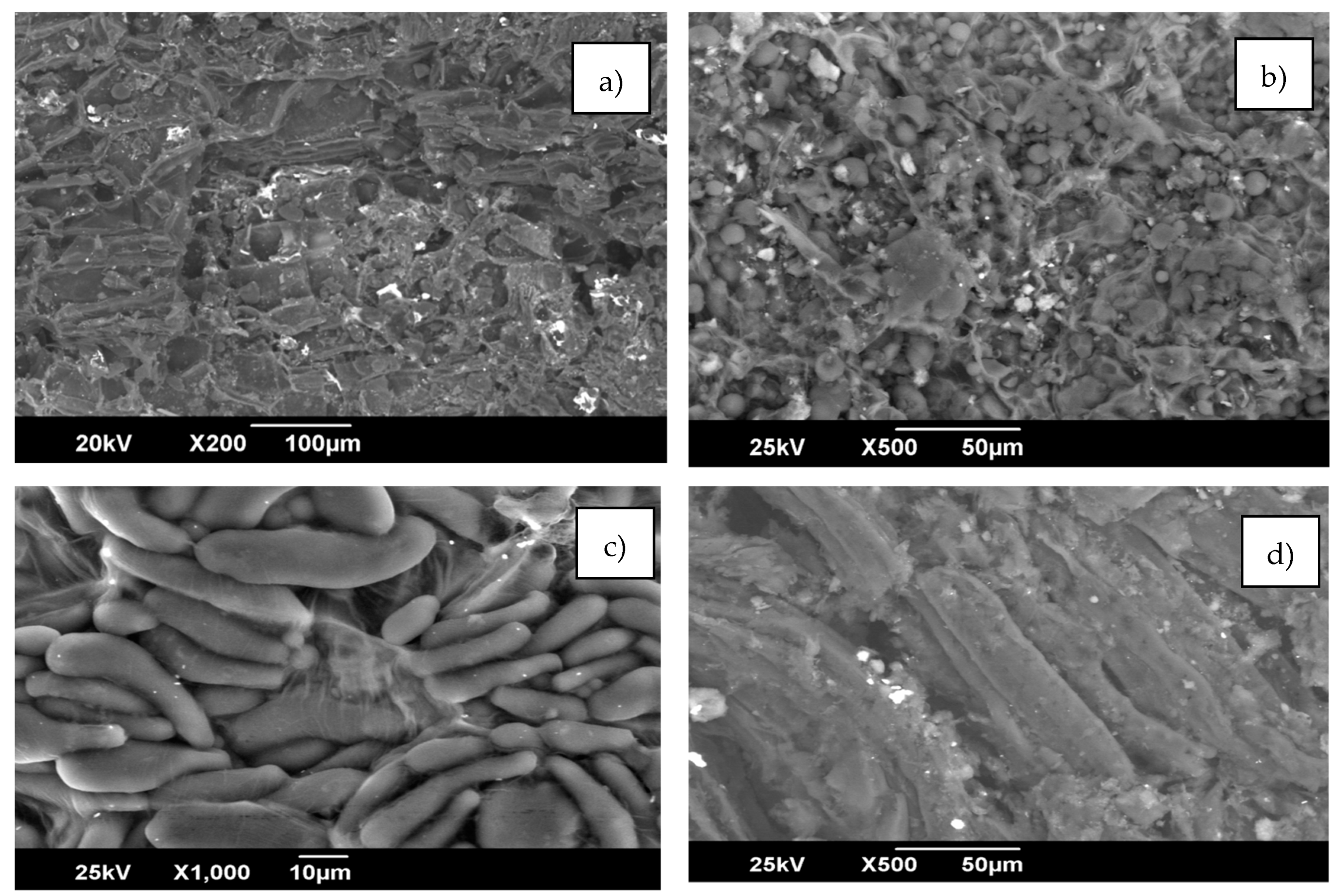
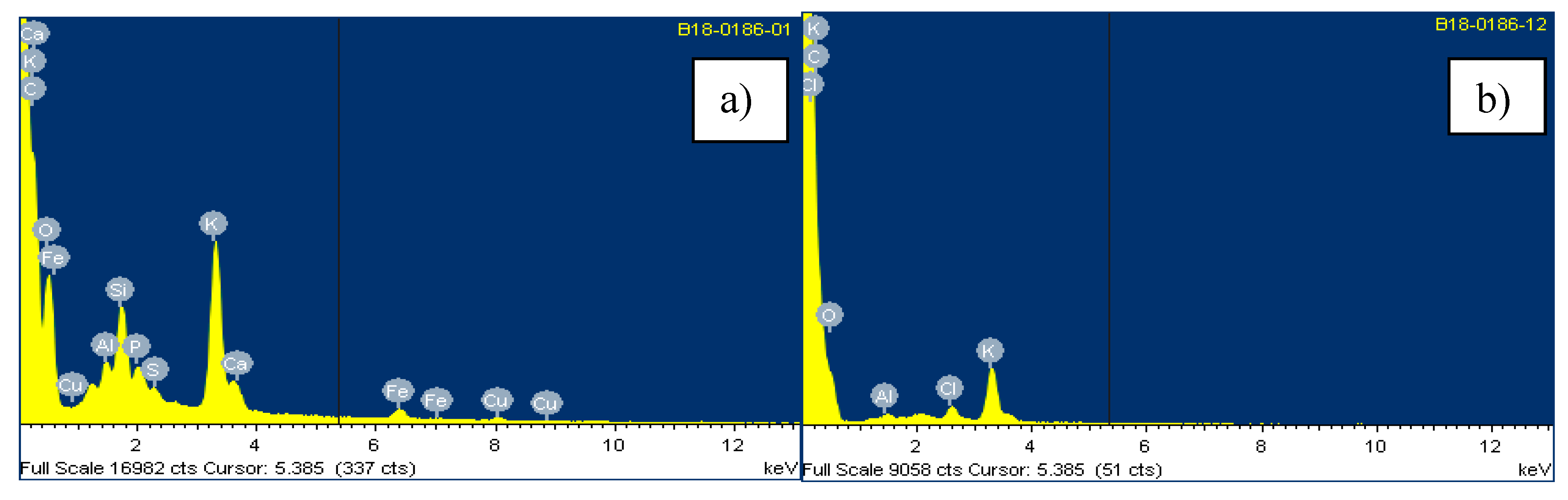
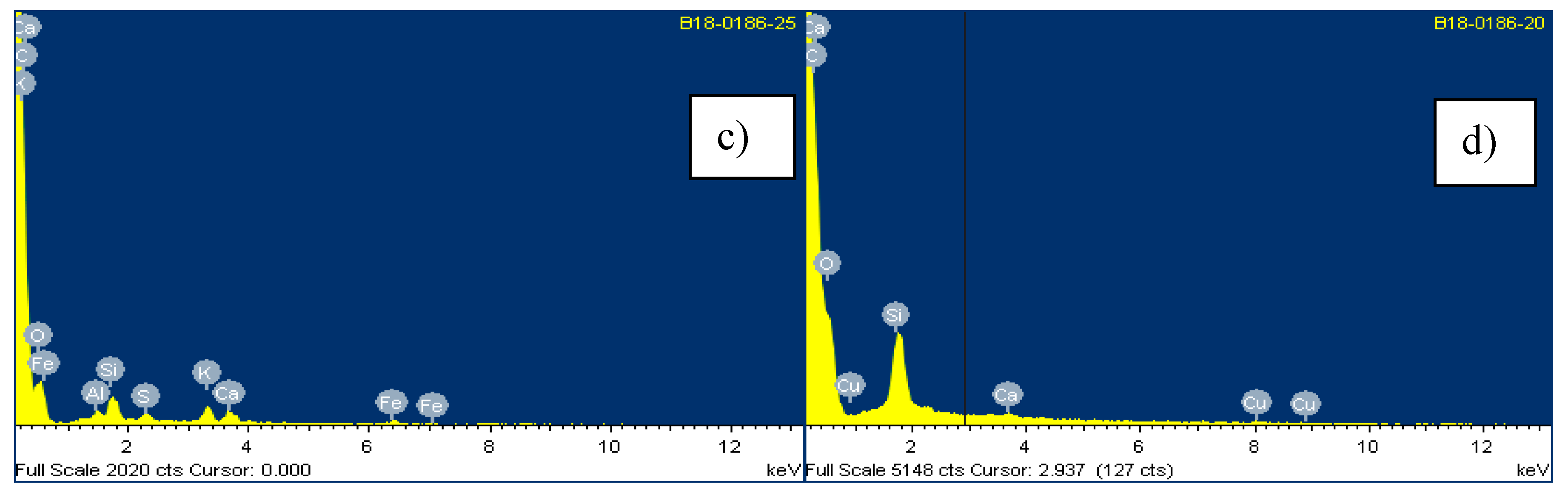
4.1. Characterization Techniques
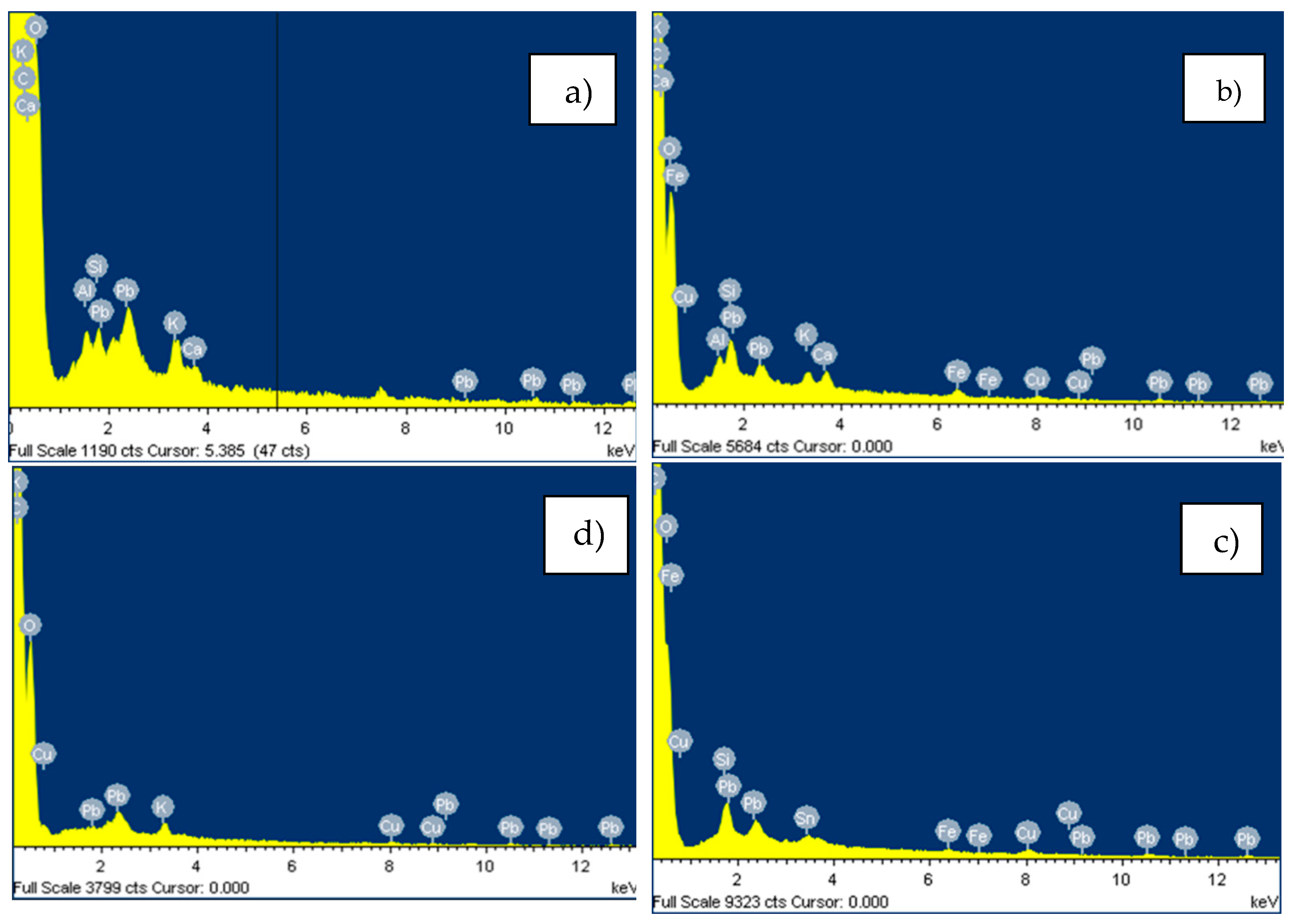
4.2. Adsorption Tests
4.3. Kinetics and Adsorption Isotherms
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Romero-Cano, L.A.; García-Rosero, H.; Gonzalez-Gutierrez, L.V.; Baldenegro-Pérez, L.A.; Carrasco-Marín, F. Functionalized adsorbents prepared from fruit peels: Equilibrium, kinetic and thermodynamic studies for copper adsorption in aqueous solution. J. Clean. Prod. 2017, 162, 195–204. [Google Scholar] [CrossRef]
- Iqbal, M.; Abbas, M.; Nisar, J.; Nazir, A.; Qamar, A.Z. Bioassays based on higher plants as excellent dosimeters for ecotoxicity monitoring: A review. Chem. Int. 2019, 5, 1–80. [Google Scholar]
- Iqbal, M. Vicia faba bioassay for environmental toxicity monitoring: A review. Chemosphere 2016, 144, 785–802. [Google Scholar] [CrossRef]
- Zaidi, N.A.H.M.; Lim, L.B.L.; Usman, A. Enhancing adsorption of Pb(II) from aqueous solution by NaOH and EDTA modified Artocarpus odoratissimus leaves. J. Environ. Chem. Eng. 2018, 6, 7172–7184. [Google Scholar] [CrossRef]
- Neris, J.B.; Luzardo, F.H.M.; da Silva, E.G.P.; Velasco, F.G. Evaluation of adsorption processes of metal ions in multi-element aqueous systems by lignocellulosic adsorbents applying different isotherms: A critical review. Chem. Eng. J. 2019, 357, 404–420. [Google Scholar] [CrossRef]
- Abbas, M.; Adil, M.; Ehtisham-ul-Haque, S.; Munir, B.; Yameen, M.; Ghaffar, A.; Abbas, G.; Asif, M.; Iqbal, M. Vibrio fischeri bioluminescence inhibition assay for ecotoxicity assessment: A review. Sci. Total Environ. 2018, 626, 1295–1309. [Google Scholar] [CrossRef] [PubMed]
- Hasan, R.; Bukhari, S.N.; Jusoh, R.; Mutamin, N.S.A.; Setiabudi, H.D. Adsorption of Pb(II) onto KCC-1 from aqueous solution: Isotherm and kinetic study. Mater. Today Proc. 2018, 5, 21574–21583. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, H.; Zhao, W.; Huang, S. Four different kinds of peels as adsorbents for the removal of Cd (II) from aqueous solution: Kinetics, isotherm and mechanism. J. Taiwan Inst. Chem. Eng. 2018, 88, 146–151. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, X.; Li, B. Adsorption of Hg2+ and Cd2+ by ethylenediamine modified peanut shells. Carbohydr. Polym. 2010, 81, 335–339. [Google Scholar] [CrossRef]
- Kang, R.; Qiu, L.; Fang, L.; Yu, R.; Chen, Y.; Lu, X.; Luo, X. A novel magnetic and hydrophilic ion-imprinted polymer as a selective sorbent for the removal of cobalt ions from industrial wastewater. Biochem. Pharmacol. 2016, 4, 2268–2277. [Google Scholar] [CrossRef]
- Moreno-Sader, K.; García-Padilla, A.; Realpe, A.; Acevedo-Morantes, M.; Soares, J. Removal of Heavy Metal Water Pollutants (Co2+ and Ni2+) Using Polyacrylamide/Sodium Montmorillonite (PAM/Na-MMT) Nanocomposites. ACS Omega 2019, 4, 10834–10844. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Guo, X.; Feng, N.; Tian, Q. Isotherms, kinetics and thermodynamic studies of adsorption of Cu2+ from aqueous solutions by Mg2+/K+ type orange peel adsorbents. J. Hazard Mater. 2010, 174, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Wei, G.; Xiong, J.; Tan, F.; He, H.; Qu, C.; Yin, H.; Zhu, J.; Zhu, R.; Qin, Z.; et al. Adsorption isotherm, mechanism, and geometry of Pb(II) on magnetites substituted with transition metals. Chem. Geol. 2017, 470, 132–140. [Google Scholar] [CrossRef]
- Villabona-Ortiz, A.; Tejada-Tovar, C.; Gonzalez-Delgado, A.; Herrera-Barros, A.; Cantillo-Arroyo, G. Immobilization of Lead and Nickel Ions from Polluted Yam Peels Biomass Using Cement-Based Solidification/Stabilization Technique. Int. J. Chem. Eng. 2019, 2019, 5413960. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Pashalidis, I.; Hosseini-Bandegharaei, A.; Giannakoudakis, D.; Robalds, A.; Usman, M.; Belen, L.; Zhou, Y.; Colmenares, J.; Núñez-Delgado, A.; et al. Agricultural biomass/waste as adsorbents for toxic metal decontamination of aqueous solutions. J. Mol. Liq. 2019, 111684. [Google Scholar] [CrossRef]
- Di Bitondo, L.; Volpe, A.; Pagano, M.; Bagnuolo, G.; Mascolo, G.; La Parola, V.; Di leo, P.; Pastore, C. Amorphous boron-doped sodium titanates hydrates: Efficient andreusable adsorbents for the removal of Pb2+from water. J. Hazard Mater. 2017, 324, 168–177. [Google Scholar] [CrossRef]
- Huang, J.; Cao, Y.; Liu, Z.; Deng, Z.; Tang, F.; Wang, W. Efficient removal of heavy metal ions from water system by titanate nanoflowers. Chem. Eng. J. 2012, 180, 75–80. [Google Scholar] [CrossRef]
- Hu, J.; Shipley, H.J. Regeneration of spent TiO2 nanoparticles for Pb (II), Cu (II), and Zn (II) removal. Environ. Sci. Pollut. Res. 2013, 20, 5125–5137. [Google Scholar] [CrossRef]
- Hu, J.; Shipley, H.J. Evaluation of desorption of Pb (II), Cu (II) and Zn (II) from titanium dioxide nanoparticles. Sci. Total. Environ. 2012, 431, 209–220. [Google Scholar] [CrossRef]
- Boeykens, S.; Redondo, N.; Alvarado, R.; Caracciolo, N.; Vazquez, C. Chromium and Lead adsorption by avocado seed biomass study through the use of Total Reflection X-Ray Fluorescence analysis. Appl. Radiat. Isot. 2019, 153, 108809. [Google Scholar] [CrossRef]
- Amin, M.; Alazba, A.; Shafiq, M. Application of biochar derived from date palm biomass for removal of lead and copper ions in a batch reactor: Kinetics and isotherm scrutiny. Chem. Phys. Lett. 2019, 722, 64–73. [Google Scholar] [CrossRef]
- Bagali, S.; Gowrishankar, B.; Roy, A. Optimization, Kinetics, and Equilibrium Studies on the Removal of Lead(II) from an Aqueous Solution Using Banana Pseudostem as an Adsorbent. Engineering 2017, 3, 409–415. [Google Scholar] [CrossRef]
- Alhogbi, B. Potential of coffee husk biomass waste for the adsorption of Pb(II) ion from aqueous solutions. Sustain. Chem. Pharm. 2017, 6, 21–25. [Google Scholar] [CrossRef]
- Mohammed, A.; Khalifa, A.; Muftah, K. Removal of Pb(II), Zn(II), Cu(II) and Cd(II) from aqueous solutions by adsorption onto olive branches activated carbon: Equilibrium and thermodynamic studies. Chem. Int. 2020, 6, 11–20, in press. [Google Scholar]
- Tejada, C.N.; Montiel, Z.; Acevedo, D. Aprovechamiento de Cáscaras de Yuca y Ñame para el Tratamiento de Aguas Residuales Contaminadas con Pb(II). Inf. Tecnol. 2016, 27, 9–20. [Google Scholar] [CrossRef]
- Tejada-Tovar, C.; Ruiz-Paternina, E.; Gallo-Mercado, J.; Moscote-Bohorquez, J. Evaluación de la biosorción con bagazo de palma africana para la eliminación de Pb (II) en solución Evaluation of the biosorption with african palm bagasse for the removal of Pb (II) in solution. Rev. Prospect. 2015, 13, 59–67. [Google Scholar] [CrossRef][Green Version]
- Tejada-Tovar, C.; Tejeda, L.; Marimón, W.; Villabona-Ortíz, A. Estudio de modificación química y física de biomasa (Citrus sinensis y Musa paradisiaca) para la adsorción de metales pesados en solución. Rev. Luna Azul 2014, 39, 124–142. [Google Scholar] [CrossRef]
- Singh, H.; Sapra, P.K.; Sidhu, B.S. Evaluation and Characterization of Different Biomass Residues through Proximate & Ultimate Analysis and Heating Value. Asian J. Eng. Appl. Technol. 2013, 2, 6–10. [Google Scholar]
- Sherlala, A.I.A.; Raman, A.A.A.; Bello, M.M.; Asghar, A. A review of the applications of organo-functionalized magnetic graphene oxide nanocomposites for heavy metal adsorption. Chemosphere 2018, 193, 1004–1017. [Google Scholar] [CrossRef] [PubMed]
- Tejada-Tovar, C.; Montiel-Rodríguez, Z.P.; Nieto-Muñoz, Y. Estudio de la Adsorción Competitiva de Pb+2 y Ni+2 Usando Cáscara de Yuca (Manihot Esculenta) Modificada Con ácido Cítrico. Ph.D. Thesis, University of Cartagena, Cartagena, Bolívar, Colombia, 2014; pp. 1–55. [Google Scholar]
- Janyasuthiwong, S.; Phiri, S.M.; Kijjanapanich, P.; Rene, E.R.; Esposito, G.; Lens, P.N.L. Copper, lead and zinc removal from metal-contaminated wastewater by adsorption onto agricultural wastes. Environ. Technol. 2015, 36, 3071–3083. [Google Scholar] [CrossRef]
- Abdolali, A.; Ngo, H.H.; Guo, W.; Zhou, J.L.; Zhang, J.; Liang, S.; Chang, S.W.; Nguyen, D.D.; Liu, Y. Application of a breakthrough biosorbent for removing heavy metals from synthetic and real wastewaters in a lab-scale continuous fixed-bed column. Bioresour. Technol. 2017, 229, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Šoštarić, T.D.; Petrović, M.S.; Pastor, F.T.; Lončarević, D.R.; Petrović, J.T.; Milojković, J.V.; Stojanović, M.D. Study of heavy metals biosorption on native and alkali-treated apricot shells and its application in wastewater treatment. J. Mol. Liq. 2018, 259, 340–349. [Google Scholar] [CrossRef]
- Lawal, O.S.; Ayanda, O.S.; Rabiu, O.O.; Adebowale, K.O. Application of black walnut (Juglans nigra) husk for the removal of lead (II) ion from aqueous solution. Water Sci. Technol. 2017, 75, 2454–2464. [Google Scholar] [CrossRef] [PubMed]
- Asuquo, E.D.; Martin, A.D. Sorption of cadmium (II) ion from aqueous solution onto sweet potato (Ipomoea batatas L.) peel adsorbent: Characterisation, kinetic and isotherm studies. J. Environ. Chem. Eng. 2016, 4, 4207–4228. [Google Scholar] [CrossRef]
- Asuquo, E.; Martin, A.; Nzerem, P.; Siperstein, F.; Fan, X. Adsorption of Cd(II) and Pb(II) ions from aqueous solutions using mesoporous activated carbon adsorbent: Equilibrium, kinetics and characterisation studies. J. Environ. Chem. Eng. 2017, 5, 679–698. [Google Scholar] [CrossRef]
- Berhe, S.; Ayele, D.; Tadesse, A.; Mulu, A. Adsorption Efficiency of Coffee Husk for Removal of Lead (II) from Industrial Effluents: Equilibrium and Kinetic Study. Int. J. Sci. Res. Publ. 2015, 5, 753–859. [Google Scholar]
- Yin, W.; Zhao, C.; Xu, J.; Zhang, J.; Guo, Z.; Shao, Y. Removal of Cd(II) and Ni(II) from aqueous solutions using activated carbon developed from powder-hydrolyzed-feathers and Trapa natans husks. Coll. Surf. A Physicochem. Eng. Asp. 2019, 560, 426–433. [Google Scholar] [CrossRef]
- Fernandez, M.E.; Ledesma, B.; Román, S.; Bonelli, P.R.; Cukierman, A.L. Development and characterization of activated hydrochars from orange peels as potential adsorbents for emerging organic contaminants. Bioresour. Technol. 2015, 183, 221–228. [Google Scholar] [CrossRef]
- Pradhan, P.; Arora, A.; Mahajani, S.M. Pilot scale evaluation of fuel pellets production from garden waste biomass. Energy Sustain. Dev. 2018, 43, 1–14. [Google Scholar] [CrossRef]
- Singh, S.A.; Shukla, S.R. Adsorptive removal of cobalt ions on raw and alkali-treated lemon peels. Int. J. Environ. Sci. Technol. 2016, 13, 165–178. [Google Scholar] [CrossRef]
- Medellin-Castillo, N. Bioadsorción de plomo (II) presente en solución acuosa sobre residuos de fibras naturales procedentes de la industria ixtlera (Agave lechuguilla Torr. y Yucca carnerosana (TREL.) MCKELVEY). Rev. Int. Contam. Ambient. 2017, 33, 269–280. [Google Scholar] [CrossRef]
- Gupta, A.; Balomajumder, C. Journal of Water Process Engineering Simultaneous removal of Cr (VI) and phenol from binary solution using Bacillus sp. immobilized onto tea waste biomass. J. Water Process Eng. 2015, 6, 1–10. [Google Scholar] [CrossRef]
- Castro, L.; Blázquez, M.L.; González, F.; Muñoz, J.A.; Ballester, A. Heavy metal adsorption using biogenic iron compounds. Hydrometallurgy 2018, 179, 44–51. [Google Scholar] [CrossRef]
- De Melo, N.H.; de Oliveira Ferreira, M.E.; Silva Neto, E.M.; Martins, P.R.; Ostroski, I.C. Evaluation of the adsorption process using activated bone char functionalized with magnetite nanoparticles. Environ. Nanotechnol. Monit. Manag. 2018, 10, 427–434. [Google Scholar] [CrossRef]
- Obike, A.I.; Igwe, J.C.; Emeruwa, C.N.; Uwakwe, K.J. Equilibrium and kinetic studies of Cu (II), Cd (II), Pb (II) and Fe (II) adsorption from aqueous solution using cocoa (Theobroma cacao) pod husk. J. Appl. Sci. Environ. Manag. 2018, 22, 182–190. [Google Scholar] [CrossRef]
- Raikar, R.V.; Correa, S.; Ghorpade, P. Removal of lead (II) from aqueous solution using natural and activated rice husk. Int. Res. J. Eng. Technol. 2015, 2, 1677–1686. [Google Scholar]
- Villabona-Ortíz, A.; Tejada-Tovar, C.; Gonzalez-Delgado, A. Application of cement-based solidification/stabilization technique for immobilizing lead and nickel ions after sorption-desorption cycles using cassava peels biomass. Indian J. Sci. Technol. 2018, 11, 1–6. [Google Scholar] [CrossRef]
| Kinetic Model | Equation | Parameters |
|---|---|---|
| Pseudo-1st-order | qe, Adsorption capacity at equilibrium (mg/g) k1, pseudo-1st-order constant (min−1) | |
| Pseudo-2nd-order | k2 pseudo-2nd-order constant (g/mg·min) qe, Adsorption capacity at equilibrium (mg/g) | |
| Elovich equation | α, Elovich constant (mg/g min) β, Elovich exponent (g/mg) |
| Kinetic Model | Equation | Parameters |
|---|---|---|
| Langmuir | qe: Adsorption capacity at equilibrium (mg/g); Cf: Final concentration of heavy metals (mg/L); qmax: Maximum adsorption capacity (mg/g) | |
| Freundlich | q: Adsorption capacity (mg/g); Ce: Heavy metal concentration at equilibrium (mg/L); K: Freundlich constant (mg/g); n: Heterogeneity factor |
| Biomass | Cellulose | Hemicellulose | Lignin | Pectin | Carbon | Nitrogen | Hydrogen | Ashes |
|---|---|---|---|---|---|---|---|---|
| BP | 20.9 | 7.92 | 18.11 | 2.84 | 36.69 | 0.69 | 3.98 | 7.2 |
| CP | 18.47 | 6.01 | 2.20 | 2.84 | 36.96 | 0.26 | 3.98 | 1.58 |
| YP | 13.08 | 6.47 | 27.73 | 10.98 | 48.14 | 0.18 | 5.44 | 4.85 |
| OPB | 19.90 | 7.00 | 17.11 | 4.88 | 38.27 | 2.03 | 4.71 | 4.23 |
| Biomass | Surface Area (m²/g) | Pore Volume (cm³/g) | Pore Size (nm) |
|---|---|---|---|
| Yam peel | 0.9463 | 0.005452 | 23.04419 |
| Banana peel | 3.0889 | 0.004977 | 6.44567 |
| Oil palm bagasse | 2.7317 | 0.011207 | 16.410 |
| Cassava peel | 2.0509 | 0.002233 | 4.35428 |
| Heavy Metal | Adsorbent | Removal Yield (%) | Metal Uptake (mg/g) |
|---|---|---|---|
| Pb(II) | Yam peel | 81.24 | 82.24 |
| Banana peel | 90.12 | 91.12 | |
| Cassava peel | 98.19 | 99.19 | |
| Oil palm bagasse | 39.41 | 40.41 |
| Model | Parameter | CP | YP | BP | OBP |
|---|---|---|---|---|---|
| Pseudo-first order | qe1 (mg/g) | 17.79 | 19.82 | 19.54 | 19.92 |
| k1 (min−1) | 249.37 | 1.53 | 1.276 | 0.38 | |
| R2 | 0.996 | 0.943 | 0.932 | 0.945 | |
| Pseudo-second order | k2 (g/mg∗min) | 1662.33 | 0.13 | 0.15 | 0.224 |
| qe (mg/g) | 17.79 | 19.97 | 19.68 | 19.97 | |
| R2 | 0.999 | 0.967 | 0.978 | 0.976 | |
| Elovich | α (mmol·g−1·min−1) | 2,070,197,227 | 2.47 × 1098 | 2.39 × 1087 | 1.90 × 10107 |
| β (g/mg) | 1.48 | 11.78 | 11.56 | 12.78 | |
| R2 | 0.853 | 0.898 | 0.896 | 0.898 |
| Model | Parameter | CP | YP | BP | OBP |
|---|---|---|---|---|---|
| Langmuir | qmax (mg/g) | 11.79 | 98.36 | 18.96 | 99.73 |
| Cf (L/g) | 7,246,598.09 | 0.16 | 724,09 | 0.05 | |
| R2 | 0.713 | 0.985 | 0.824 | 0.954 | |
| Freundlich | K (mg/g) | 9.57 | 13.42 | 14.78 | 8.38 |
| n | 0.242 | 0.86 | 0.376 | 1.87 | |
| R2 | 0.972 | 0.999 | 0.982 | 0.835 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tejada-Tovar, C.; Gonzalez-Delgado, A.D.; Villabona-Ortiz, A. Characterization of Residual Biomasses and Its Application for the Removal of Lead Ions from Aqueous Solution. Appl. Sci. 2019, 9, 4486. https://doi.org/10.3390/app9214486
Tejada-Tovar C, Gonzalez-Delgado AD, Villabona-Ortiz A. Characterization of Residual Biomasses and Its Application for the Removal of Lead Ions from Aqueous Solution. Applied Sciences. 2019; 9(21):4486. https://doi.org/10.3390/app9214486
Chicago/Turabian StyleTejada-Tovar, Candelaria, Angel Darío Gonzalez-Delgado, and Angel Villabona-Ortiz. 2019. "Characterization of Residual Biomasses and Its Application for the Removal of Lead Ions from Aqueous Solution" Applied Sciences 9, no. 21: 4486. https://doi.org/10.3390/app9214486
APA StyleTejada-Tovar, C., Gonzalez-Delgado, A. D., & Villabona-Ortiz, A. (2019). Characterization of Residual Biomasses and Its Application for the Removal of Lead Ions from Aqueous Solution. Applied Sciences, 9(21), 4486. https://doi.org/10.3390/app9214486







