Analysis of Seaweeds from South West England as a Biorefinery Feedstock
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Identification of Samples
2.2. Preparation of Seaweed
2.3. Pigment Extraction
2.4. CHN/Protein
2.5. Ash
2.6. Metal Analysis
2.7. Lipids
2.8. Phytohormones
3. Results and Discussion
3.1. Lipid and High-Value Omega-3 Oil Content
3.2. Pigments
3.3. Macronutrients—Carbohydrate and Protein
3.4. Minerals
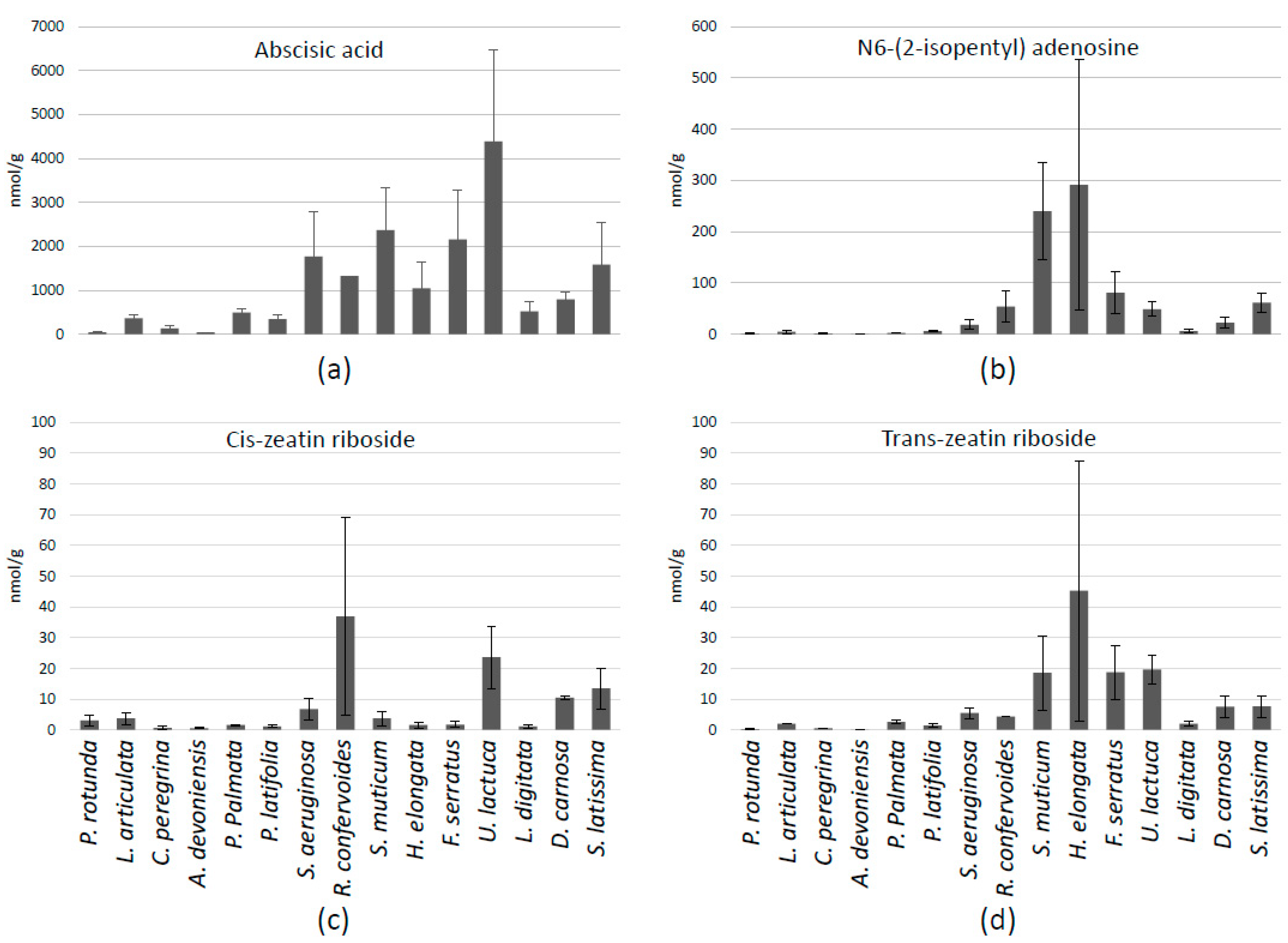
3.5. Phytohormones
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FOA. The State of World Fisheries and Aquaculture 2016, Contributing to Food Security and Nutrition for All; Food and Agriculture Organization of the United Nations: Rome, Italy, 2016. [Google Scholar]
- Kinley, R.D.; de Nys, R.; Vucko, M.J.; Machado, L.; Tomkins, N.W. The red macroalgae Asparagopsis taxiformis is a potent natural antimethanogenic that reduces methane production during in vitro fermentation with rumen fluid. Anim. Prod. Sci. 2016, 56, 282–289. [Google Scholar] [CrossRef]
- Balina, K.; Romagnoli, F.; Blumberga, D. Seaweed biorefinery concept for sustainable use of marine resources. Energy Procedia 2017, 128, 504–511. [Google Scholar] [CrossRef]
- Dang Xuan, T.; Sakanishi, K.; Nakagoshi, N.; Fujimoto, S.; Minowa, T. Biorefinery: Concepts, Current Status, and Development Trends. Int. J. Biomass Renew. 2012, 1, 1–8. [Google Scholar]
- Capuzzo, E.; McKie, T. Seaweed in the UK and Abroad–Status, Products, Limitations, Gaps and Cefas Role. 22 April 2016. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/546679/FC002I__Cefas_Seaweed_industry_report_2016_Capuzzo_and_McKie.pdf (accessed on 4 October 2019).
- Raikova, S.; Le, C.D.; Beacham, T.A.; Jenkins, R.W.; Allen, M.J.; Chuck, C.J. Towards a marine biorefinery through the hydrothermal liquefaction of macroalgae native to the united kingdom. Biomass Bioenergy 2017, 107, 244–253. [Google Scholar] [CrossRef]
- Mead, A. Sustainable Seaweed Farming, Southwest England; Case ref: MLA/2018/00506; Marine Managment Organisation: Newcastle, UK, 2018. [Google Scholar]
- Michler-Cieluch, T.; Kodeih, S. Mussel and Seaweed Cultivation in Offshore Wind Farms: An Opinion Survey. Coast. Manag. 2008, 36, 392–411. [Google Scholar] [CrossRef]
- Bunker, F.; Brodie, J.A.; Maggs, C.A.; Bunker, A.R. Seaweeds of Britain and Ireland, 2nd ed.; Wild Nature Press: Plymouth, UK, 2017; p. 312. [Google Scholar]
- Wei, N.; Quarterman, J.; Jin, Y.-S. Marine macroalgae: An untapped resource for producing fuels and chemicals. Trends Biotechnol. 2013, 31, 70–77. [Google Scholar] [CrossRef]
- Hayward, P.; Nelson-Smith, T.; Sheilds, C. Sea Shore of Britain and Northern Europe, 1st ed.; HarperCollins: London, UK, 1996; p. 352. [Google Scholar]
- Zapata, M.; Rodríguez, F.; Garrido, J.L. Separation of chlorophylls and carotenoids from marine phytoplankton: A new HPLCmethod using a reversed phase c8 column and pyridine-containing mobile phases. Mar. Ecol. Prog. Ser. 2000, 195, 29–45. [Google Scholar] [CrossRef]
- Van Heukelem, L.; Hooker, S. The importance of a quality assurance plan for method validation and minimizing uncertainties in the HPLC analysis of phytoplankton pigments. In Phytoplankton Pigments: Characterization, Chemotaxonomy and Applications in Oceanography; Roy, S., Llewellyn, C.A., Skarstad Egeland, E., Johnsen, G., Eds.; Cambridge University Press: Cambridge, UK, 2011; pp. 195–242. [Google Scholar]
- Angell, A.R.; Mata, L.; de Nys, R.; Paul, N.A. The protein content of seaweeds: A universal nitrogen-to-protein conversion factor of five. J. Appl. Phycol. 2016, 28, 511–524. [Google Scholar] [CrossRef]
- Jones, D.B. Factors for Converting Percentages of Nitrogen in Foods and Feeds into Percentages of Protein; Circular No. 183; US Department of Agriculture: Washington, DC, USA, 1941. [Google Scholar]
- Van Ginneken, V.J.; Helsper, J.P.; de Visser, W.; van Keulen, H.; Brandenburg, W.A. Polyunsaturated fatty acids in various macroalgal species from north atlantic and tropical seas. Lipids Health Dis. 2011, 10, 104. [Google Scholar] [CrossRef]
- Kendel, M.; Wielgosz-Collin, G.; Bertrand, S.; Roussakis, C.; Bourgougnon, N.; Bedoux, G. Lipid composition, fatty acids and sterols in the seaweeds ulva armoricana, and solieria chordalis from Brittany (France): An analysis from nutritional, chemotaxonomic, and antiproliferative activity perspectives. Mar. Drugs 2015, 13, 5606–5628. [Google Scholar] [CrossRef]
- Stratistics Market Research Consulting Pvt Ltd. Nutraceuticals—Global Market Outlook (2017–2026); SMRC17563; Research and Markets: Dublin, Ireland, 2018; p. 144. [Google Scholar]
- Patel, A.; Matsakas, L.; Hruzova, K.; Rova, U.; Christakopoulos, P. Biosynthesis of nutraceutical fatty acids by the oleaginous marine microalgae Phaeodactylum tricornutum utilizing hydrolysates from organosolv-pretreated birch and spruce biomass. Mar. Drugs 2019, 17, 119. [Google Scholar] [CrossRef] [PubMed]
- Yi, T.; Li, S.-M.; Fan, J.-Y.; Fan, L.-L.; Zhang, Z.-F.; Luo, P.; Zhang, X.-J.; Wang, J.-G.; Zhu, L.; Zhao, Z.-Z.; et al. Comparative analysis of epa and dha in fish oil nutritional capsules by gc-ms. Lipids Health Dis. 2014, 13, 190. [Google Scholar] [CrossRef] [PubMed]
- Grand View Research. Dyes & Pigments Market Analysis by Product [Dyes (Reactive, Vat, Acid, Direct, Disperse), Pigments (Organic, Inorganic)], by Application (Dyes, Pigments), and Segment Forecasts, 2018–2025; GVR-1–68038–545–8; Grand View Research: San Francisco, CA, USA, 2017; p. 130. [Google Scholar]
- Rajauria, G.; Abu-Ghannam, N. Isolation and partial characterization of bioactive fucoxanthin from Himanthalia elongata brown seaweed: A TLC-based approach. Int. J. Anal. Chem. 2013, 2013, 802573. [Google Scholar] [CrossRef] [PubMed]
- Satomi, Y. Antitumor and cancer-preventative function of fucoxanthin: A marine carotenoid. Anticancer Res. 2017, 37, 1557–1562. [Google Scholar] [CrossRef] [PubMed]
- Sathasivam, R.; Ki, J.-S. A review of the biological activities of microalgal carotenoids and their potential use in healthcare and cosmetic industries. Mar. Drugs 2018, 16, 26. [Google Scholar] [CrossRef]
- Scotter, M.J. Emerging and persistent issues with artificial food colours: Natural colour additives as alternatives to synthetic colours in food and drink. Qual. Assur. Saf. Crops Foods 2011, 3, 28–39. [Google Scholar] [CrossRef]
- Jayasinghe, P.S.; Pahalawattaarachchi, V.; Ranaweera, K.K.D.S. Seaweed extract as a natural food coloring agent in jelly desserts on chemical, microbial and sensory quality. Acad. Agric. J. 2016, 1, 65–69. [Google Scholar]
- Humphrey, A.M. Chlorophyll as a Color and Functional Ingredient. J. Food Sci. 2004, 69, C422–C425. [Google Scholar] [CrossRef]
- Mourelle, M.L.; Gómez, C.P.; Legido, J.L. The potential use of marine microalgae and cyanobacteria in cosmetics and thalassotherapy. Cosmetics 2017, 4, 46. [Google Scholar] [CrossRef]
- Yuan, D.; Bassie, L.; Sabalza, M.; Miralpeix, B.; Dashevskaya, S.; Farre, G.; Rivera, S.M.; Banakar, R.; Bai, C.; Sanahuja, G.; et al. The potential impact of plant biotechnology on the millennium development goals. Plant Cell Rep. 2011, 30, 249–265. [Google Scholar] [CrossRef]
- Torregrosa-Crespo, J.; Montero, Z.; Fuentes, J.L.; Reig García-Galbis, M.; Garbayo, I.; Vílchez, C.; Martínez-Espinosa, R.M. Exploring the valuable carotenoids for the large-scale production by marine microorganisms. Mar. Drugs 2018, 16, 203. [Google Scholar] [CrossRef] [PubMed]
- Ambati, R.R.; Phang, S.M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A Review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef] [PubMed]
- Milledge, J.J.; Nielsen, B.V.; Bailey, D. High-value products from macroalgae: The potential uses of the invasive brown seaweed Sargassum muticum. Rev. Environ. Sci. BioTechnol. 2016, 15, 67–88. [Google Scholar] [CrossRef]
- Yu, Z.; Zhu, X.; Jiang, Y.; Luo, P.; Hu, C. Bioremediation and fodder potentials of two Sargassum spp. In coastal waters of Shenzhen, South China. Mar. Pollut. Bull. 2014, 85, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Cordell, D.; Rosemarin, A.; Schröder, J.J.; Smit, A.L. Towards global phosphorus security: A systems framework for phosphorus recovery and reuse options. Chemosphere 2011, 84, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Childers, D.L.; Corman, J.; Edwards, M.; Elser, J.J. Sustainability challenges of phosphorus and food: Solutions from closing the human phosphorus cycle. BioScience 2011, 61, 117–124. [Google Scholar] [CrossRef]
- Chen, Q.; Pan, X.-D.; Huang, B.-F.; Han, J.-L. Distribution of metals and metalloids in dried seaweeds and health risk to population in southeastern china. Sci. Rep. 2018, 8, 3578. [Google Scholar] [CrossRef]
- Matsushima, F.; Meshitsuka, S.; Nose, T. Aluminum contents in dried and cooked sea vegetables. Nihon Eiseigaku Zasshi Jpn. J. Hyg. 1997, 51, 763–769. [Google Scholar] [CrossRef][Green Version]
- Imadi, S.R.; Waseem, S.; Kazi, A.G.; Azooz, M.M.; Ahmad, P. Chapter 1—Aluminum toxicity in plants: An overview. In Plant Metal Interaction; Ahmad, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–20. [Google Scholar]
- Piccini, M.; Raikova, S.; Allen, M.; Chuck, C. A synergistic use of microalgae and macroalgae for heavy metal bioremediation and bioenergy production through hydrothermal liquefaction. Sustain. Energy Fuels 2019, 3, 292–301. [Google Scholar] [CrossRef]
| Species | Common Name | Taxon |
|---|---|---|
| Ulva lactuca | Sea lettuce | Chlorophyta |
| Spongomorpha aeruginosa | Spongy weed | Chlorophyta |
| Polyides rotunda | Discoid fork weed | Rhodophyta |
| Lomentaria articulata | Bunny ears | Rhodophyta |
| Ahnfeltiopsis devoniensis | Devonshire fan weed | Rhodophyta |
| Palmaria palmata | Dulse | Rhodophyta |
| Rhodomela confervoides | Straggly bush weed | Rhodophyta |
| Dilsea carnosa | Red rags | Rhodophyta |
| Calliblepharis spp. | Eye lash weed | Rhodophyta |
| Gastroclonium ovatum | Red grape weed | Rhodophyta |
| Sargassum muticum | Wireweed | Phaeophyta |
| Himanthalia elongata | Thong weed | Phaeophyta |
| Fucus serratus | Serrated wrack | Phaeophyta |
| Laminaria digitata | Oar weed/tangle | Phaeophyta |
| Saccharina latissima | Sugar kelp | Phaeophyta |
| Punctaria latifolia | n/a | Phaeophyta |
| Colpomenia peregrina | Oyster thief | Phaeophyta |
| Fatty Acid (% Total FAME) | Total Lipid | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C14:0 | C16:0 | C16:1 | C18:0 | C18:1 | C18:2 | C18:3 (γ) | C18:3 (α) | C20:4 | C20:5 | (mg/g) | (% Dry Biomass) | |
| U. lactuca | 0.0 | 36.4 | 0.0 | 9.1 | 18.2 | 18.2 | 0.0 | 18.2 | 0.0 | 0.0 | 18.09 | 1.8 |
| S. aeruginosa | 0.0 | 33.3 | 0.0 | 11.1 | 11.1 | 11.1 | 0.0 | 22.2 | 0.0 | 11.1 | 13.89 | 1.4 |
| P. rotunda | 10.0 | 30.0 | 0.0 | 10.0 | 10.0 | 0.0 | 0.0 | 0.0 | 20.0 | 20.0 | 12.92 | 1.3 |
| L. articulata | 14.3 | 28.6 | 0.0 | 14.3 | 14.3 | 0.0 | 0.0 | 0.0 | 14.3 | 14.3 | 12.15 | 1.2 |
| A. devoniensis | 11.1 | 22.2 | 0.0 | 11.1 | 11.1 | 0.0 | 0.0 | 0.0 | 22.2 | 22.2 | 12.36 | 1.2 |
| P. palmata | 7.1 | 21.4 | 0.0 | 7.1 | 7.1 | 0.0 | 0.0 | 0.0 | 7.1 | 50.0 | 23.25 | 2.3 |
| R. confervoides | 14.2 | 28.6 | 0.0 | 14.2 | 14.2 | 0.0 | 0.0 | 0.0 | 14.2 | 14.2 | 8.58 | 0.9 |
| D. carnosa | 9.1 | 27.3 | 0.0 | 9.1 | 9.1 | 0.0 | 0.0 | 0.0 | 18.2 | 27.3 | 18.83 | 1.9 |
| S. muticum | 8.3 | 25.0 | 8.3 | 8.3 | 8.3 | 8.3 | 0.0 | 8.3 | 16.7 | 8.3 | 15.79 | 1.6 |
| H. elongata | 7.7 | 23.1 | 0.0 | 7.7 | 15.4 | 7.7 | 0.0 | 7.7 | 15.4 | 15.4 | 19.26 | 1.9 |
| F. serratus | 12.5 | 20.8 | 4.2 | 4.2 | 25.0 | 8.3 | 0.0 | 4.2 | 12.5 | 8.3 | 29.13 | 2.9 |
| L. digitata | 8.3 | 16.6 | 0.0 | 8.3 | 16.6 | 8.3 | 0.0 | 8.3 | 16.6 | 16.6 | 15.62 | 1.6 |
| S. latissima | 8.3 | 16.7 | 0.0 | 8.3 | 16.7 | 8.3 | 8.3 | 8.3 | 16.7 | 8.3 | 18.52 | 1.9 |
| C. peregrina | 16.7 | 16.7 | 0.0 | 16.7 | 16.7 | 0.0 | 0.0 | 0.0 | 16.7 | 16.7 | 7.50 | 0.8 |
| P. latifolia | 8.3 | 16.7 | 8.3 | 8.3 | 8.3 | 8.3 | 0.0 | 8.3 | 16.7 | 16.7 | 16.48 | 1.6 |
| Pigment µg/g | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chl-c | Chl-b | Chl-a | Fucoxanthin | α Carotene | β Carotene | Lutein | Zeaxanthin | Neoxanthin | Violaxanthin | Antheraxanthin | Total Pigment | |
| U. lactuca | 0.0 | 110.6 | 155.0 | 2.3 | 0.8 | 8.4 | 34.0 | 1.5 | 80.2 | 17.3 | 0.0 | 422 |
| S. aeruginosa | 0.7 | 98.7 | 154.9 | 2.9 | 0.0 | 10.6 | 27.7 | 3.9 | 7.4 | 11.1 | 0.0 | 334 |
| P. rotunda | 0.7 | 2.8 | 76.6 | 3.6 | 4.1 | 2.8 | 15.2 | 0.8 | 0.0 | 0.4 | 0.0 | 129 |
| L. articulata | 0.0 | 2.6 | 119.7 | 4.1 | 7.7 | 1.0 | 19.4 | 0.8 | 0.0 | 0.0 | 0.0 | 173 |
| A. devoniensis | 0.0 | 1.6 | 26.3 | 0.9 | 0.6 | 1.4 | 5.0 | 0.8 | 0.0 | 0.0 | 0.0 | 37 |
| P. palmata | 0.0 | 0.0 | 213.1 | 6.2 | 12.1 | 11.0 | 56.4 | 1.1 | 0.0 | 1.5 | 0.0 | 315 |
| R. confervoides | 5.7 | 4.0 | 179.0 | 22.6 | 5.7 | 7.9 | 29.4 | 2.0 | 0.0 | 1.4 | 2.5 | 270 |
| D. carnosa | 0.0 | 10.1 | 133.6 | 1.9 | 11.6 | 5.9 | 33.9 | 0.0 | 1.2 | 0.7 | 0.0 | 271 |
| Calliblepharis spp. | 0.9 | 5.9 | 197.5 | 4.6 | 9.1 | 9.6 | 44.1 | 0.8 | 0.0 | 0.0 | 0.0 | 283 |
| S. muticum | 99.2 | 2.1 | 473.5 | 292.7 | 0.0 | 35.7 | 0.7 | 10.2 | 0.0 | 80.2 | 38.6 | 1045 |
| H. elongata | 52.8 | 0.0 | 379.5 | 195.7 | 0.0 | 27.0 | 0.3 | 3.1 | 0.0 | 64.9 | 21.0 | 749 |
| F. serratus | 32.1 | 0.0 | 62.5 | 131.8 | 0.0 | 13.4 | 0.4 | 1.5 | 0.0 | 53.7 | 14.9 | 313 |
| L. digitata | 65.4 | 0.0 | 387.1 | 230.8 | 0.0 | 20.6 | 0.3 | 2.7 | 0.0 | 57.4 | 28.9 | 801 |
| S. latissima | 73.0 | 0.0 | 462.6 | 298.2 | 0.0 | 17.7 | 0.5 | 7.7 | 0.0 | 36.9 | 34.3 | 945 |
| P. latifolia | 139.0 | 0.0 | 612.7 | 340.4 | 0.0 | 46.8 | 0.5 | 6.8 | 0.0 | 106.3 | 54.1 | 1319 |
| C. peregrina | 8.5 | 1.0 | 70.7 | 38.8 | 0.0 | 1.9 | 0.9 | 1.0 | 0.0 | 6.6 | 4.7 | 135 |
| G. ovatum | 1.8 | 0.0 | 337.0 | 7.6 | 6.4 | 27.1 | 71.3 | 11.8 | 0.0 | 0.0 | 1.3 | 484 |
| Component % of Dry Biomass | |||||
|---|---|---|---|---|---|
| C | N | Protein | Ash | Carbohydrate | |
| U. lactuca | 30.8 | 1.09 | 5.45 | 22.7 | 71.8 |
| S. aeruginosa | 20.3 | 1.06 | 8.01 | 46.4 | 45.6 |
| P. rotunda | 31.0 | 3.53 | 17.65 | 29.6 | 52.7 |
| L. articulata | 20.2 | 2.75 | 13.77 | 50.1 | 36.2 |
| A. devoniensis | 31.8 | 1.87 | 9.37 | 24.2 | 66.4 |
| P. palmata | 38.3 | 2.5 | 12.50 | 21.4 | 66.1 |
| R. confervoides | 26.2 | 2.51 | 12.54 | 45.8 | 41.6 |
| D. carnosa | 36.3 | 2.91 | 14.55 | 18.2 | 67.2 |
| S. muticum | 34.5 | 1.39 | 4.64 | 26.4 | 69.0 |
| H. elongata | 35.8 | 1.13 | 5.65 | 24.6 | 69.7 |
| F. serratus | 39.3 | 1.8 | 8.98 | 21.5 | 69.5 |
| L. digitata | 34.2 | 1.37 | 6.87 | 27.8 | 65.3 |
| S. latissima | 36.2 | 1.21 | 6.03 | 20.9 | 73.0 |
| C. peregrina | 13.8 | 0.58 | 2.48 | 85.3 | 12.2 |
| P. latifolia | 28.8 | 1.15 | 5.73 | 43.8 | 50.5 |
| Mineral (ppm) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fe | Sn | Mn | Al | Si | K | P | S | Ca | Cu | Zn | |
| S. aeruginosa | 620 | 0 | 0 | 1120 | 2530 | 35,020 | 910 | 1070 | 7370 | 0 | 10 |
| L. articulata | 4040 | 0 | 260 | 9090 | 74,010 | 16,320 | 3010 | 1290 | 46,780 | 0 | 80 |
| A. devoniensis | 180 | 20 | 70 | 230 | 1980 | 30,480 | 1430 | 390 | 12,720 | 0 | 20 |
| P. palmata | 0 | 0 | 30 | 0 | 0 | 105,740 | 2390 | 0 | 2550 | 0 | 10 |
| R. confervoides | 900 | 0 | 1420 | 1740 | 6450 | 77,280 | 1970 | 260 | 11,960 | 0 | 200 |
| D. carnosa | 0 | 70 | 0 | 0 | 0 | 41,130 | 1650 | 0 | 9030 | 0 | 10 |
| C. spp. | 1320 | 0 | 160 | 2290 | 12,400 | 25,130 | 1620 | 1400 | 16,250 | 0 | 20 |
| S. muticum | 80 | 60 | 10 | 280 | 1990 | 75,760 | 1340 | 0 | 11,200 | 0 | 10 |
| H. elongata | 0 | 0 | 20 | 200 | 1350 | 72,290 | 1140 | 0 | 7530 | 10 | 20 |
| F. serratus | 440 | 0 | 10 | 730 | 3950 | 34,320 | 890 | 730 | 5110 | 0 | 10 |
| L. digitata | 210 | 30 | 10 | 120 | 2800 | 34,560 | 2850 | 0 | 3160 | 0 | 20 |
| S. latissima | 120 | 10 | 70 | 0 | 0 | 39,460 | 1670 | 0 | 8670 | 0 | 30 |
| P. latifolia | 260 | 20 | 10 | 510 | 7420 | 123,530 | 1270 | 420 | 12,340 | 0 | 30 |
| C. peregrina | 9310 | 50 | 230 | 19,610 | 252,290 | 46,930 | 670 | 3350 | 55,640 | 20 | 50 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beacham, T.A.; Cole, I.S.; DeDross, L.S.; Raikova, S.; Chuck, C.J.; Macdonald, J.; Herrera, L.; Ali, T.; Airs, R.L.; Landels, A.; et al. Analysis of Seaweeds from South West England as a Biorefinery Feedstock. Appl. Sci. 2019, 9, 4456. https://doi.org/10.3390/app9204456
Beacham TA, Cole IS, DeDross LS, Raikova S, Chuck CJ, Macdonald J, Herrera L, Ali T, Airs RL, Landels A, et al. Analysis of Seaweeds from South West England as a Biorefinery Feedstock. Applied Sciences. 2019; 9(20):4456. https://doi.org/10.3390/app9204456
Chicago/Turabian StyleBeacham, Tracey A., Isobel S. Cole, Louisa S. DeDross, Sofia Raikova, Christopher J. Chuck, John Macdonald, Leopoldo Herrera, Tariq Ali, Ruth L. Airs, Andrew Landels, and et al. 2019. "Analysis of Seaweeds from South West England as a Biorefinery Feedstock" Applied Sciences 9, no. 20: 4456. https://doi.org/10.3390/app9204456
APA StyleBeacham, T. A., Cole, I. S., DeDross, L. S., Raikova, S., Chuck, C. J., Macdonald, J., Herrera, L., Ali, T., Airs, R. L., Landels, A., & Allen, M. J. (2019). Analysis of Seaweeds from South West England as a Biorefinery Feedstock. Applied Sciences, 9(20), 4456. https://doi.org/10.3390/app9204456





