Featured Application
The synthetic peptide of amelogenin exon 5 might be a useful application to the pulp capping treatment.
Abstract
Amelogenin is a complex enamel matrix protein that consists of various molecular-size proteins and amino acids. A spliced form of amelogenin was identified that included exons 2, 3, 5, 6, and 7. However, the biological function of amelogenin exon 5 on dental pulp remains unknown. We designed a synthetic amelogenin exon 5 encoded peptide (SP), which was based on a protein produced by cells in response to the enamel matrix derivative (EMD). We investigated the effect of the SP on potentiation of osteogenesis and its signal pathway in dental pulp stem cells (DPSCs). DPSCs are an important cell for pulp tissue homeostasis. DPSCs were cultured with SP to examine the effect of cell proliferation and osteogenic differentiation. We also investigated the mitogen-activated protein kinase (MAPK) signaling pathway. SP significantly enhanced cell proliferation and the expression of osteogenic differentiation. Moreover, SP promoted the expression of the MAPK signaling pathway. Therefore, amelogenin exon 5 might contribute to dental pulp capping.
1. Introduction
There are various types of enamel matrix proteins. The enamel matrix proteins exhibit a variety of isoforms, generated by mRNA splicing. Among the enamel proteins, amelogenin is suspected to have at least six molecular types, and many of their biological functions remain unknown [1,2,3].
Amelogenin is synthesized by ameloblasts and is a major component of enamel matrix proteins; notably, amelogenin is an extracellular matrix protein that consists of various molecular-size proteins and amino acids. In the early stages of tooth development, amelogenin contributes to regulating mineralization in enamel and bone regeneration [4]. Although there are applications for amelogenin in clinical treatment, its biological functions remain unknown. In general, amelogenin is used in periodontal tissue engineering as an enamel matrix derivative (EMD).
EMD—an extract of porcine fetal tooth material—promotes cell proliferation and mineralization in dental pulp cells [5,6]. EMD has been used in periodontal regenerative surgery, and there is evidence that EMD can induce new cementum and bone tissue [7,8].
Previously, we found that the injections of EMD on the backs of rats can induce hard tissue formation and eosinophilic round bodies (ERBs) [9]. We investigated these ERBs and found fragments of amelogenin exon 5. We examined ERBs using Matrix Assisted Laser Desorption Ionization-Time of Flight (MMALDI-TOF) and found fragments of amelogenin exon5. We synthesized a seven-amino acid (WYQNMIR) peptide based on these fragments. We made a synthetic amelogenin exon 5 encoded peptide (SP) to see if it would behave in a manner similar to EMD (i.e., whether it would induce formation of cementum and bone-like tissue) [10]. Our previous study showed that SP induced bone and cementum tissue in rats [11]. We also showed that SP promotes cell viability in human periodontal ligament (PDL) fibroblasts [12], and promotes the osteogenic differentiation of mesenchymal stem cells (MSCs) [13] and PDL stem cells [14], as well as EMD.
Gronthos found dental pulp stem cells (DPSCs) and contributed to the homeostasis of dental pulp tissue [15]. These cells have proliferative potential and osteogenic differentiation potential; thus, they may be useful in bone regeneration [16,17,18,19]. The function of amelogenin in dental pulp cells (DPCs) has been elucidated in some studies, but the biological effects of each portion of amelogenin are not clear.
Therefore, the aim of our study was to examine the biological function of the amelogenin exon 5-encoded peptide on human DPSCs, and to investigate whether the amelogenin exon 5-encoded peptide acts through the mitogen-activated protein kinase (MAPK) signal pathway.
2. Materials and Methods
2.1. Cell Culture
Human DPSCs were purchased from Lonza (Tokyo, Japan). DPSCs were positive for CD73, CD90, CD105, and CD106; they were negative for CD34, CD45, and CD133. DPSCs at passage 3–4 were used for experiments. We used osteogenic medium containing 50 µM L-ascorbic acid 2-phosphate (Nacalai Tesque, Kyoto, Japan), 10 mM β-glycerophosphate (Wako Pure Chemical Industries Ltd., Tokyo, Japan), and 10 nM dexamethasone (Wako Pure Chemical Industries Ltd Tokyo, Japan), according previous our study [12,13,14].
2.2. Cell Proliferation Assay
DPSCs were cultured with normal culture medium. After 24 h, the medium was changed with medium, including the SP (0, 10, 100, 1000 ng/mL), and DPSCs were then cultured for 1, 3, and 7 days. We determined the cell proliferation using cell count reagent SF (Nacalai Tesque, Kyoto, Japan).
2.3. Alkaline Phosphatase (ALP) Activity and Osteocalcin (OCN)
DPSCs were cultured for 7 or 14 days. The measurement of ALP activity involved a one-step p-nitrophenyl phosphate (pNPP) (Pierce Biotechnology Inc., Rockford, IL, USA). We normalized ALP activity to the amount of DNA. We measured the DNA volume using a PicoGreen dsDNA Assay kit (Invitrogen, Paisley, UK). The cultured supernatant (at 7 and 14 days) was collected to quantify OCN levels using an Enzyme-Linked Immuno Sorbent Assay (ELISA) kit (Takara Inc., Shiga, Japan).
2.4. Extracellular Matrix Mineralization
DPSCs were cultured for 7 or 14 days. DPSCs were dissolved with 10% formic acid. We measured calcium deposition (Ca) using a Calcium detection kit (Wako Pure Chemical Industries Ltd Tokyo, Japan). The amount of phosphate was examined by P test kit (Bio Assay Systems, Hayward, CA, USA).
For qualitative histology, other cultures of DPSCs at 7 and 14 days were fixed using 70% ethanol (Nacalai Tesque, Kyoto, Japan). DPSCs were then stained using 1% Alizarin Red S. Calcified nodules were imaged by a BZ-II all-in-one fluorescence microscope (Keyence Corporation, Osaka, Japan).
2.5. Gene Expression of Osteogenic Differentiation
DPSCs were cultured for 7 or 14 days. We extracted RNA using a RNeasy Mini Kit (Qiagen, Venlo, The Netherlands), and then RNA samples were reverse transcribed into cDNA by PrimeScript Reagent kit (Takara). Gene expression was examined by real-time PCR assays (Thermo Fisher Scientific, Waltham, MA, USA). The mRNA expression of collagen type 1 alpha 1 (Col1A1), osteonectin (ON), and Runx2 were evaluated using quantitative real-time PCR, in accordance with standard protocols.
2.6. Western Blot Analyses
Adherent DPSCs were cultured for 0, 30, and 60 min in the presence of SP. Total protein was extracted by a Radio-Immunoprecipitation Assay (RIPA) buffer solution (Thermo Fisher Scientific). Samples were separated by electrophoresis on 12.5% sodium dodecyl sulfate-polyacrylamide gels, and then transferred onto polyvinylidene difluoride membranes. The membranes were incubated with primary antibodies (Extracellular Signal-regulated Kinase (ERK), phospho-ERK, c-Jun N-terminal kinase (JNK), phospho-JNK, p38, and phospho-p38). Immunoreactive signals were analyzed with the ChemiDoc MP System (BioRad, Hercules, CA, USA).
2.7. Statistical Analysis
We performed the one-way analysis of variance followed by Bonferroni post hoc test. P values < 0.05 were determined to be statistically significant.
3. Results
3.1. Cell Proliferation
We examined four concentrations of the SP (0, 10, 100, or 1000 ng/mL) on DPSC cells to evaluate the optimal concentration. We found that the SP significantly promoted DPSC proliferation at 1, 3, and 7 days (Figure 1, P < 0.05), with a concentration of 100 ng/mL generating the highest change in cell proliferation. Therefore, we used 100 ng/mL SP as the optimal concentration for following experiments.
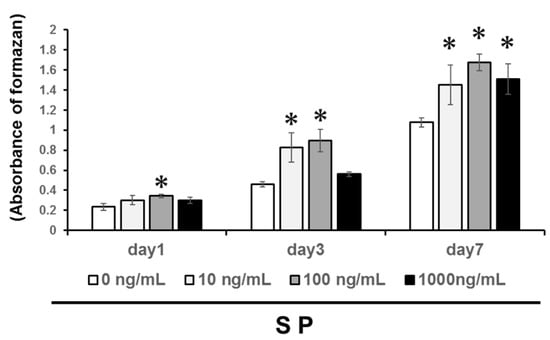
Figure 1.
Dental pulp stem cell (DPSCs) were cultured with 0, 10, 100, and 1000 ng/mL synthetic amelogenin exon 5 encoded peptide (SP). Measurement of cell proliferation was investigated for 1, 3, and 7 days. Significant differences were determined compared with the control (SP, 0 ng/mL) (* P > 0.05).
3.2. ALP Activity and OCN Production
ALP activity and OCN secretion in the SP group were significantly enhanced compared with osteogenic media alone (Control group; Figure 2A,B; P < 0.05).
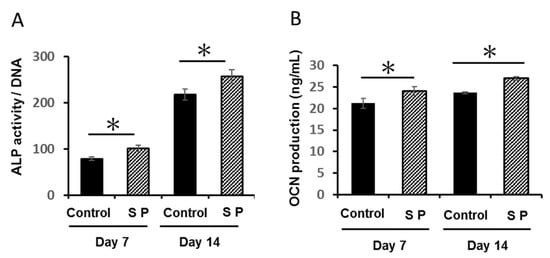
Figure 2.
Effect of synthetic amelogenin exon 5 encoded peptide (SP) on DPSCs in osteogenic differentiation and mineralization. Confluent DPSCs were cultured with osteogenic medium and SP (0, 100 ng/mL) for 7 and 14 days (* P < 0.05). (A) alkaline phosphatase (ALP) activity and (B) osteocalcin (OCN) production (A,B).
3.3. Osteogenic Gene Expression
The mRNA expression of Col1A1, Runx2, and ON were all significantly enhanced in SP group, compared with their levels in the control group at both time points (Figure 3A–C) (* P < 0.05).
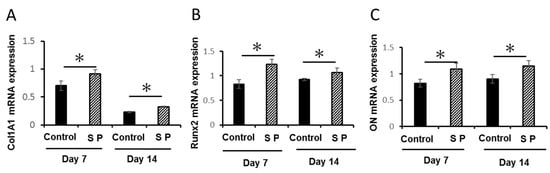
Figure 3.
Effects of the SP on mRNA expression of (A) collagen 1-alpha 1 (COL1A1), (B) Runx2, and (C) osteonectin (ON). The gene expression was investigated by quantitative RT-PCR (* P < 0.05).
3.4. Extracellular Matrix Mineralization
The mineralized nodules stained with alizarin red were larger in SP group compared with control group (Figure 4A). Calcium deposition in SP group was significantly promoted at both 7 and 14 days, compared with deposition in control group (Figure 4B; P < 0.05); the ratio of Ca/P at 7 days showed a similar trend (Figure 4C; P < 0.05).
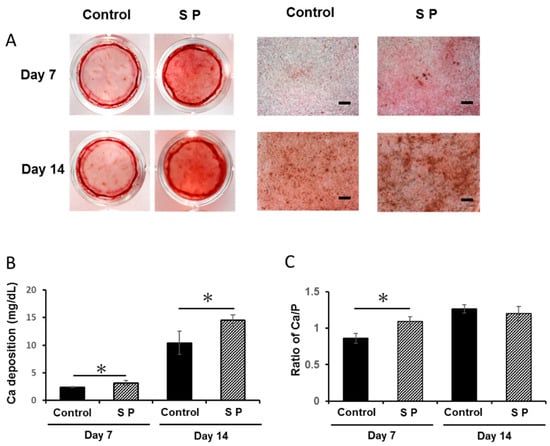
Figure 4.
SP enhanced the mineralized nodule formation under growth conditions, as measured by (A) alizarin red staining, (B) calcium deposition, and (C) phosphate deposition (ratio of Ca/P) in the extracellular matrix. Scale bar in (A), 100 µm.
3.5. Phosphorylation of the MAPK Signaling Pathway
The SP-activated expression of phospho-ERK, phospho-p-38, and phospho-JNK in SP (Figure 5A). The expression levels of the phosphor-ERK/ERK ratio were increased at both 30 and 60 min (Figure 5B). The expression levels of the phospho-p-38/p-38 ratio were increased at 30 min (Figure 5C). The expression levels of the phospho-JNK/JNK ratio were increased at both 30 and 60 min (Figure 5D).
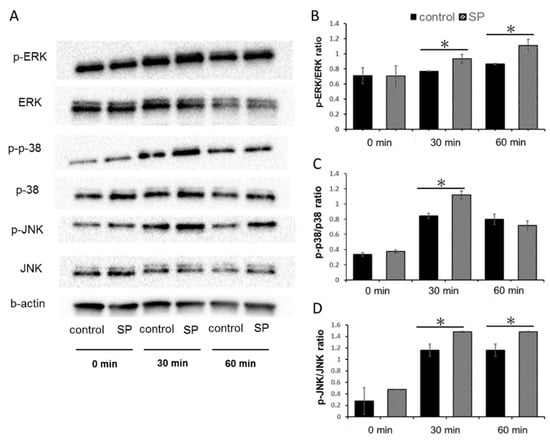
Figure 5.
Phosphorylation of the mitogen-activated protein kinase (MAPK) signaling pathway of SP. Dental pulp stem cells (DPSCs) were treated with SP (0 or 100 ng/mL) for 0, 30, or 60 min. Protein expression was evaluated by immunoblotting analysis. (A) Protein expression of ERK 1/2, JNK, p-38, phospho-ERK 1/2, phospho-JNK, and phospho-p-38. (B) The ratio of phospho-ERK 1/2 intensity: The expression levels of the phospho-ERK 1/2/ERK 1/2 ratio were increased at both 30 and 60 min. (C) The ratio of phospho-p38 intensity: The expression levels of the phospho-p38/ p38 ratio were increased at 30 min. (D) The ratio of phospho-JNK intensity: The expression levels of the phospho-JNK/ JNK ratio were increased at both 30 and 60 min. (* P > 0.05).
4. Discussion
The present study indicated that the amelogenin exon 5-encoded peptide (SP) promoted cell proliferation and osteogenic differentiation of human DPSCs. Moreover, we found that SP activated the MAPK pathway. EMD and another enamel proteins promote the cell viability of dental pulp cells [20]. In our previous study, we found that SP could also promote PDL cell proliferation [21].
In this study, we found that a concentration of 100 ng/mL SP most enhanced DPSC proliferation; this was similar to our previous reported study [14]. Although, the cell proliferation at 1000 ng/mL SP was decreased compared with 100 ng/mL SP, the cell proliferation was increased compared with 0 ng/mL SP. Therefore, the concentration of 1000 ng/mL was not toxic. From these results, we used this concentration to further investigate the effects of 100 ng/mL SP on osteogenic differentiation.
ALP and OCN are regarded as markers of the osteogenesis phenotype [22,23]. In this study, we found that SP promoted the activity of ALP and the production of OCN. This result is similar to the effect of EMD and SP in human MSCs [13] and PDL stem cells [14]. Thus, we concluded that SP can also induce differentiation of DPSCs. Runx2, ON, and COL1A1 are essential factors required during the early stages of osteogenic differentiation [24,25,26]. In particular, COL1A1 has a critical role in the formation of new hard tissue [27]. Previous work has shown that amelogenin and EMD can promote the expression of all three genes in human osteoblasts and human dental pulp cells [28], as well as in human MSCs [29]. Similarly, SP can promote expression of ON in human PDL stem cells [13], and it promoted the expression of type I collagen in our previous in vivo study [11]. In this study, the SP enhanced the expression of Runx2, ON, and COL1A1 in DPSCs, suggesting that the SP enhances osteogenic differentiation in DPSCs during early osteogenic differentiation.
Previous work has shown that EMD promotes the mineralization of dental pulp cells [28], and that SP could also increase the mineralization of PDL stem cell [14]. Here, we examined qualitatively and quantitatively changes in calcified nodule formation induced by SP. We found that SP induced greater nodule formation and calcium deposition at 7 and 14 days compared with cultures that lacked SP. Furthermore, SP promoted an increased Ca/P ratio at 7 days of culture compared with control conditions. The theoretical Ca/P ratio of 1:67 is indicative of stoichiometrically pure hydroxyapatite [30]. The Ca/P ratio at day 7 is significantly different, but day 14 is no different. These results suggest that SP induces high-quality calcified nodule formation on dental pulp at an early stage (day 7). However, the effect is limited, meaning the late stage (day14) is no different. We consider that forming calcifications at an early stage in protecting the dental pulp is very important in endodontic treatments. Therefore, we believe that SP induces the dentin bridge at an early stage, and could contribute to the successes of endodontic treatments.
The MAPK signal is an essential regulator of cell viability and osteogenic differentiation in dental tissue cells [31,32,33]; however, the mechanism by which the amelogenin exon 5 peptide exerts its effects remains unclear.
Figure 5 shows that SP activated the phosphorylation of MAPK components in human DPSCs. EMD and another amelogenin peptides have the ability to regulate cellular functions through MAPK signaling. Our findings support the role of the SP in regulating cellular function through MAPK signaling in a manner similar to that of EMD and other enamel proteins. Amelogenin has various biological functions, such as cell proliferation and differentiation. As well known, amelogenin can activate physiological actions through various signaling pathways. In our previous study, SP promotes the cell proliferation via the ERK pathway in human MSCs [13]. Therefore, we believe that SP promotes the cell proliferation via MAPK, such as ERK signaling. However, our previous studies fragmentally elucidated the effects of SP. Thus, we consider that the effects of SP on other signaling pathways need to be investigated.
Our previous study showed that SP promoted cell viability and odontogenic differentiation in rat odontoblast-like cells [34]. Thus, we assumed that SP induces calcification nodules, such as the dentin bridge, and could contribute to protection of dental pulp tissues. However, we only evaluated the cell response in this study; we should examine additional experiments in future research using an animal model study.
Previous reports showed that other amelogenin peptides regulate the biological function through LAMP or CD63, and then the signaling cascade induces the activation of the MAPK pathway in a cytoplasmic reaction [35,36,37,38]. Therefore, we believe this SP also regulates these signaling cascades, similar to any growth factor.
5. Conclusions
In summary, we showed that a synthetic form of the amelogenin exon 5 peptide can promote cell proliferation and osteogenic differentiation of DPSCs in vitro. The amelogenin exon 5 could contribute to dental pulp tissue repair and mineralization.
Author Contributions
Writing—original draft preparation, H.K. and Y.T.; conceptualization, H.K.; methodology, I.Y., Y.R., Y.N., and Q.W.; data curation, N.T. and T.N.; supervision, M.N.; project administration, M.U.; funding acquisition, Y.T. and M.U.
Funding
This research was funded by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (No. 16K 20476 and 17K 11818).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lyngstadaas, S.P.; Risnes, S.; Nordbo, H.; Flones, A.G. Amelogenin gene similarity in vertebrates: DNA sequences encoding amelogenin seem to be conserved during evolution. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 1990, 160, 469–472. [Google Scholar] [CrossRef]
- Fincham, A.G.; Belcourt, A.B.; Termine, J.D.; Butler, W.T.; Cothran, W.C. Amelogenins. Sequence homologies in enamel-matrix proteins from three mammalian species. Biochem. J. 1983, 211, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Tompkins, K.; Alvares, K.; George, A.; Veis, A. Two related low molecular mass polypeptide isoforms of amelogenin have distinct activities in mouse tooth germ differentiation in vitro. J. Bone Miner. Res. 2005, 20, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Amin, H.D.; Olsen, I.; Knowles, J.C.; Donos, N. Differential effect of amelogenin peptides on osteogenic differentiation in vitro: Identification of possible new drugs for bone repair and regeneration. Tissue Eng. A 2012, 18, 1193–1202. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Hammarström, L.; Matsumoto, K.; Lyngstadaas, S.P. The induction of reparative dentine by enamel proteins. Int. Endod. J. 2002, 35, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Slaby, I.; Matsumoto, K.; Ritchie, H.H.; Lyngstadaas, S.P. Immunohistochemical characterization of rapid dentin formation induced by enamel matrix derivative. Calcif. Tissue Int. 2004, 75, 243–252. [Google Scholar] [CrossRef]
- Hammarström, L. Enamel matrix, cementum development and regeneration. J. Clin. Periodontol. 1997, 24, 658–668. [Google Scholar] [CrossRef]
- Hammarström, L.; Heijl, L.; Gestrelius, S. Periodontal regeneration in a buccal dehiscence model in monkeys after application of enamel matrix proteins. J. Clin. Periodontol. 1997, 24, 669–677. [Google Scholar] [CrossRef]
- Kim, N.H.; Tominaga, K.; Tanaka, A. Analysis of eosinophilic round bodies formed after injection of enamel matrix derivative into the backs of rats. J. Periodontol. 2005, 76, 1934–1941. [Google Scholar] [CrossRef]
- Hida, T.; Tominaga, K.; Tanaka, A. Tissue Reaction to synthetic oligopeptide derived from enamel matrix derivative in rats. Oral Sci. Int. 2010, 7, 26–33. [Google Scholar] [CrossRef]
- Noguchi, M.; Tominaga, K.; Tanaka, A.; Ueda, M. Hard tissue formation induced by synthetic oligopeptide derived from an enamel matrix derivative. Oral. Med. Pathol. 2012, 16, 75–80. [Google Scholar] [CrossRef]
- Taguchi, Y.; Takahashi, S.; Tominaga, K.; Komasa, S.; Shida, M.; Hayashi, H.; Tanaka, A.; Umeda, M. Effect of oligopeptide derived from enamel matrix derivatives on the proliferation, adhesion migration of human periodontal ligament cells. J. Conserv. Dent. 2012, 55, 227–235. (In Japanese) [Google Scholar]
- Katayama, N.; Kato, H.; Taguchi, Y.; Tanaka, A.; Umeda, M. The effects of synthetic oligopeptide derived from enamel matrix derivative on cell proliferation and osteoblastic differentiation of human mesenchymal stem cells. Int. J. Mol. Sci. 2014, 15, 14026–14043. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Katayama, N.; Taguchi, Y.; Tominaga, K.; Umeda, M.; Tanaka, A. A synthetic oligopeptide derived from enamel matrix derivative promotes the differentiation of human periodontal ligament stem cells into osteoblast-like cells with increased mineralization. J. Periodontol. 2013, 84, 1476–1483. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Brahim, J.; Li, W.; Fisher, L.W.; Cherman, N.; Boyde, A.; DenBesten, P.; Robey, P.G.; Shi, S. Stem cell properties of human dental pulp stem cells. J. Dent. Res. 2002, 81, 531–535. [Google Scholar] [CrossRef]
- Yamada, Y.; Nakamura, S.; Ito, K.; Sugito, T.; Yoshimi, R.; Nagasaka, T.; Ueda, M. A feasibility of useful cell-based therapy by bone regeneration with deciduous tooth stem cells, dental pulp stem cells, or bone-marrow-derived mesenchymal stem cells for clinical study using tissue engineering technology. Tissue Eng. Part A 2010, 16, 1891–1900. [Google Scholar] [CrossRef] [PubMed]
- Saghiri, M.A.; Asatourian, A.; Sorenson, C.M.; Sheibani, N. Role of angiogenesis in endodontics: Contributions of stem cells and proangiogenic and antiangiogenic factors to dental pulp regeneration. J. Endod. 2015, 41, 797–803. [Google Scholar] [CrossRef]
- Moonesi Rad, R.; Atila, D.; Akgün, E.E.; Evis, Z.; Keskin, D.; Tezcaner, A. Evaluation of human dental pulp stem cells behavior on a novel nanobiocomposite scaffold prepared for regenerative endodontics. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 100, 928–948. [Google Scholar] [CrossRef]
- Noda, S.; Kawashima, N.; Yamamoto MHashimoto, K.; Nara, K.; Sekiya, I.; Okiji, T. Effect of cell culture density on dental pulp-derived mesenchymal stem cells with reference to osteogenic differentiation. Sci. Rep. 2019, 9, 5430. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; Ge, L. Effects of the enamel matrix derivative on the proliferation and odontogenic differentiation of human dental pulp cells. J. Dent. 2014, 42, 53–59. [Google Scholar] [CrossRef]
- Taguchi, Y.; Yasui, N.; Takahashi, S.; Tominaga, K.; Kato, H.; Komasa, S.; Shida, M.; Hayashi, H.; Tanaka, A.; Umeda, M. Hard tissue formation by human periodontal ligament fibroblast Cells treated with an emdogain-derived oligopeptide in vitro. J. Hard Tissue Biol. 2012, 21, 375–384. [Google Scholar] [CrossRef]
- Weinreb, M.; Shinar, D.; Rodan, G.A. Different pattern of alkaline phosphatase, osteopontin, and osteocalcin expression in developing rat bone visualized by in situ hybridization. J. Bone Miner. Res. 1990, 5, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Nomura, S.; Yamaguchi, A.; Suda, T.; Yoshiki, S. In situ hybridization of bone matrix proteins in undecalcified adult rat bone sections. J. Histochem. Cytochem. 1992, 40, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Ishigaki, R.; Takagi, M.; Igarashi, M.; Ito, K. Gene expression and immunohistochemical localization of osteonectin in association with early bone formation in the developing mandible. Histochem. J. 2002, 34, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Aubin, J.E.; Liu, F.; Malaval, L.; Gupta, A.K. Osteoblast and chondroblast differentiation. Bone 1995, 17, 77S–83S. [Google Scholar] [CrossRef]
- Ohyama, M.; Suzuki, N.; Yamaguchi, Y.; Maeno, M.; Otsuka, K.; Ito, K. Effect of enamel matrix derivative on the differentiation of C2C12 cells. J. Periodontol. 2002, 73, 543–550. [Google Scholar] [CrossRef]
- Wiesmann, H.P.; Meyer, U.; Plate, U.; Höhling, H.J. Aspects of collagen mineralization in hard tissue formation. Int. Rev. Cytol. 2005, 242, 121–156. [Google Scholar]
- Karanxha, L.; Park, S.J.; Son, W.J.; Nör, J.E.; Min, K.S. Combined effects of simvastatin and enamel matrix derivative on odontoblastic differentiation of human dental pulp cells. J. Endod. 2013, 39, 76–82. [Google Scholar] [CrossRef]
- Tanimoto, K.; Huang, Y.C.; Tanne, Y.; Kunimatsu, R.; Michida, M.; Yoshioka, M.; Ozaki, N.; Sasamoto, T.; Yoshimi, Y.; Kato, Y.; et al. Amelogenin Enhances the Osteogenic Differentiation of Mesenchymal Stem Cells Derived from Bone Marrow. Cells Tissues Organs. 2012, 196, 411–419. [Google Scholar] [CrossRef]
- Wang, W.; Yi, X.; Ren, Y.; Xie, Q. Effects of adenosine triphosphate on proliferation and odontoblastic differentiation of human dental pulp cells. J. Endod. 2016, 42, 1483–1489. [Google Scholar] [CrossRef]
- Junttila, M.R.; Li, S.P.; Westermarck, J. Phosphatase-mediated crosstalk between MAPK signaling pathways in the regulation of cell survival. FASEB J. 2008, 22, 954–965. [Google Scholar] [CrossRef] [PubMed]
- Pullikuth, A.K.; Catling, A.D. Scaffold mediated regulation of MAPK signaling and cytoskeletal dynamics: A perspective. Cell Signal. 2007, 19, 1621–1632. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, N.; Horikawa, M.; Watanabe, M.; Kitagawa, S.; Kudo, Y.; Takata, T. Possible involvement of extracellular signal-regulated kinases 1/2 in mitogenic response of periodontal ligament cells to enamel matrix derivative. Eur. J. Oral Sci. 2002, 110, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Taguchi, Y.; Imai, K.; Ruan, Y.; Tsai, Y.W.; Chen, Y.C.; Shida, M.; Taguchi, R.; Tominaga, K.; Umeda, M. The Enhancing Effects of Amelogenin Exon 5-Encoded Peptide from Enamel Matrix Derivative on Odontoblast-Like KN-3 Cells. Appl. Sci. 2018, 8, 1890. [Google Scholar] [CrossRef]
- Ando, K.; Kunimatsu, R.; Awada, T.; Yoshimi, Y.; Tsuka, Y.; Sumi, K.; Horie, K.; Abe, T.; Nakajima, K.; Tanimoto, K. Effects of Human Full-length Amelogenin and C-terminal Amelogenin Peptide on the Proliferation of Human Mesenchymal Stem Cells Derived from Adipose Tissue. Curr. Pharm. Des. 2018, 24, 2993–3001. [Google Scholar] [CrossRef]
- Hakki, S.S.; Bozkurt, S.B.; Türkay, E.; Dard, M.; Purali, N.; Götz, W. Recombinant amelogenin regulates the bioactivity of mouse cementoblasts in vitro. Int. J. Oral Sci. 2018, 10, 15. [Google Scholar] [CrossRef]
- Huang, Y.C.; Tanimoto, K.; Tanne, Y.; Kamiya, T.; Kunimatsu, R.; Michida, M.; Yoshioka, M.; Yoshimi, Y.; Kato, Y.; Tanne, K. Effects of human full-length amelogenin on the proliferation of human mesenchymal stem cells derived from bone marrow. Cell Tissue Res. 2010, 342, 205–212. [Google Scholar] [CrossRef]
- Kunimatsu, R.; Tanimoto, K.; Tanne, Y.; Kamiya, T.; Ohkuma, S.; Huang, Y.C.; Yoshimi, Y.; Miyauchi, M.; Takata, T.; Tanne, K. Amelogenin enhances the proliferation of cementoblast lineage cells. J. Periodontol. 2011, 82, 1632–1638. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).