Toward Development of a Vocal Fold Contact Pressure Probe: Bench-Top Validation of a Dual-Sensor Probe Using Excised Human Larynx Models
Abstract
Featured Application
Abstract
1. Introduction
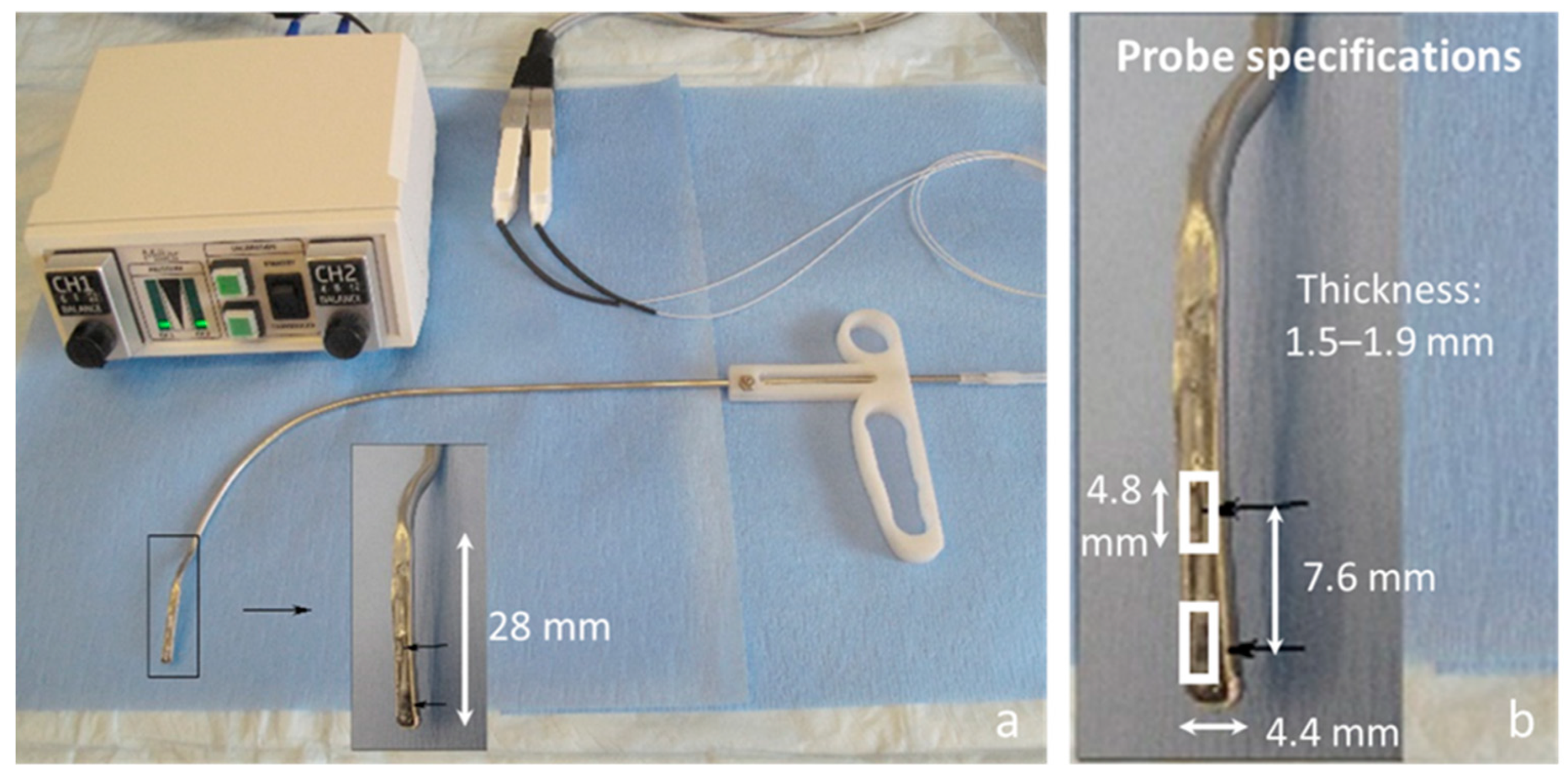
2. In Vivo Intraglottal/Subglottal Pressure (ISP) Probe
3. Proof-of-Concept Using an Excised Whole-Mount Human Larynx Model
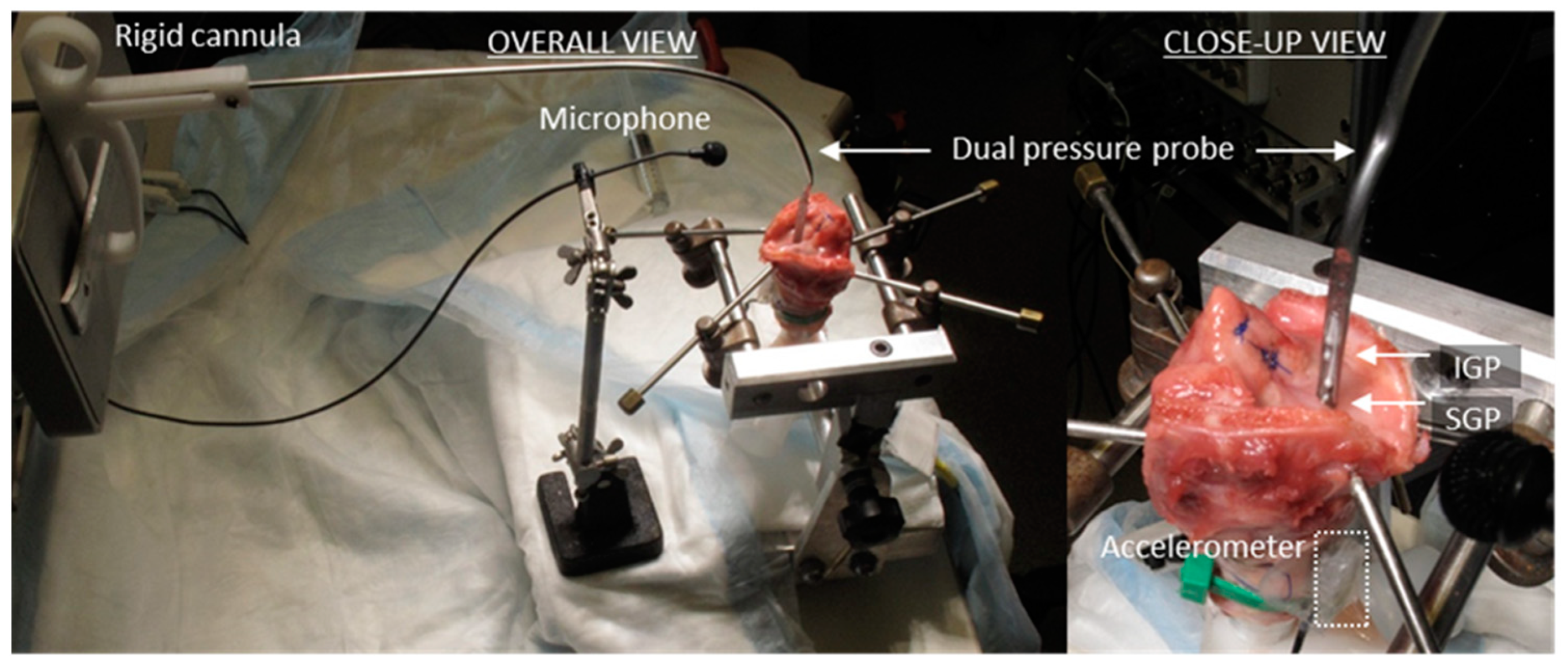
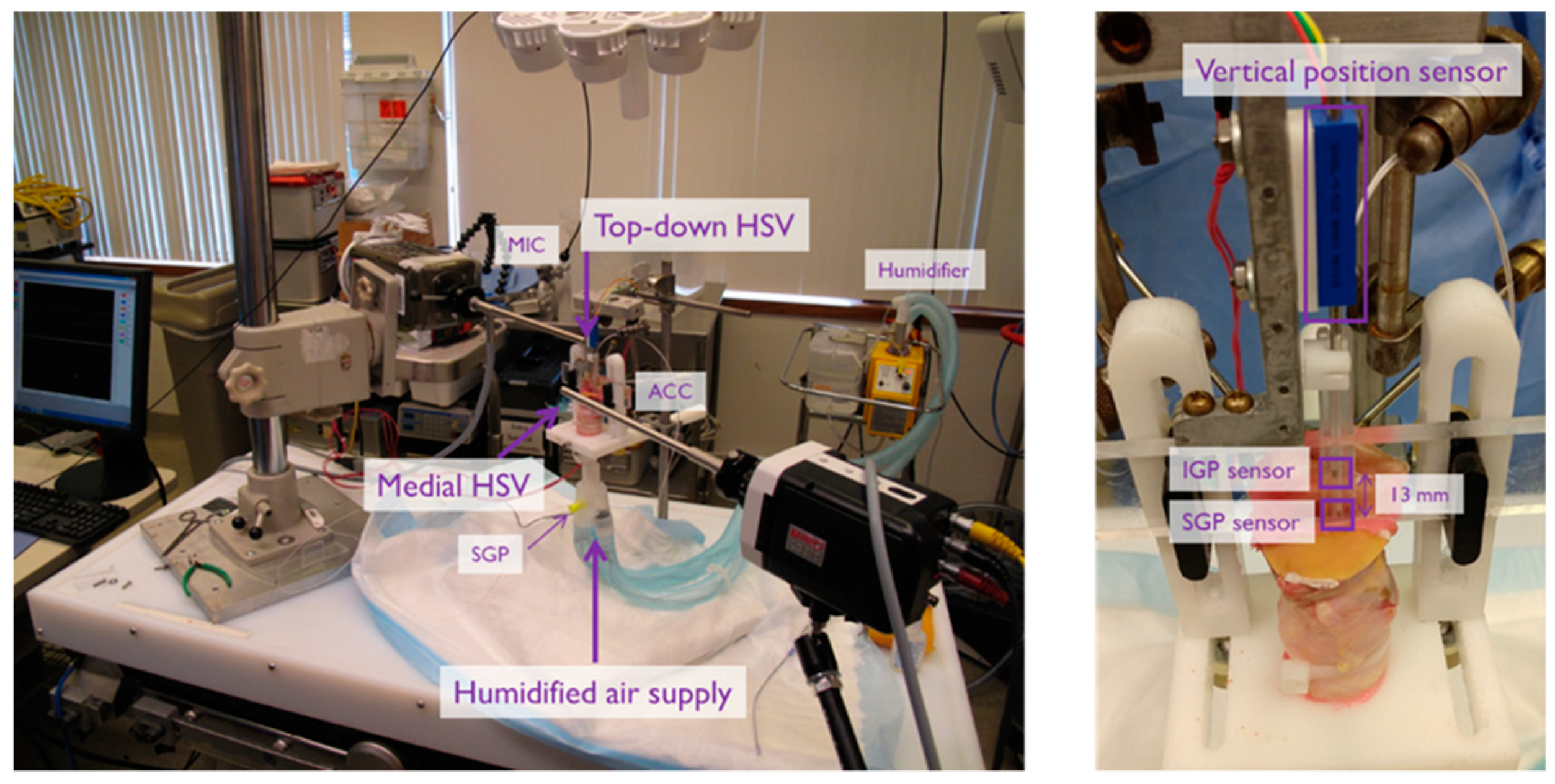
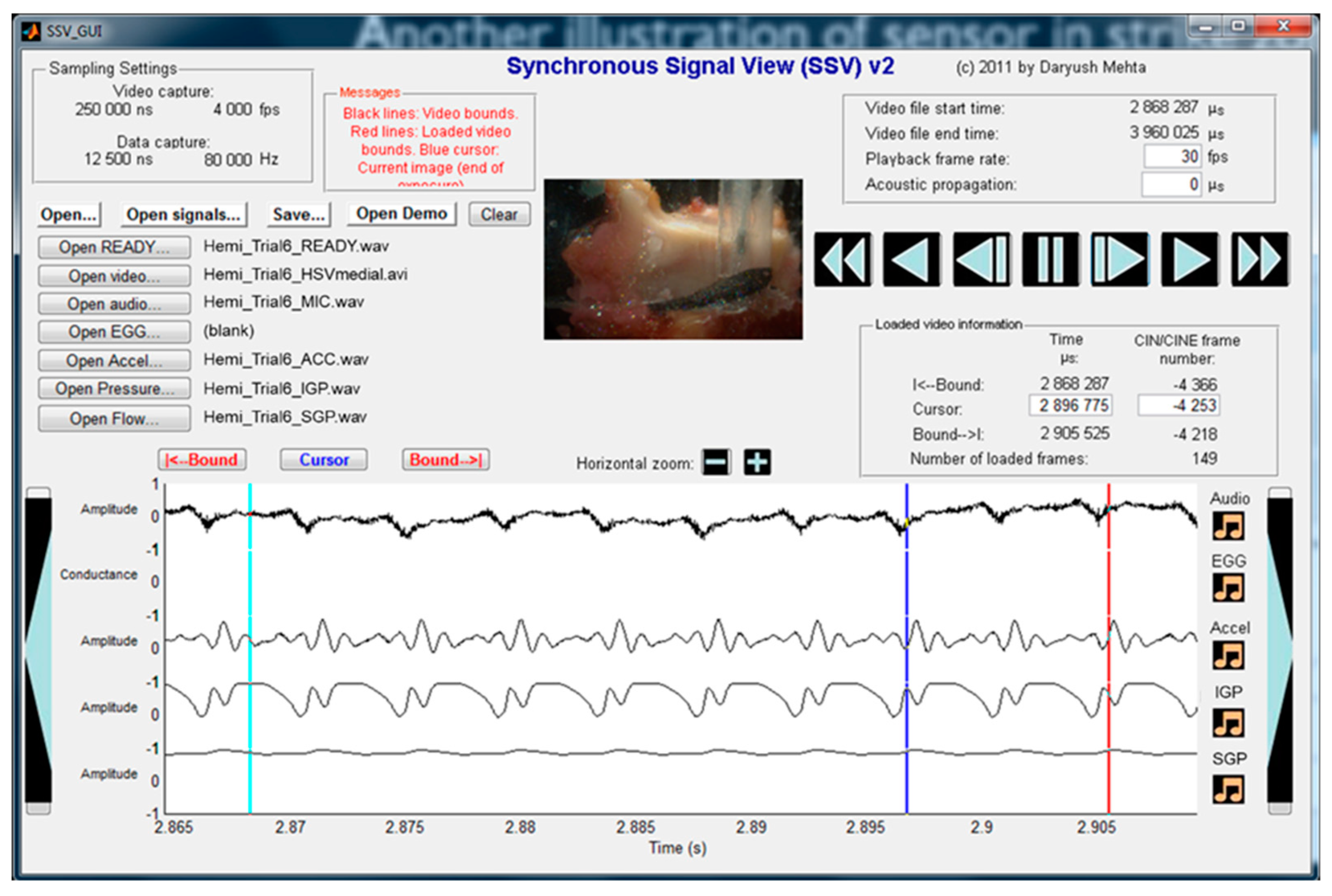
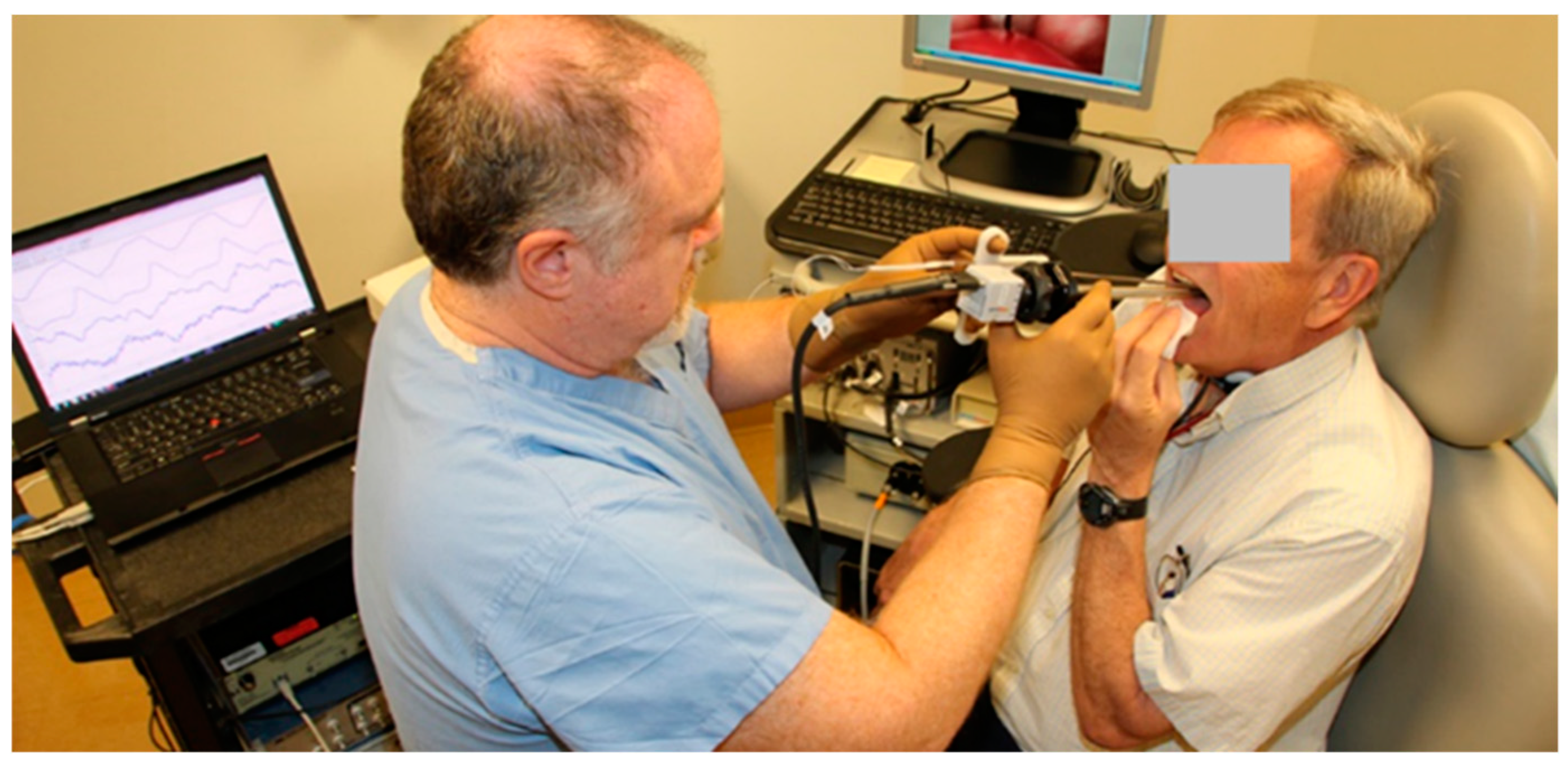
3.1. Materials and Methods
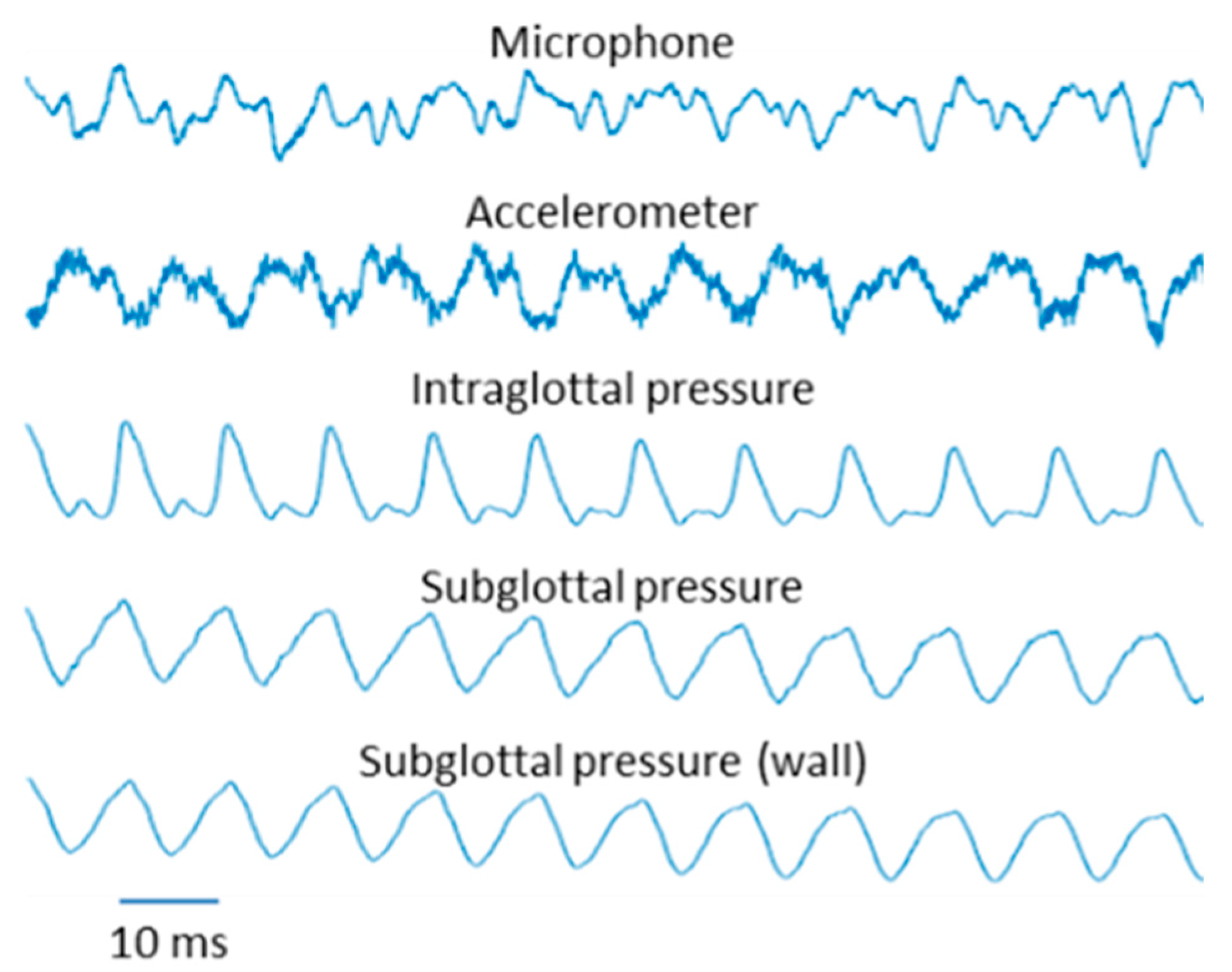
3.2. Results
4. Importance of a Flat Pressure Sensing Surface
5. Excised Human Hemilarynx Experiment
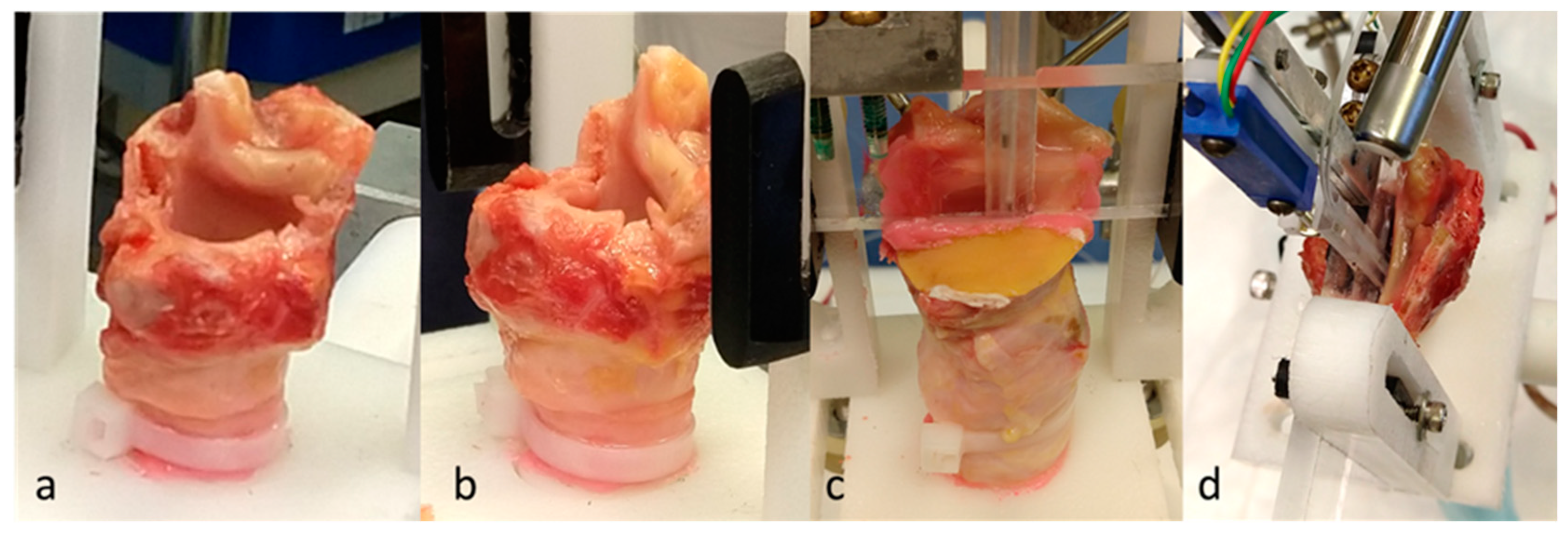
5.1. Materials and Methods
5.2. Results
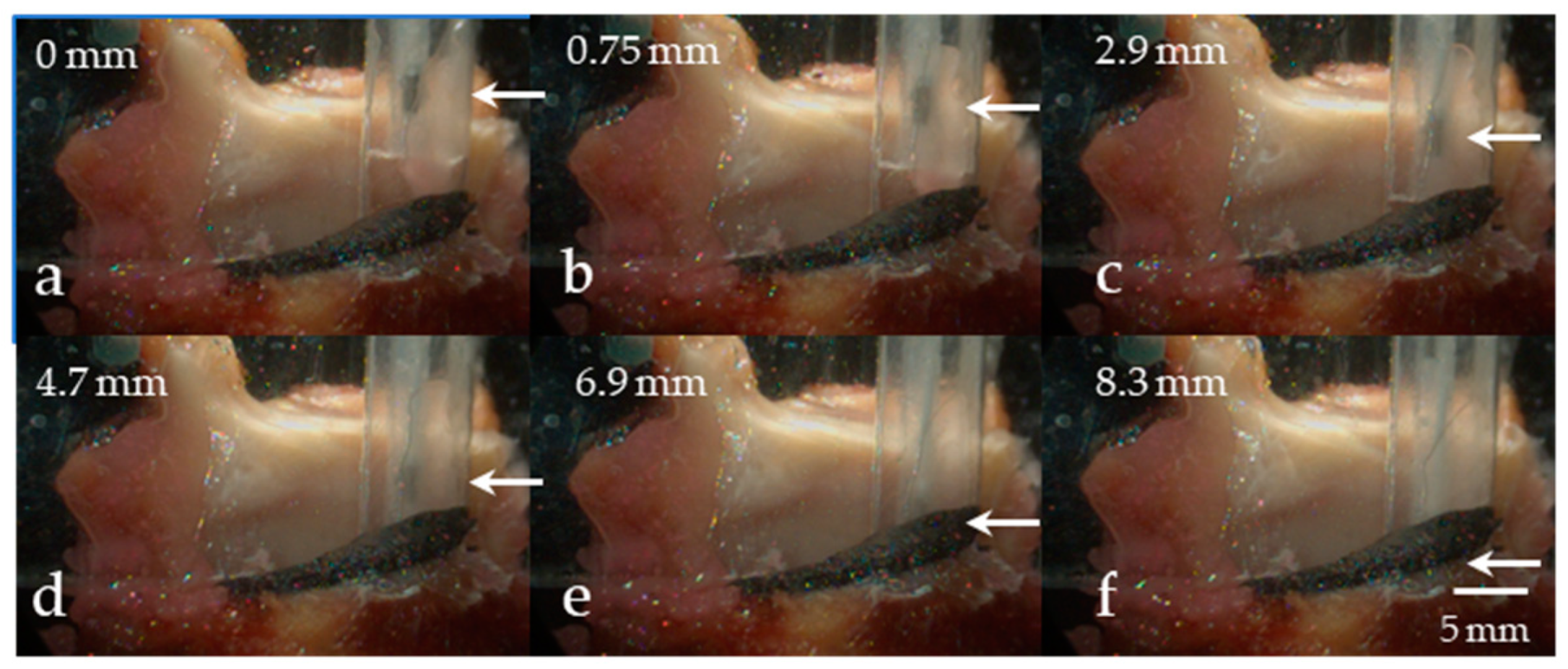
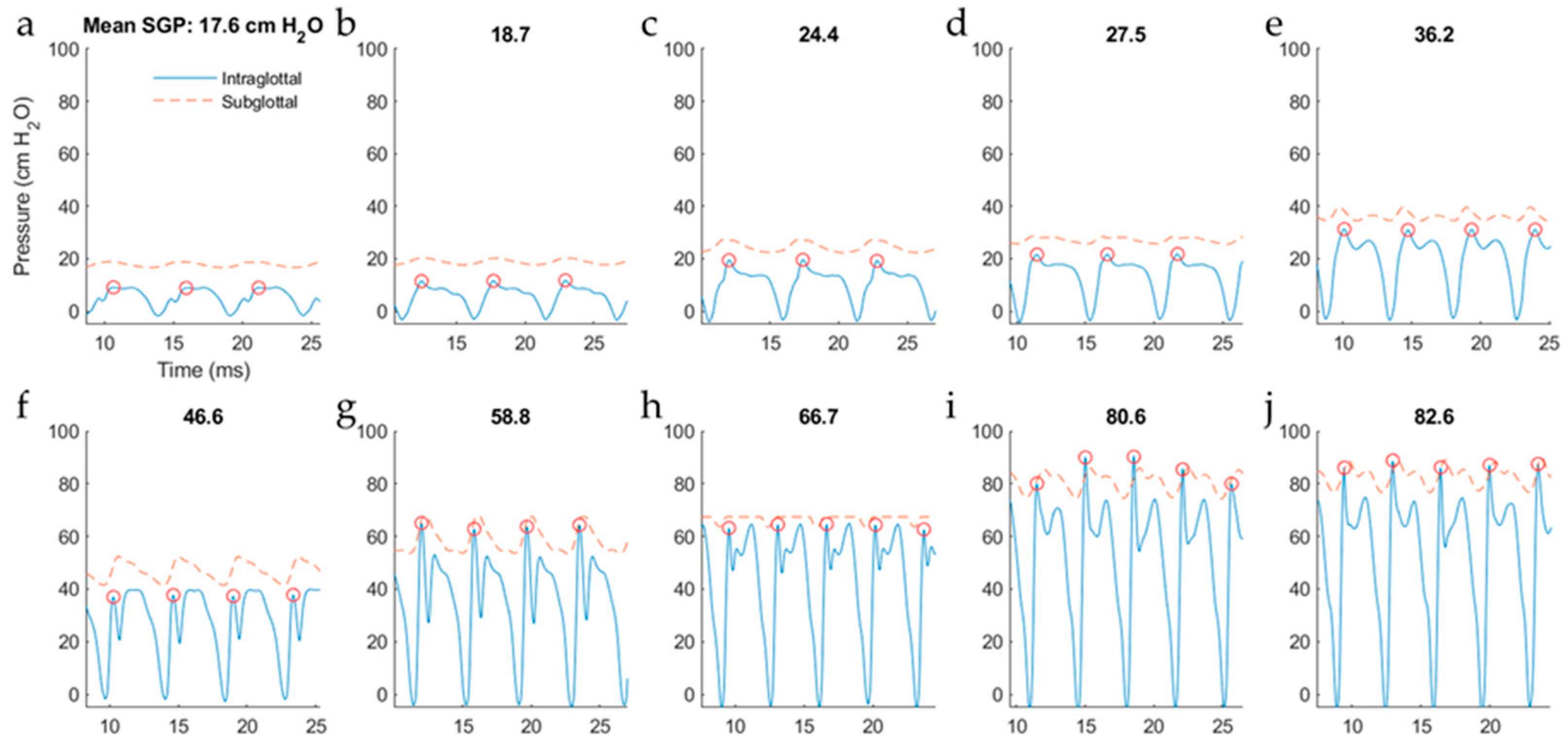
5.2.1. Variation in Superior–Inferior Position of the Intraglottal Pressure Sensor
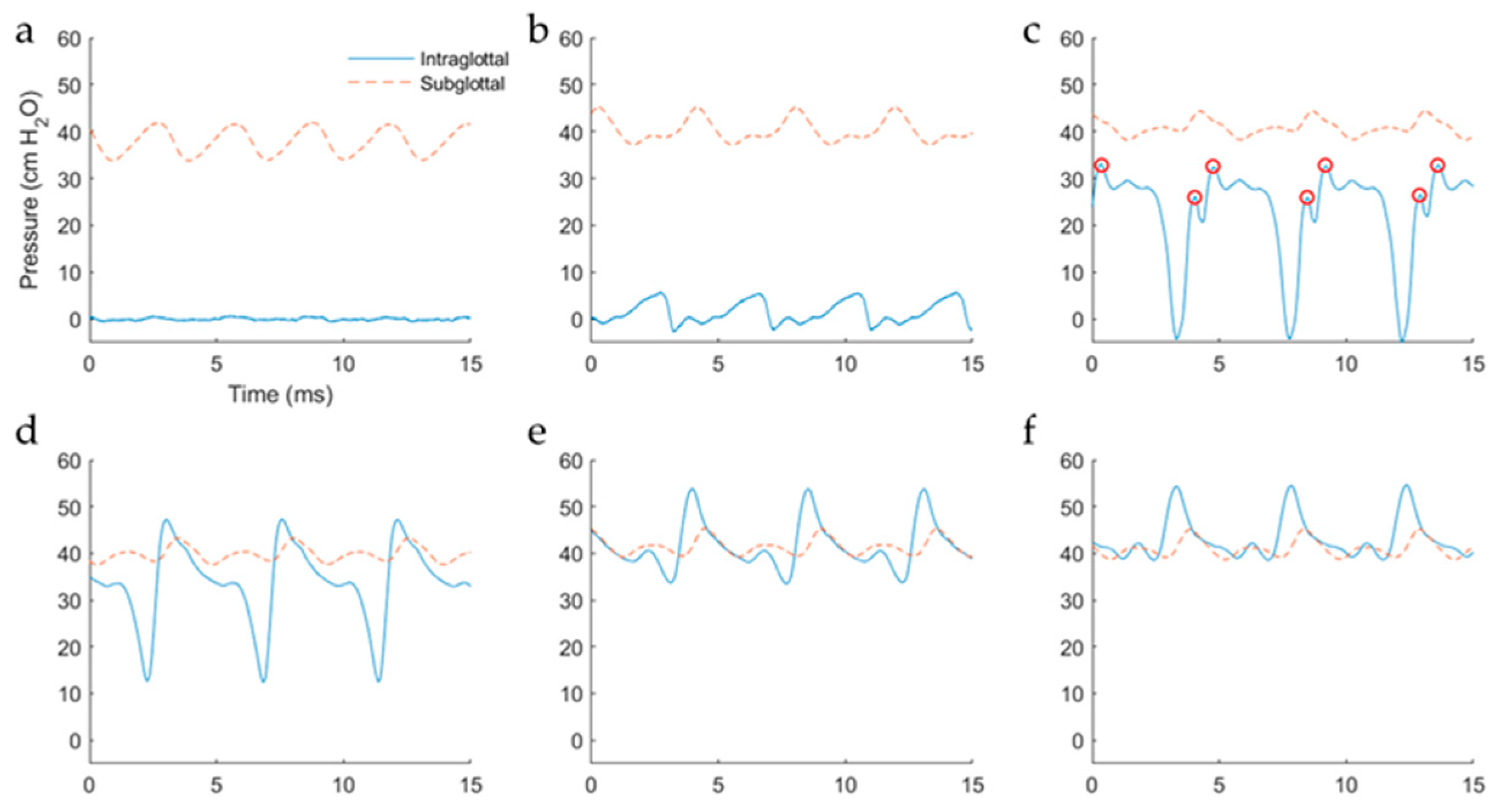
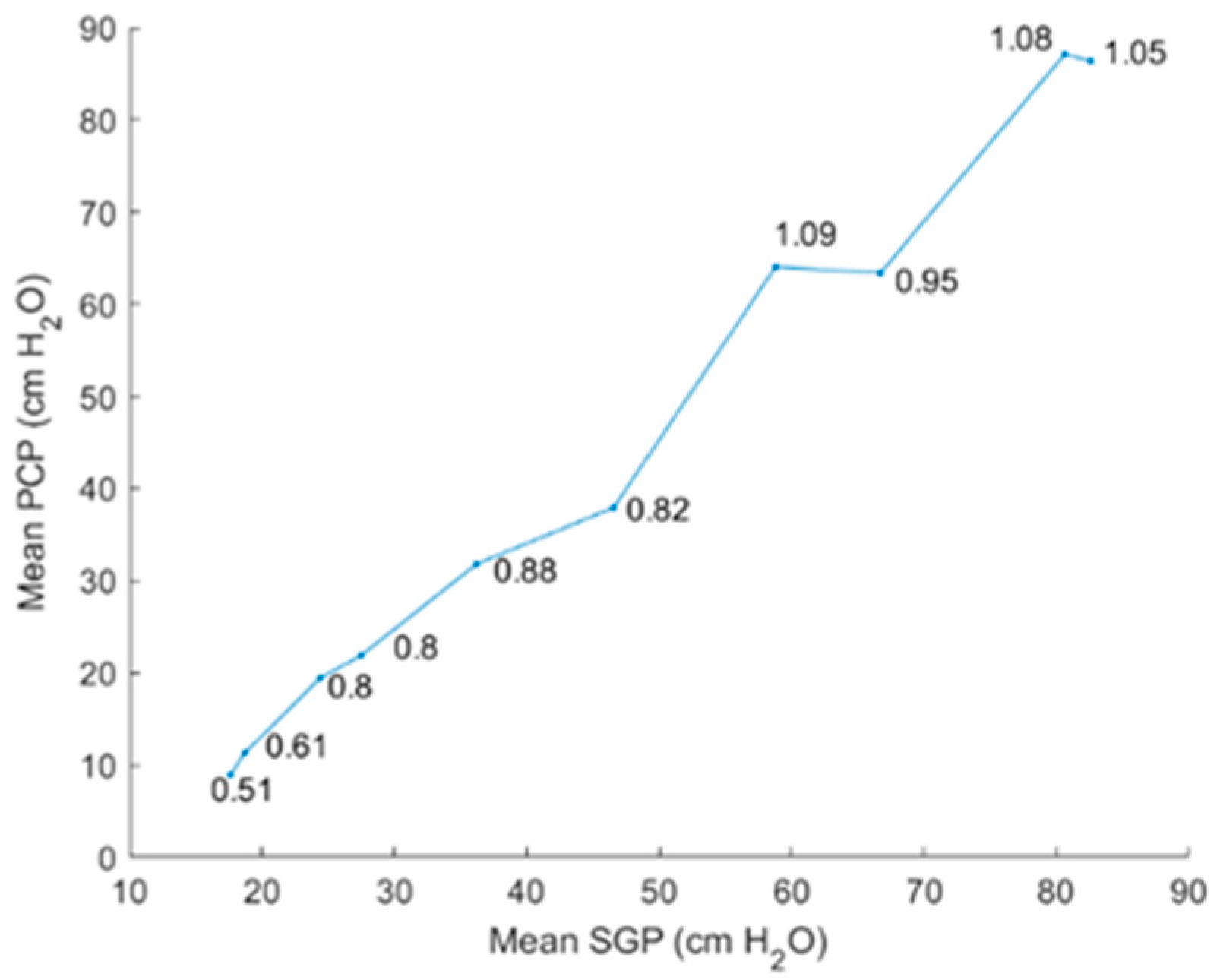
5.2.2. Intraglottal Pressure Waveform Characteristics for Varying Subglottal Pressure
6. Discussion
6.1. Comparison of Results to Prior Work
6.2. ISP Probe Considerations in Practice
- Endoscopic visualization of the larynx during phonation via videostroboscopy or high-speed videoendoscopy for general placement of the ISP probe such that the proximal sensor is positioned intraglottally and the distal sensor subglottally.
- In patients with a unilateral cordectomy, placement of the ISP probe on the non-vibrating flap of tissue to maintain a flat surface on which the contralateral, functioning vocal fold can come into contact.
- Real-time monitoring of both pressure sensor waveforms to verify that the two waveforms are distinct from each other. In particular, the proximal pressure sensor would be in the strike zone during phonation when the intraglottal pressure waveform exhibits:
- An impulsive peak in the direction of increasing pressure at the start of the closed phase;
- A rounded peak following the impulsive peak during the open phase;
- A minimum value reaching a zero or negative value during the open phase.
6.3. Implications for Vocal Dose Measures
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Titze, I.R.; Švec, J.G.; Popolo, P.S. Vocal dose measures: Quantifying accumulated vibration exposure in vocal fold tissues. J. Speech Lang. Hear. Res. 2003, 46, 919–932. [Google Scholar] [CrossRef]
- Czerwonka, L.; Jiang, J.J.; Tao, C. Vocal Nodules and Edema May Be Due to Vibration-Induced Rises in Capillary Pressure. Laryngoscope 2008, 118, 748–752. [Google Scholar] [CrossRef] [PubMed]
- Karkos, P.D.; McCormick, M. The etiology of vocal fold nodules in adults. Curr. Opin. Otolaryngol. Head Neck Surg. 2009, 17, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Tao, C.; Jiang, J.J.; Czerwonka, L. Liquid accumulation in vibrating vocal fold tissue: A simplified model based on a fluid-saturated porous solid theory. J. Voice 2010, 24, 260–269. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gunter, H.E. A mechanical model of vocal-fold collision with high spatial and temporal resolution. J. Acoust. Soc. Am. 2003, 113, 994–1000. [Google Scholar] [CrossRef]
- Kvit, A.A.; Devine, E.E.; Jiang, J.J.; Vamos, A.C.; Tao, C. Characterizing liquid redistribution in a biphasic vibrating vocal fold using finite element analysis. J. Voice 2015, 29, 265–272. [Google Scholar] [CrossRef][Green Version]
- Verdolini, K.; Hess, M.M.; Titze, I.R.; Bierhals, W.; Gross, M. Investigation of vocal fold impact stress in human subjects. J. Voice 1999, 13, 184–202. [Google Scholar] [CrossRef]
- Hess, M.M.; Verdolini, K.; Bierhals, W.; Mansmann, U.; Gross, M. Endolaryngeal contact pressures. J. Voice 1998, 12, 50–67. [Google Scholar] [CrossRef]
- Gunter, H.E.; Howe, R.D.; Zeitels, S.M.; Kobler, J.B.; Hillman, R.E. Measurement of vocal fold collision forces during phonation: Methods and preliminary data. J. Speech Lang. Hear. Res. 2005, 48, 567–576. [Google Scholar] [CrossRef]
- Motie-Shirazi, M.; Zañartu, M.; Peterson, S.D.; Mehta, D.D.; Kobler, J.B.; Hillman, R.E.; Erath, B.D. Toward Development of a Vocal Fold Contact Pressure Probe: Sensor Characterization and Validation Using Synthetic Vocal Fold Models. Appl. Sci. 2019, 9, 3002. [Google Scholar] [CrossRef]
- Jiang, J.J.; Titze, I.R. A Methodological Study of Hemilaryngeal Phonation. Laryngoscope 1993, 103, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.J.; Titze, I.R. Measurement of vocal fold intraglottal pressure and impact stress. J. Voice 1994, 8, 132–144. [Google Scholar] [CrossRef]
- Zhang, Z. Vocal fold contact pressure in a three-dimensional body-cover phonation model. J. Acoust. Soc. Am. 2019, 146, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Erath, B.D.; Zañartu, M.; Peterson, S.D. Modeling viscous dissipation during vocal fold contact: The influence of tissue viscosity and thickness with implications for hydration. Biomech. Model. Mechanobiol. 2017, 16, 947–960. [Google Scholar] [CrossRef] [PubMed]
- Galindo, G.E.; Peterson, S.D.; Erath, B.D.; Castro, C.; Hillman, R.E.; Zañartu, M. Modeling the Pathophysiology of Phonotraumatic Vocal Hyperfunction With a Triangular Glottal Model of the Vocal Folds. J. Speech Lang. Hear. Res. 2017, 60, 2452–2471. [Google Scholar] [CrossRef]
- Hadwin, P.J.; Motie-Shirazi, M.; Erath, B.D.; Peterson, S.D. Bayesian Inference of Vocal Fold Material Properties from Glottal Area Waveforms Using a 2D Finite Element Model. Appl. Sci. 2019, 9, 2735. [Google Scholar] [CrossRef]
- Díaz-Cádiz, M.E.; Peterson, S.D.; Galindo, G.E.; Espinoza, V.M.; Motie-Shirazi, M.; Erath, B.D.; Zañartu, M. Estimating Vocal Fold Contact Pressure from Raw Laryngeal High-Speed Videoendoscopy Using a Hertz Contact Model. Appl. Sci. 2019, 9, 2384. [Google Scholar] [CrossRef]
- Holmberg, E.B.; Hillman, R.E.; Perkell, J.S. Glottal airflow and transglottal air pressure measurements for male and female speakers in soft, normal, and loud voice. J. Acoust. Soc. Am. 1988, 84, 511–529. [Google Scholar] [CrossRef]
- Ford, C.N. A Multipurpose Laryngeal Injector Device. Otolaryngol. Neck Surg. 1990, 103, 135–137. [Google Scholar] [CrossRef]
- Chan, R.W.; Titze, I.R. Effect of postmortem changes and freezing on the viscoelastic properties of vocal fold tissues. Ann. Biomed. Eng. 2003, 31, 482–491. [Google Scholar] [CrossRef]
- Lulich, S.M.; Alwan, A.; Arsikere, H.; Morton, J.R.; Sommers, M.S. Resonances and wave propagation velocity in the subglottal airways. J. Acoust. Soc. Am. 2011, 130, 2108–2115. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.A.; Montequin, D.W.; Tayama, N. High-speed digital imaging of the medial surface of the vocal folds. J. Acoust. Soc. Am. 2001, 110, 2539–2547. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.A.; Montequin, D.W.; Clark, M.J.O.; Titze, I.R. Characterization of the medial surface of the vocal folds. Ann. Otol. Rhinol. Laryngol. 2001, 110, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Doellinger, M.; Berry, D.A.; Berke, G.S. A Quantitative Study of the Medial Surface Dynamics of an In Vivo Canine Vocal Fold during Phonation. Laryngoscope 2005, 115, 1646–1654. [Google Scholar] [CrossRef] [PubMed]
- Döllinger, M.; Tayama, N.; Berry, D.A. Empirical Eigenfunctions and medial surface dynamics of a human vocal fold. Methods Inf. Med. 2005, 44, 384–391. [Google Scholar]
- Döllinger, M.; Berry, D.A.; Berke, G.S. Medial surface dynamics of an in vivo canine vocal fold during phonation. J. Acoust. Soc. Am. 2005, 117, 3174–3383. [Google Scholar] [CrossRef] [PubMed]
- Döllinger, M.; Berry, D.A. Computation of the three-dimensional medial surface dynamics of the vocal folds. J. Biomech. 2006, 39, 369–374. [Google Scholar] [CrossRef]
- Doellinger, M.; Berry, D.A. Visualization and Quantification of the Medial Surface Dynamics of an Excised Human Vocal Fold During Phonation. J. Voice 2006, 20, 401–413. [Google Scholar] [CrossRef]
- Döllinger, M.; Kobler, J.; Berry, D.A.; Mehta, D.D.; Luegmair, G.; Bohr, C. Experiments on Analysing Voice Production: Excised (Human, Animal) and In Vivo (Animal) Approaches. Curr. Bioinform. 2011, 6, 286–304. [Google Scholar] [CrossRef][Green Version]
- Mehta, D.D.; Deliyski, D.D.; Zeitels, S.M.; Zañartu, M.; Hillman, R.E. Integration of transnasal fiberoptic high-speed videoendoscopy with time-synchronized recordings of vocal function. In Normal & Abnormal Vocal Folds Kinematics: HSDP, OCT & NBI®, Volume I: Technology; Izdebski, K., Yan, Y., Ward, R.R., Wong, B.J.F., Cruz, R.M., Eds.; Pacific Voice & Speech Foundation: San Francisco, CA, USA, 2015; Volume 1, pp. 105–114. [Google Scholar]
- Titze, I.R. Vocal efficiency. J. Voice 1992, 6, 135–138. [Google Scholar] [CrossRef]
- Thomson, S.L.; Mongeau, L.; Frankel, S.H. Aerodynamic transfer of energy to the vocal folds. J. Acoust. Soc. Am. 2005, 118, 1689–1700. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.J.; Shah, A.G.; Hess, M.M.; Verdolini, K.; Banzali, F.M., Jr.; Hanson, D.G. Vocal fold impact stress analysis. J. Voice 2001, 15, 4–14. [Google Scholar] [CrossRef]
- Alipour, F.; Montequin, D.; Tayama, N. Aerodynamic profiles of a hemilarynx with a vocal tract. Ann. Otol. Rhinol. Laryngol. 2001, 110, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Döllinger, M.; Berry, D.A.; Kniesburges, S. Dynamic vocal fold parameters with changing adduction in ex-vivo hemilarynx experiments. J. Acoust. Soc. Am. 2016, 139, 2372–2385. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-J.; Mongeau, L. Verification of two minimally invasive methods for the estimation of the contact pressure in human vocal folds during phonation. J. Acoust. Soc. Am. 2011, 130, 1618–1627. [Google Scholar] [CrossRef] [PubMed]
- Alipour, F.; Scherer, R.C. Dynamic glottal pressures in an excised hemilarynx model. J. Voice 2000, 14, 443–454. [Google Scholar] [CrossRef]
- Verdolini, K.; Chan, R.; Titze, I.R.; Hess, M.; Bierhals, W. Correspondence of electroglottographic closed quotient to vocal fold impact stress in excised canine larynges. J. Voice 1998, 12, 415–423. [Google Scholar] [CrossRef]
- Spencer, M.; Siegmund, T.; Mongeau, L. Determination of superior surface strains and stresses, and vocal fold contact pressure in a synthetic larynx model using digital image correlation. J. Acoust. Soc. Am. 2008, 123, 1089–1103. [Google Scholar] [CrossRef]
- Tao, C.; Jiang, J.J.; Zhang, Y. Simulation of vocal fold impact pressures with a self-oscillating finite-element model. J. Acoust. Soc. Am. 2006, 119, 3987–3994. [Google Scholar] [CrossRef]
- Zeitels, S.M.; Jarboe, J.; Franco, R.A. Phonosurgical Reconstruction of Early Glottic Cancer. Laryngoscope 2001, 111, 1862–1865. [Google Scholar] [CrossRef]
- Zeitels, S.M.; Hillman, R.E.; Franco, R.A.; Bunting, G.W. Voice and treatment outcome from phonosurgical management of early glottic cancer. Ann. Otol. Rhinol. Laryngol. 2002, 111 (Suppl. S190), 1–20. [Google Scholar] [CrossRef]
- Zeitels, S.M. Optimizing voice after endoscopic partial laryngectomy. Otolaryngol. Clin. N. Am. 2004, 37, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Titze, I.R.; Hunter, E.J. Comparison of Vocal Vibration-Dose Measures for Potential-Damage Risk Criteria. J. Speech Lang. Hear. Res. 2015, 58, 1425–1439. [Google Scholar] [CrossRef] [PubMed]
- Mehta, D.D.; Zañartu, M.; Feng, S.W.; Cheyne, H.A.; Hillman, R.E. Mobile Voice Health Monitoring Using a Wearable Accelerometer Sensor and a Smartphone Platform. IEEE Trans. Biomed. Eng. 2012, 59, 3090–3096. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehta, D.D.; Kobler, J.B.; Zeitels, S.M.; Zañartu, M.; Erath, B.D.; Motie-Shirazi, M.; Peterson, S.D.; Petrillo, R.H.; Hillman, R.E. Toward Development of a Vocal Fold Contact Pressure Probe: Bench-Top Validation of a Dual-Sensor Probe Using Excised Human Larynx Models. Appl. Sci. 2019, 9, 4360. https://doi.org/10.3390/app9204360
Mehta DD, Kobler JB, Zeitels SM, Zañartu M, Erath BD, Motie-Shirazi M, Peterson SD, Petrillo RH, Hillman RE. Toward Development of a Vocal Fold Contact Pressure Probe: Bench-Top Validation of a Dual-Sensor Probe Using Excised Human Larynx Models. Applied Sciences. 2019; 9(20):4360. https://doi.org/10.3390/app9204360
Chicago/Turabian StyleMehta, Daryush D., James B. Kobler, Steven M. Zeitels, Matías Zañartu, Byron D. Erath, Mohsen Motie-Shirazi, Sean D. Peterson, Robert H. Petrillo, and Robert E. Hillman. 2019. "Toward Development of a Vocal Fold Contact Pressure Probe: Bench-Top Validation of a Dual-Sensor Probe Using Excised Human Larynx Models" Applied Sciences 9, no. 20: 4360. https://doi.org/10.3390/app9204360
APA StyleMehta, D. D., Kobler, J. B., Zeitels, S. M., Zañartu, M., Erath, B. D., Motie-Shirazi, M., Peterson, S. D., Petrillo, R. H., & Hillman, R. E. (2019). Toward Development of a Vocal Fold Contact Pressure Probe: Bench-Top Validation of a Dual-Sensor Probe Using Excised Human Larynx Models. Applied Sciences, 9(20), 4360. https://doi.org/10.3390/app9204360




