Biodegradation of Sulfamethoxazole in Milkfish (Chanos chanos) Pond Sediments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sediment Sampling and Sampling Site
2.3. Experimental Setting
2.4. Analysis of Sulfonamides
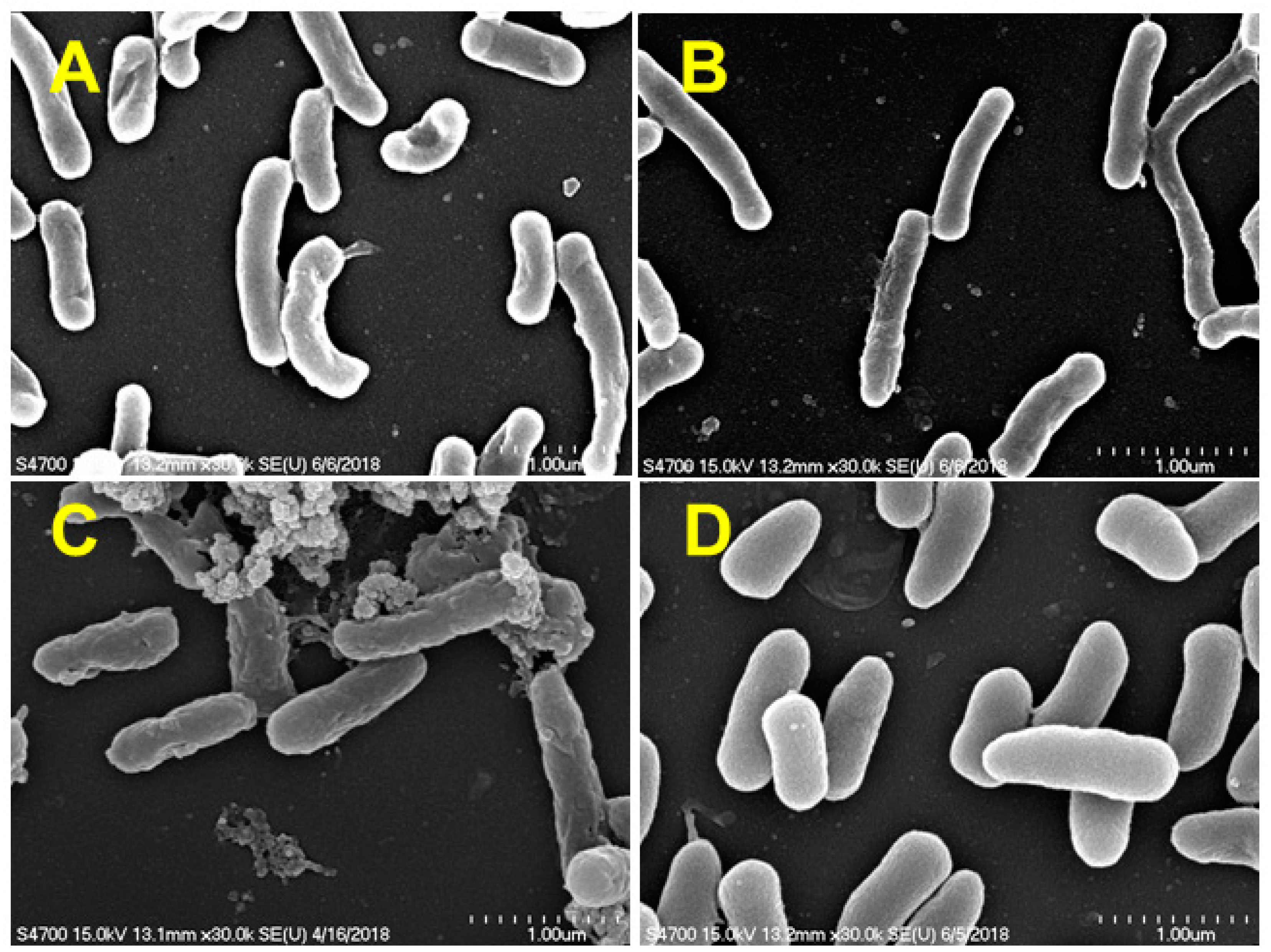
2.5. Scanning Electron Microscope
2.6. Next-Generation Sequencing and Data Analysis
3. Results and Discussion
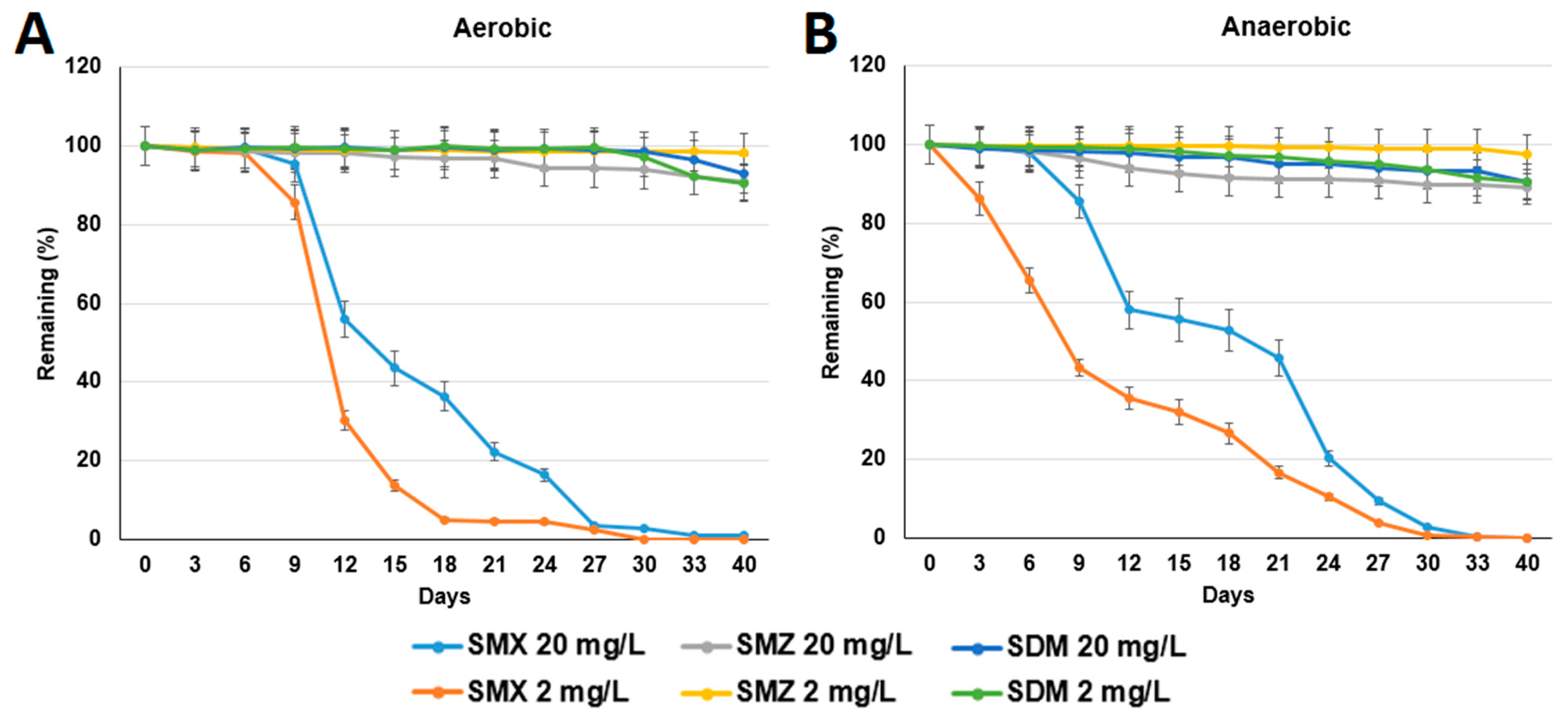
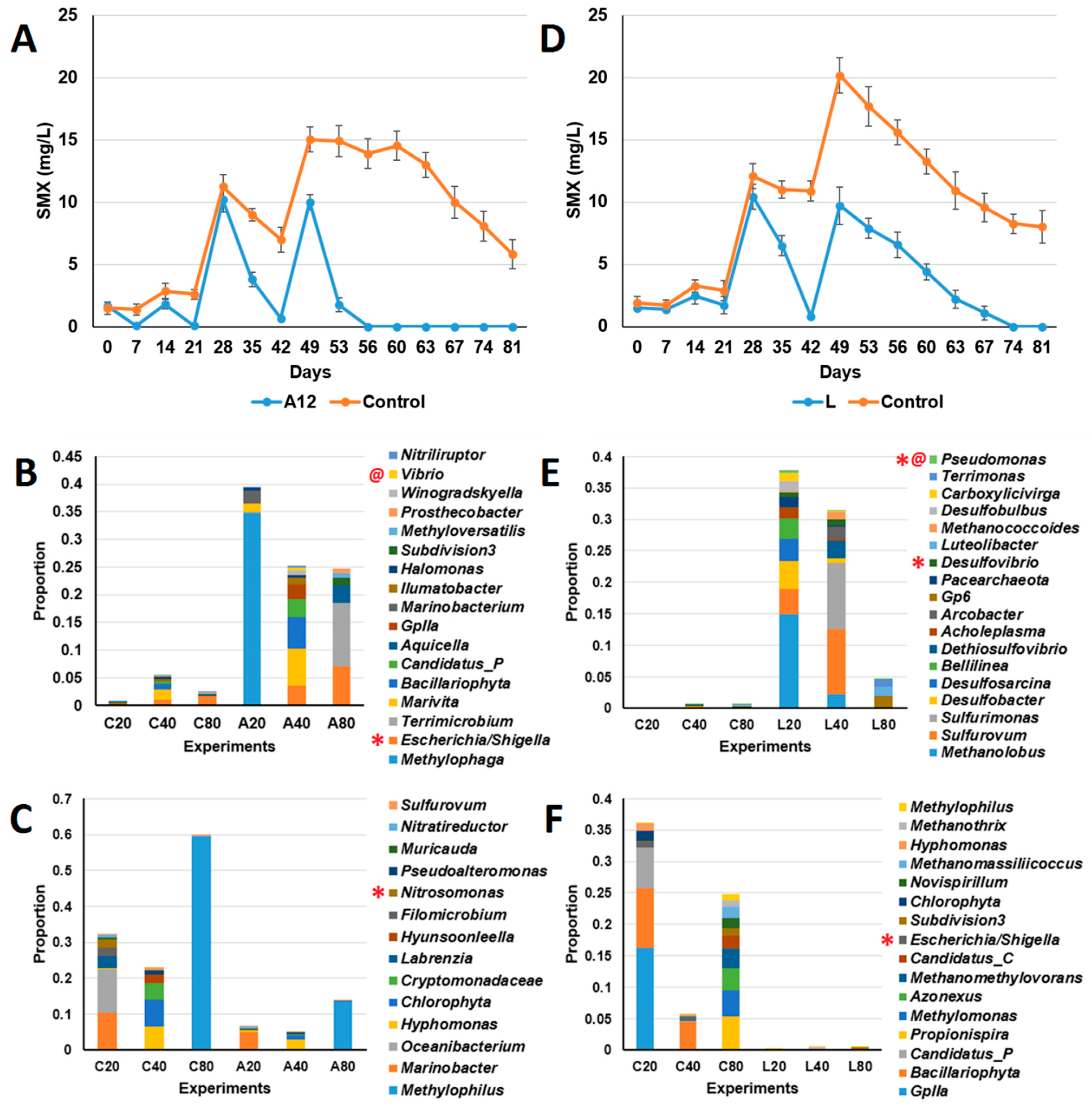
3.1. Test of Sulfonamide Degradation in Milkfish Pond Sediments
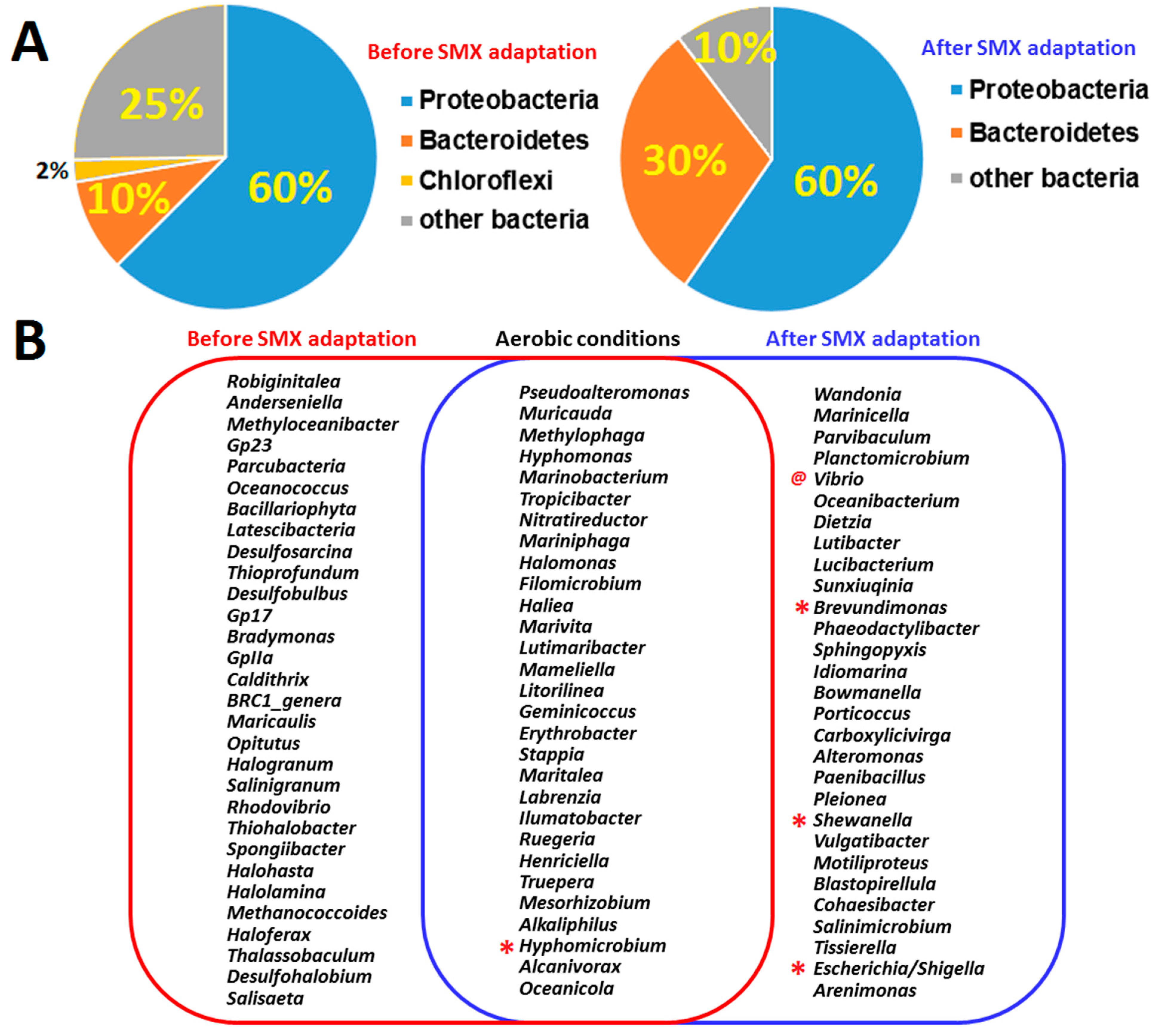
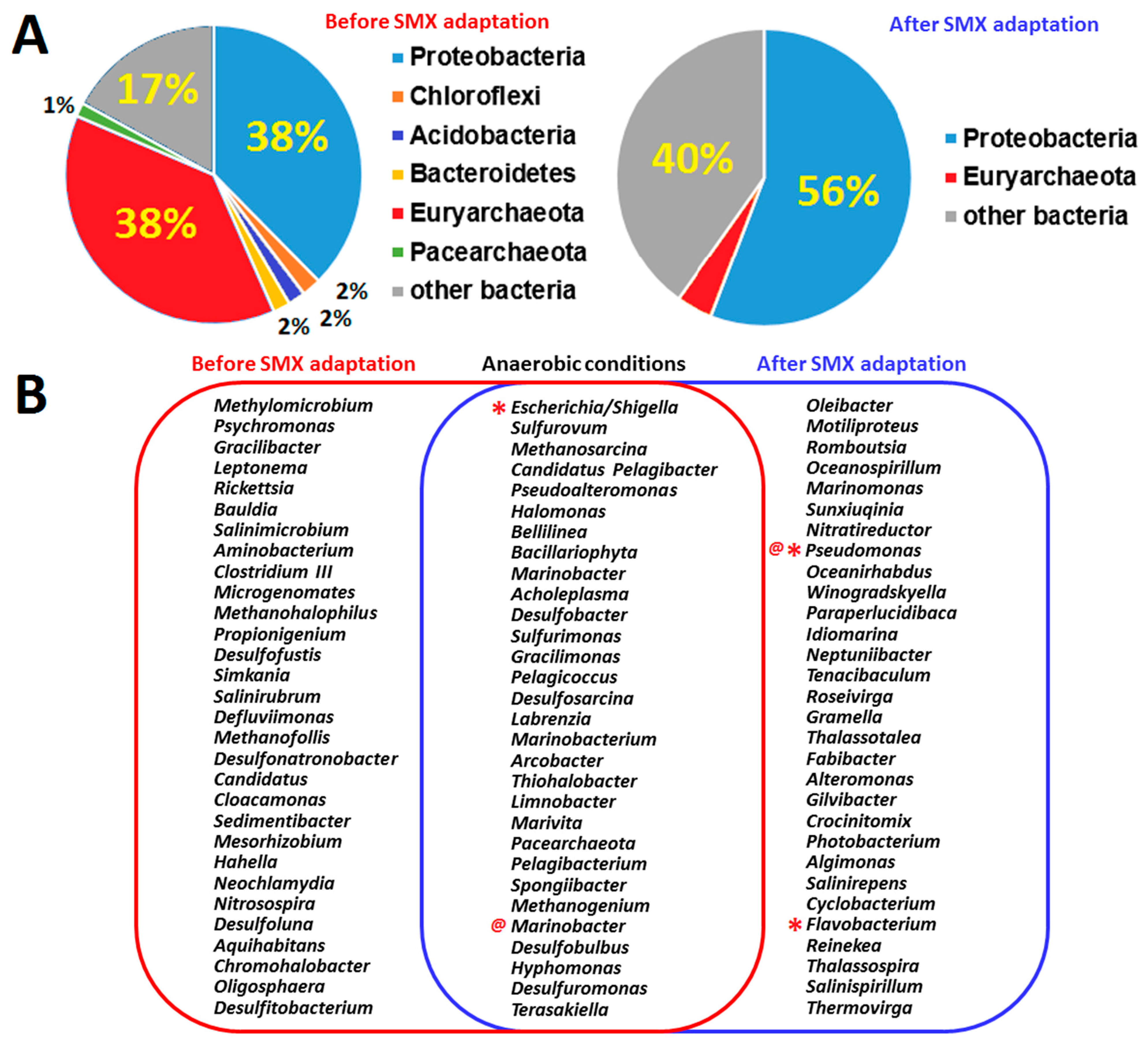
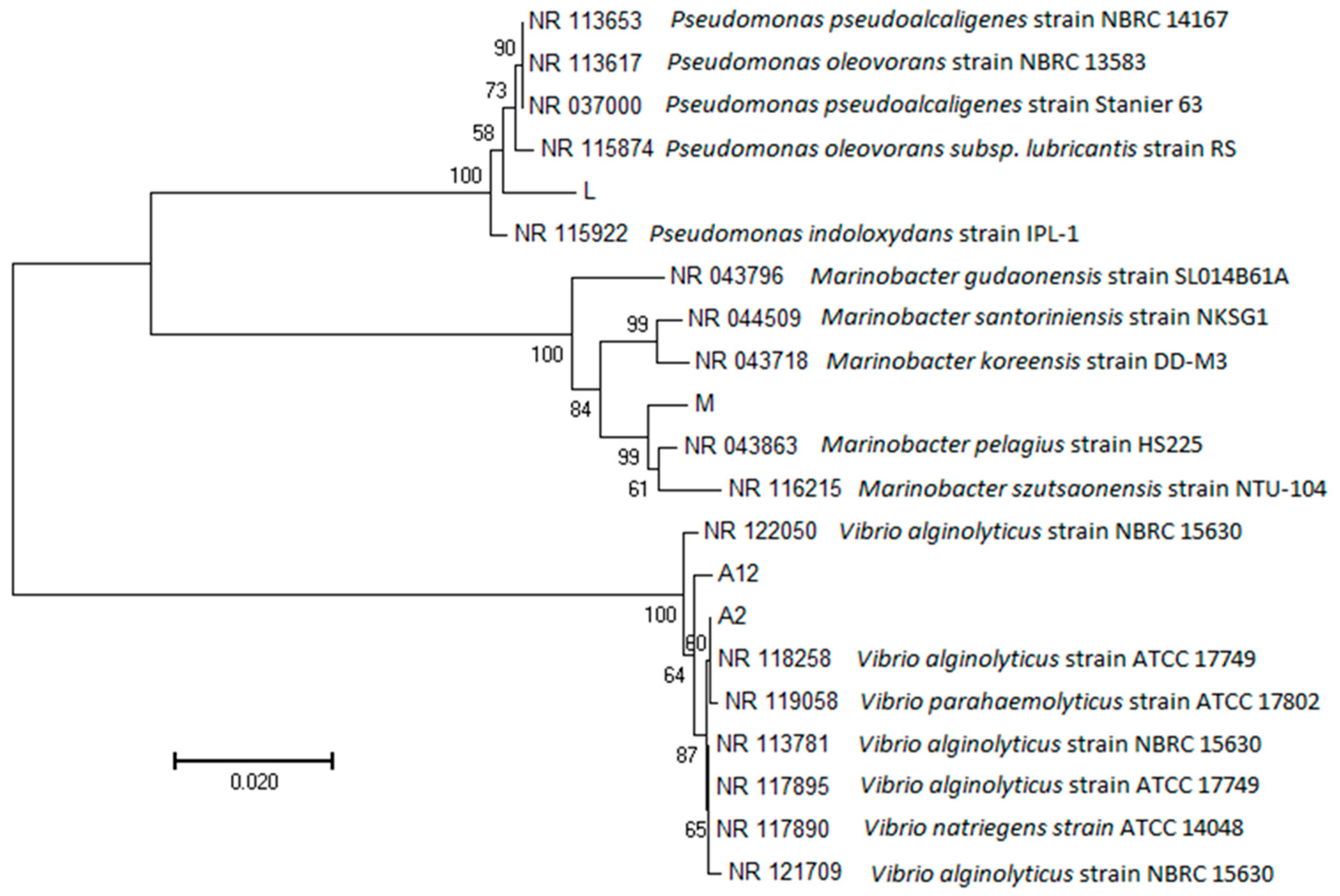
3.2. Isolation and Identification of Sulfamethoxazole-Degrading Bacteria
3.3. Test of Sulfamethoxazole-Degrading Ability of Isolated Bacteria
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Opiyo, M.A.; Marijani, E.; Muendo, P.; Odede, R.; Leschen, W.; Charo-Karisa, H. A review of aquaculture production and health management practices of farmed fish in Kenya. Int. J. Vet. Sci. Med. 2018, 6, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, A.; Takada, H.; Koike, T.; Takeshita, A.; Saha, M.; Rinawati; Nakada, N.; Murata, A.; Suzuki, T.; Suzuki, S.; et al. Ubiquitous occurrence of sulfonamides in tropical Asian waters. Sci. Total Environ. 2013, 452–453, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, N.; Wang, B.; Fang, H.; Fu, C.; Tang, C.; Jiang, F.; Zhou, Y.; He, G.; Zhao, Q.; et al. Antibiotics detected in urines and adipogenesis in school children. Environ. Int. 2016, 89, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Li, L.; Zhang, C.; Kamira, B.; Qiu, L.; Fan, L.; Wu, W.; Meng, S.; Hu, G.; Chen, J. Occurrence and human dietary assessment of sulfonamide antibiotics in cultured fish around Tai Lake, China. Environ. Sci. Pollut. Res. 2017, 24, 17493–17499. [Google Scholar] [CrossRef] [PubMed]
- Ezzariai, A.; Hafidi, M.; Khadra, A.; Aemig, Q.; El Fels, L.; Barret, M.; Merlina, G.; Patureau, D.; Pinelli, E. Human and veterinary antibiotics during composting of sludge or manure: Global perspectives on persistence, degradation, and resistance genes. J. Hazard. Mater. 2018, 359, 465–481. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, S. Microbial degradation of sulfamethoxazole in the environment. Appl. Microbiol. Biotechnol. 2018, 102, 3573–3582. [Google Scholar] [CrossRef] [PubMed]
- Rabanal, H.R. Development of the aquaculture industry in Southeast Asia. In Perspectives in Aquaculture Development in Southeast Asia and Japan; Juario, J.V., Benitez, L.V., Eds.; SEAFDEC Aquaculture Department: Tigbauan, Philippines, 1988; pp. 3–37. [Google Scholar]
- Martinez, F.S.; Tseng, M.C.; Yeh, S.P. Milkfish (Chanos chanos) culture: Situations and trends. J. Fish. Soc. Taiwan 2006, 33, 229–244. [Google Scholar]
- OECD/OCDE. Aerobic Mineralisation in Surface Water—Simulation Biodegradation Test. In Oecd Guideline for the Testing of Chemicals; INGENTA: Oxford, UK, 2004; p. 309. [Google Scholar]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.W.; Tsai, L.L.; Chang, B.V. Anaerobic degradation of sulfamethoxazole in mangrove sediments. Sci. Total Environ. 2018, 643, 1446–1455. [Google Scholar] [CrossRef] [PubMed]
- Zahed, M.A.; Aziz, H.A.; Isa, M.H.; Mohajeri, L.; Mohajeri, S.; Kutty, S.R. Kinetic modeling and half life study on bioremediation of crude oil dispersed by Corexit 9500. J. Hazard. Mater. 2011, 185, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Benotti, M.J.; Brownawell, B.J. Microbial degradation of pharmaceuticals in estuarine and coastal seawater. Environ. Pollut. 2009, 157, 994–1002. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chang, Q.; Li, S.; Gao, M.; She, Z.; Guo, L.; Zhao, Y.; Jin, C.; Zheng, D.; Xu, Q. Impact of sulfadiazine on performance and microbial community of a sequencing batch biofilm reactor treating synthetic mariculture wastewater. Bioresour. Technol. 2017, 235, 122–130. [Google Scholar] [CrossRef]
- Herzog, B.; Lemmer, H.; Horn, H.; Muller, E. Characterization of pure cultures isolated from sulfamethoxazole-acclimated activated sludge with respect to taxonomic identification and sulfamethoxazole biodegradation potential. BMC Microbiol. 2013, 13, 276. [Google Scholar] [CrossRef]
- Zhang, W.W.; Wen, Y.Y.; Niu, Z.L.; Yin, K.; Xu, D.X.; Chen, L.X. Isolation and characterization of sulfonamide-degrading bacteria Escherichia sp. HS21 and Acinetobacter sp. HS51. World J. Microbiol. Biotechnol. 2012, 28, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, Y.; Ma, J.; Zhao, F. Rapid degradation of sulphamethoxazole and the further transformation of 3-amino-5-methylisoxazole in a microbial fuel cell. Water Res. 2016, 88, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Walker, N. A soil Flavobacterium sp. that degrades sulphanilamide and asulam. J. Appl. Bacteriol. 1978, 45, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Dzierzewicz, Z.; Cwalina, B.; Weglarz, L.; Wi?niowska, B.; Szczerba, J. Susceptibility of Desulfovibrio desulfuricans intestinal strains to sulfasalazine and its biotransformation products. Med. Sci. Monit. 2004, 10, BR185–BR190. [Google Scholar] [PubMed]
- Xie, H.; Wang, X.; Chen, J.; Li, X.; Jia, G.; Zou, Y.; Zhang, Y.; Cui, Y. Occurrence, distribution and ecological risks of antibiotics and pesticides in coastal waters around Liaodong Peninsula, China. Sci. Total Environ. 2019, 656, 946–951. [Google Scholar] [CrossRef] [PubMed]
- Limbu, S.M.; Zhou, L.; Sun, S.X.; Zhang, M.L.; Du, Z.Y. Chronic exposure to low environmental concentrations and legal aquaculture doses of antibiotics cause systemic adverse effects in Nile tilapia and provoke differential human health risk. Environ. Int. 2018, 115, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Steele, J.C.; Meng, X.Z. Usage, residue, and human health risk of antibiotics in Chinese aquaculture: A review. Environ. Pollut. 2017, 223, 161–169. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, B.-V.; Chao, W.-L.; Yeh, S.-L.; Kuo, D.-L.; Yang, C.-W. Biodegradation of Sulfamethoxazole in Milkfish (Chanos chanos) Pond Sediments. Appl. Sci. 2019, 9, 4000. https://doi.org/10.3390/app9194000
Chang B-V, Chao W-L, Yeh S-L, Kuo D-L, Yang C-W. Biodegradation of Sulfamethoxazole in Milkfish (Chanos chanos) Pond Sediments. Applied Sciences. 2019; 9(19):4000. https://doi.org/10.3390/app9194000
Chicago/Turabian StyleChang, Bea-Ven, Wei-Liang Chao, Shinn-Lih Yeh, Dong-Lin Kuo, and Chu-Wen Yang. 2019. "Biodegradation of Sulfamethoxazole in Milkfish (Chanos chanos) Pond Sediments" Applied Sciences 9, no. 19: 4000. https://doi.org/10.3390/app9194000
APA StyleChang, B.-V., Chao, W.-L., Yeh, S.-L., Kuo, D.-L., & Yang, C.-W. (2019). Biodegradation of Sulfamethoxazole in Milkfish (Chanos chanos) Pond Sediments. Applied Sciences, 9(19), 4000. https://doi.org/10.3390/app9194000




