1. Introduction
Applications including particle accelerators and vacuum electronic devices (VEDs) require materials with the highest electrical and thermal conductivity, as well as ultra-high vacuum compatibility. The copper used in these applications approaches the theoretical maximum achievable quality in terms of the purity, density, and metallurgical properties, such as crystallographic texture and grain size. These applications also require intricate designs and extensive metallurgical processing routes, followed by the assembly and brazing or welding of multiple components into a final part. The consolidation of the component assemblies into complex monolithic parts, and the potential for novel designs achievable through additive manufacturing (AM), is desired in order to reduce the cost and improve the component performance and reliability [
1,
2,
3,
4,
5]. The underlying challenges associated with the AM of high-purity copper, particularly for electron beam powder bed fusion (EB-PBF) and laser powder bed fusion (L-PBF), have been discussed in several recent studies [
1,
6,
7,
8,
9,
10,
11]. The high thermal conductivity of copper rapidly removes heat from the melt pool, promoting high local and global thermal gradients; residual stress accumulation; and distortion, which is exacerbated by the significant difference in thermal conductivity between the consolidated material and the surrounding powder bed [
12,
13,
14]. The resulting rapid solidification of the melt pool, coupled with the low viscosity of molten copper, also tends to retain defects such as keyhole porosity [
15]. Additionally, the low absorptivity of copper for the lasers used in many commercial L-PBF systems (~1060 nm wavelength) necessitates the use of high-power lasers increasing the recoil pressure, vaporization, spatter, and related defects [
16]. However, even with these challenges, several promising results have been reported with densities ranging above 99.95% for non-electronic grade copper [
1,
7,
8,
9] for EB-PBF, and 96.6% for Nd:YAG fiber laser AM processing [
17,
18,
19,
20,
21,
22,
23].
Despite the progress, achieving the high purity requirements for copper using AM has remained a significant challenge. For accelerator applications, copper typically needs to meet or exceed that of ASTM F68 for Class 1 oxygen free electronic (OFE) copper, which is 0.15176 ohms g/m
2 or 101.0% of the minimum International Annealed Copper Standard (IACS) at 20 °C [
24]. The maximum oxygen content for OFE copper is 0.0005 wt% (5 wt. ppm). The negative influence of oxygen contamination on the electrical, thermal, and mechanical properties of copper is small, but not insignificant. The incoherent Cu
2O found along the grain boundaries has an effect similar to porosity, and less of an influence on electron scattering than the solute contamination that strains the copper lattice. For instance, electrolytic tough pitch (ETP) copper has an oxygen content of ~0.04% (400 ppm) and typically exceeds 100–101% IACS [
24,
25,
26]. Much more significant is the adverse effect of embrittlement in the copper components caused by an excessive oxygen content during the downstream hydrogen brazing processes [
27]. It should be noted that the copper AM literature has either resulted in specimens with a high oxygen content (similar to ETP Cu), or has not reported an oxygen content in the fabricated samples. Of chief concern is the powder feedstock purity. The powder feedstock commonly used in AM is subject to oxygen contamination during handling, screening, loading, and transport after atomization. At ambient conditions, the oxygen solubility in pure copper is less than 2 wt. ppm [
24,
28], where the excess oxygen reacts to form cuprous oxide, Cu
2O, as a non-passivating surface film or along the grain boundaries. At higher temperatures (>300 °C), cupric oxide (CuO) and other variants may also form [
29,
30].
This poses a unique obstacle for the copper powder used in AM, which is typically produced by nitrogen gas atomization, and quickly oxidizes if exposed to ambient conditions at any point in the powder fabrication and handling process. Because of the very high surface area of these small diameter particles, the relative contribution of oxygen from the surface oxide film typically causes the feedstock to exceed 400–600 wt. ppm oxygen. For example, in their study on the EB-PBF fabrication of copper, Raab et al. [
7], Lodes et al. [
8], and Guschlbauer et al. [
9] utilize copper powder feedstocks with reported purities of 99.90 wt%, 99.94 wt%, and 99.95 wt%, respectively, while Frigola et al. [
1] and Ramirez et al. [
10] used powders with 99.99 wt% and 99.80 wt% purity, respectively. In our preliminary studies, a chemical analysis conducted directly after the powder atomization process, and after the screening, indicates that much of the oxygen pickup in the copper powder occurs during the handling, screening, and packaging processes, which are typically carried out in ambient atmosphere.
Preserving even these oxygen contents during the AM melting and solidification process is extremely challenging, and requires near perfect conditions, as imperfect inert or vacuum atmospheres, moisture absorption, complex thermal cycles, powder recycling, and storage exacerbate oxygen pickup. Frigola et al. [
1] showed a significant oxidation of feedstock powders after several EB-PBF reuse cycles. Once the oxygen contamination is present in the powder feedstock as oxide films or grain boundary particles, the contamination is directly transferred to the fabricated AM components [
11]. This combination of factors hinders the fabrication of quality copper components by most powder bed AM processes.
Reduction processes may be employed in order to improve the copper feedstock purity for AM processing. Hydrogen, carbon monoxide, and ethanol are commonly employed reducing agents of Cu
2O and CuO in thin films [
31,
32,
33]. El-Wardany et al. [
6] treated copper powders for L-PBF in a heated forming gas atmosphere (4% H
2 + Ar bal.), theorizing that the hydrogen would reduce the surface Cu
2O films into Cu + H
2O, however, no quantitative data on the composition of the powders or solid samples produced were provided [
6]. In addition, this study only considered the reduction of oxides from the surface of the Cu powders, and did not address the possibility that hydrogen also reduces the internal oxides. Previous work has shown that the diffusivity of hydrogen in copper is high at elevated temperatures (e.g., ~9.58 m/s
2 at 400 °C) [
34], and that at temperatures above about 400 °C, hydrogen reduces the grain boundary oxides, forming H
2O gas. The larger H
2O molecules do not diffuse, and the resulting pressure forms high pressure steam pores along the grain boundaries [
28,
35,
36]. This well-known embrittlement mechanism is considered detrimental for most traditional copper powder processing routes because of their significant impacts on downstream processing, such as swelling, porosity, and embrittlement, through plastic deformation, work hardening, the reduction of grain boundary area, and material failure at elevated temperatures [
28,
36,
37,
38], as Lin and Hwang demonstrated with the swelling and cracking of copper powders during the sintering of copper heat pipes [
36].
On the other hand, PBF processes utilize thin layers of powder, which are spread over a substrate and selectively melted by a focused energy source. In the present work, we hypothesize that this localized processing can be leveraged to liberate the retained H2O from the hydrogen heat-treated copper powder during melting on a layer-by-layer basis. This results in a significantly higher copper purity, without the detrimental effects of embrittlement observed in traditional powder metal processes.
Here, the mechanisms for H2O removal from copper powders is identified through a series of experiments, one building upon the next. First, copper powders of varying initial oxygen contents were treated in a hydrogen atmosphere at elevated temperatures. The temperature that H2O escapes from these copper powders was determined using thermogravimetric analysis. The powder cracking during H2O released at these temperatures was observed directly using a scanning electron microscope (SEM) equipped with a heated stage. We confirm the release of H2O in a single layer melt in a custom EB-PBF setup using residual gas analysis (RGA), and the subsequent chemical analysis of the melted layers. The single melt pool wide thin walled wafers produced with multiple layers of varying hatch spacings confirm the H2O release from the copper powder in the heated affected zone of the melt pool, and high-speed videos of these melt pools also show that a proportion of this H2O escapes from the molten during melting. The efficacy of this approach is validated with the fabrication of fully dense parts, and multi-layer samples with a very low oxygen content, with RGA monitoring of H2O outgassing on a layer by layer basis.
2. Materials and Methods
The experiments were performed in order to understand the influence of a hydrogen heat treatment on copper powder feedstocks of varying initial oxygen contents. We evaluated three levels of oxygen content, with and without hydrogen heat treatment. To generate these six conditions, the following methodology was used.
Two separate batches of nitrogen gas atomized copper powder were acquired. For the purposes of this discussion, the powders were categorized based on the initial measured oxygen content. The lowest oxygen content powder received was between 100–200 wt. ppm oxygen, and is referred to as low oxygen copper (LO-Cu). The second powder batch with 400–700 wt. ppm oxygen is referred to as medium oxygen content powder (MO-Cu). A selected amount of the received MO-Cu powder was intentionally oxidized to above 1000 wt. ppm oxygen, and is referred as high oxygen content copper (HO-Cu).
2.1. Feedstock Preparation
Spherical MO-Cu powder was received with a nominal 15–53 µm particle size distribution (d10–d90), smaller than what is typical for other materials used in EB-PBF (e.g., Ti6Al4V). Nevertheless, it is consistent with previous studies in copper AM by our group [
4], and compared to early work by our group and others, also results in a significantly reduced surface roughness [
1]. In order to maintain a comparatively low oxygen content in the LO-Cu powder, special consideration was given to the packaging, handling, and screening steps post atomization. This powder was received in the as-atomized (−250 µm) condition, directly from the manufacturer, and screened to a nominal 15–53 µm particle size distribution within an argon glove box. To generate the HO-Cu powder, a methodology similar to that of Nieh and Nix [
28,
30] was utilized. A portion of MO-Cu was treated at 150 °C for 1 h in one atmosphere of air, and cooled at a rate of 5 °C per minute. The temperature was kept lower in the current study, compared with the 800 °C used by Nieh and Nix, to prevent the sintering of the small powder size distribution. Given that the diffusion of oxygen in copper is 2e
−11 m
2/s at 400 °C [
39], the oxygen penetration depth exceeded the particle diameters used in this study during the 2-h treatment, which is confirmed by subsequent focused ion beam (FIB) sectioning of individual powder particles.
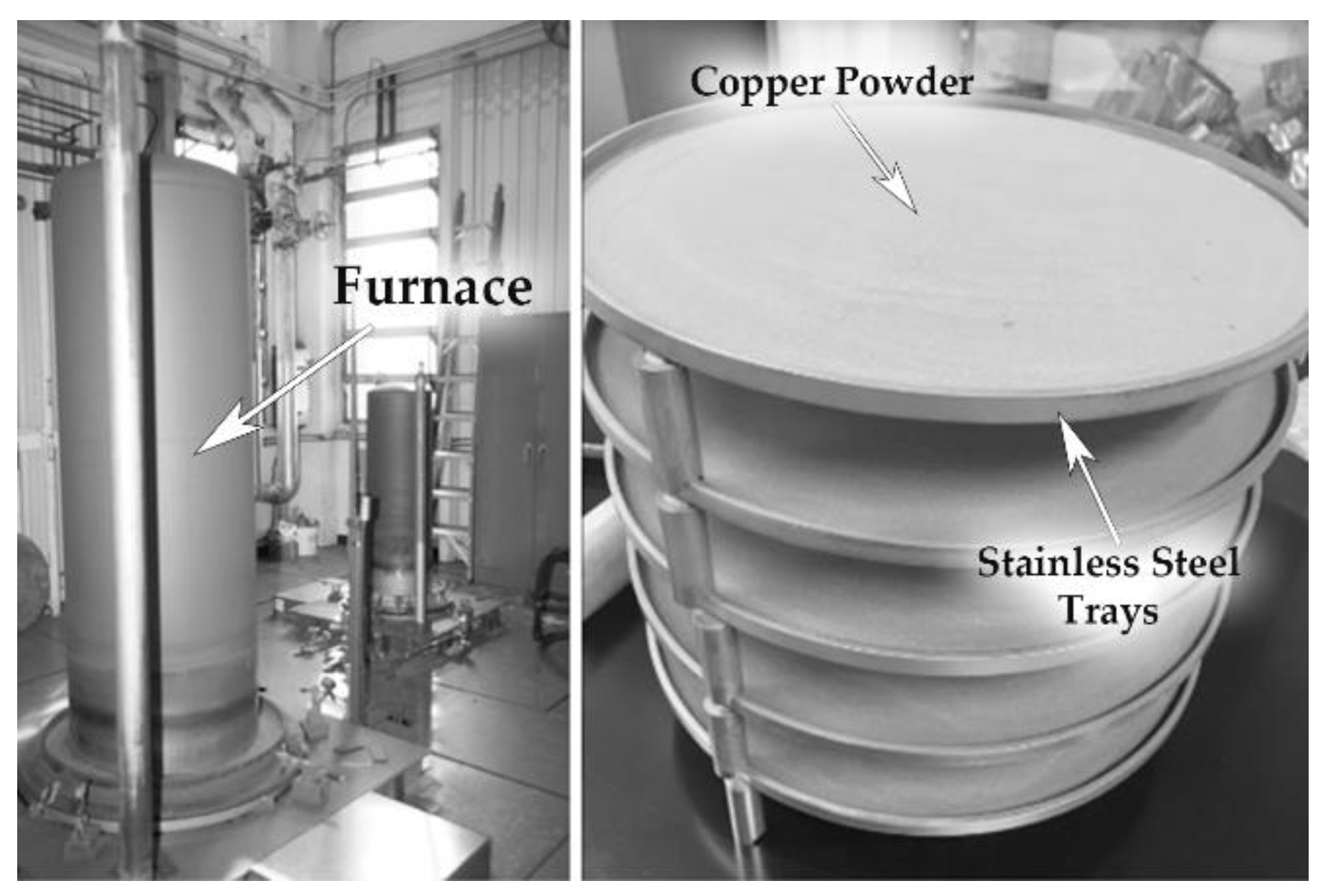
Portions of the powder from each of the three batches, LO-Cu, MO-Cu, and HO-Cu, were then exposed to hydrogen treatment at 400 °C for 4 h under one atmosphere of flowing pure hydrogen, and cooled at a rate of 5 °C per minute in a dry hydrogen atmosphere heating cover retort furnace. A custom stainless-steel fixture comprised of stacked circular trays allowed the processing of 1 kg of powder per treatment cycle in 2 mm thick layers, as shown in
Figure 1. To avoid oxygen contamination after hydrogen treatment, the powders were packed in vacuum-sealed foil bags after purging with dry nitrogen. The vacuum bags were then sealed in a second bag filled with dry nitrogen. All of the copper powders were subsequently stored, handled, and sampled in an argon or nitrogen atmosphere.
2.2. Feedstock Characterization
Each of the three powder conditions were characterized before and after hydrogen treatment, for size distribution by laser diffraction (Microtrac S3500, Microtrac Inc., Montgomeryville, PA, USA), and density by helium pycnometry (ASTM B923-16, Quantachrome MicroUltrapyc 1200e, Quantachrome Instruments, Boynton Beach, Florida, USA). The oxygen and hydrogen content of all the powders were quantified by inert gas fusion analysis (LECO OHN836, LECO, St. Joseph, MI, USA). The reported values herein were acquired immediately prior to the experiments. The powder morphology was characterized by a JOEL 6010LA scanning electron microscope (JEOL USA, Inc., Peabody, MA, USA). An additional powder characterization was performed on individual powder particles using focused ion beam (FIB) milling for the cross-sectional analysis by secondary electron and gallium ion contrast on a FEI Quanta 3D Field Emission Gun (FEG) (Thermo Fisher Scientific, Hillsboro, Oregon, USA). Scanning transmission electron microscopy (STEM) analysis and energy dispersive spectroscopy (EDS) were performed on a FEI Talos (Thermo Fisher Scientific, Hillsboro, Oregon, USA), to assess the chemical composition, size of surface, and internal oxide species.
A thermogravimetric analysis (TGA) was performed using a TA SDT 650 Simultaneous Thermal Analyzer (TA Instruments, New Castle, DE, USA) under flowing nitrogen, while the samples were heated at 5 °C/s to 600 °C in an alumina sample holder in order to identify the temperature at which the H2O can escape from the treated copper powders.
2.3. In-Situ Heated Stage
In-situ heated stage SEM degassing experiments were performed on a FEI Quanta 3D FEG so as to observe the mechanism of H2O gas release from a single copper powder particle. Both hydrogen heat treated copper particles and untreated copper particles were separated from the adjacent powder feedstock with a single fiber brush using a light microscope at 50× magnification. Individual powder particles were then placed onto a SiC heated stage (Protochips Fusion) for controlled heating under standard SEM operating conditions. The stage was ramped to a maximum temperature of 400 °C at 100 °C/s, and image sequences were made at 30 fps with secondary electron detectors.
2.4. Customized EB-PBF Platform
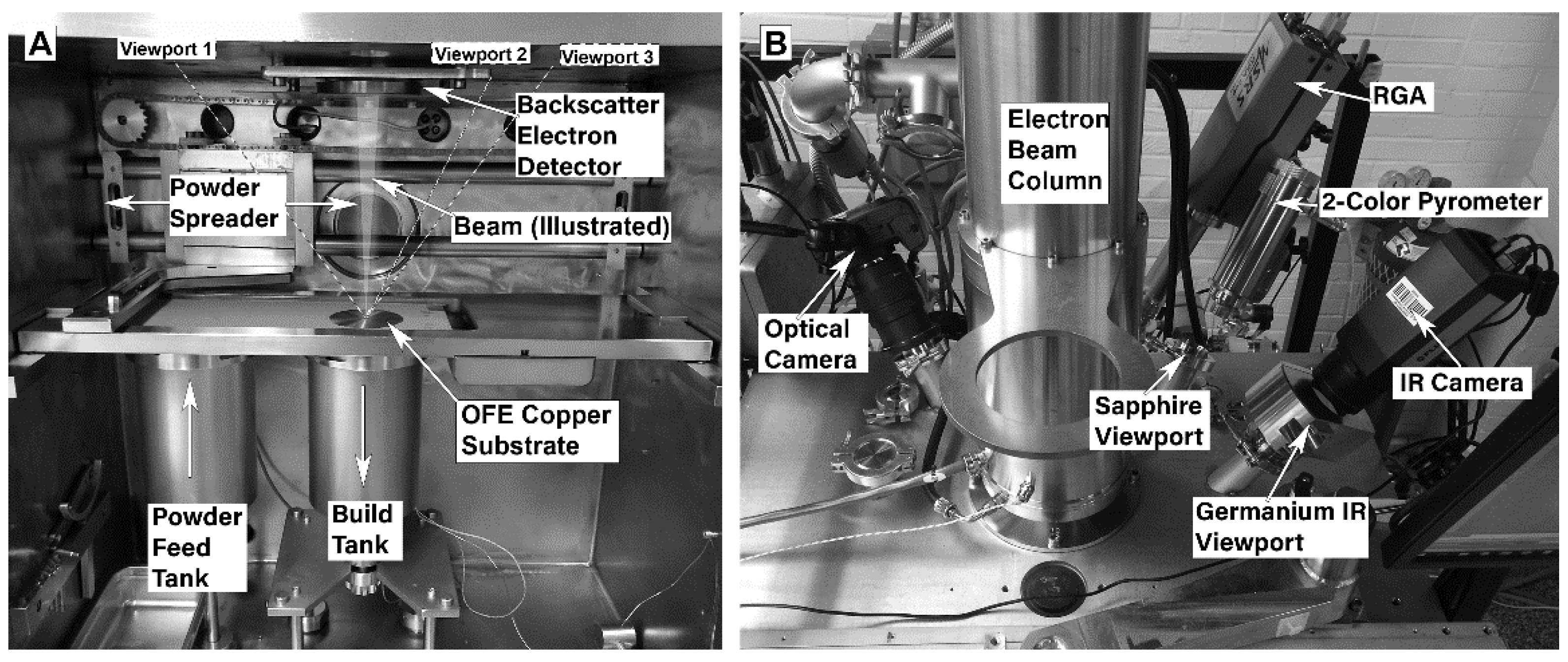
All of the electron beam melting (EBM) experiments were conducted using a customized Arcam A2 EB-PBF system (Arcam Build Control Software V3.2, SP2, Arcam AB, Sweden). While the EB-PBF operation and process parameters have been described in detail elsewhere [
40], it is important to highlight that in this work, the internal components of the commercial system have been removed and replaced with a custom 304 stainless steel build tank, platform, and powder feeder system. The customized chamber was designed with viewports that are oriented radially about the vertical axis of the electron beam column at a 55° angle to the surface of the experimental substrate/fixture, in order to view the entire build surface. The experiments were monitored with by an emissivity corrected single color pyrometer (Fluke Process Endurance Pyrometer, Fluke, Everett, WA, USA), a visible light camera (Nikon D7000, Nikon, Melville, NY, USA), a 200-amu residual gas analyzer (Stanford Research Systems, Sunnyvale, CA, USA), and an IR thermal imaging camera (FLIR A655sc, FLIR, Wilsonville, OR, USA). The viewport window materials varied as a function of the transmittance requirements for each instrument.
Figure 2 shows a photograph of the customized setup.
2.5. Simulation and Validation of Variable Spacing Single Melt Track Width Wafers
We hypothesized that during melting, H
2O vapor escapes from both the liquid melt pool and from powder in the heat affected zone. For the powder adjacent to the melt pool, the internal pressure of the H
2O vapor overcomes the mechanical strength of the grain boundary, and the pore begins to expand in size. This leads to the fracturing of the particle along the grain boundaries and a release of H
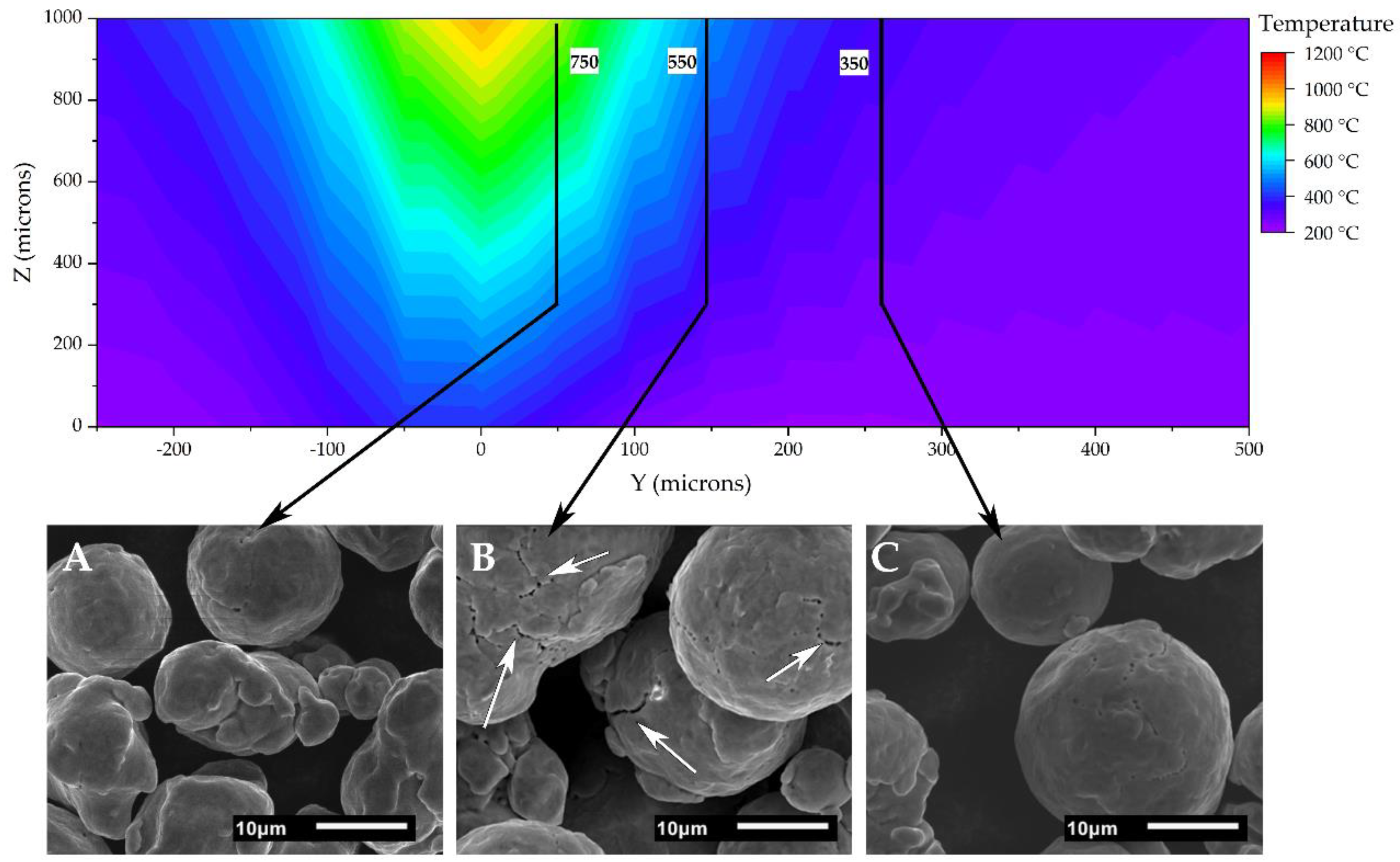
2O vapor into the vacuum system. To explore this, single melt track width scenarios were simulated at various hatch spacings, utilizing the 3D transient heat transfer model developed by Lee et al. [
41] at the Oak Ridge National Laboratory. Calculations were made using FEniCS software (2019.1.0, FEniCS), utilizing python scripts, and the thermal model output was analyzed using ParaView visualization software (v5.7, Kitware Inc., Clifton Park, NY, USA). The simulation used variables including heat input, beam speed, thermal conductivity, and so on, to model the EB-PBF process and the temperature profile across a solid plate with the thermophysical properties of a comparable copper powder bed during different operating conditions. The simulation variables used in this study are listed in
Appendix A.
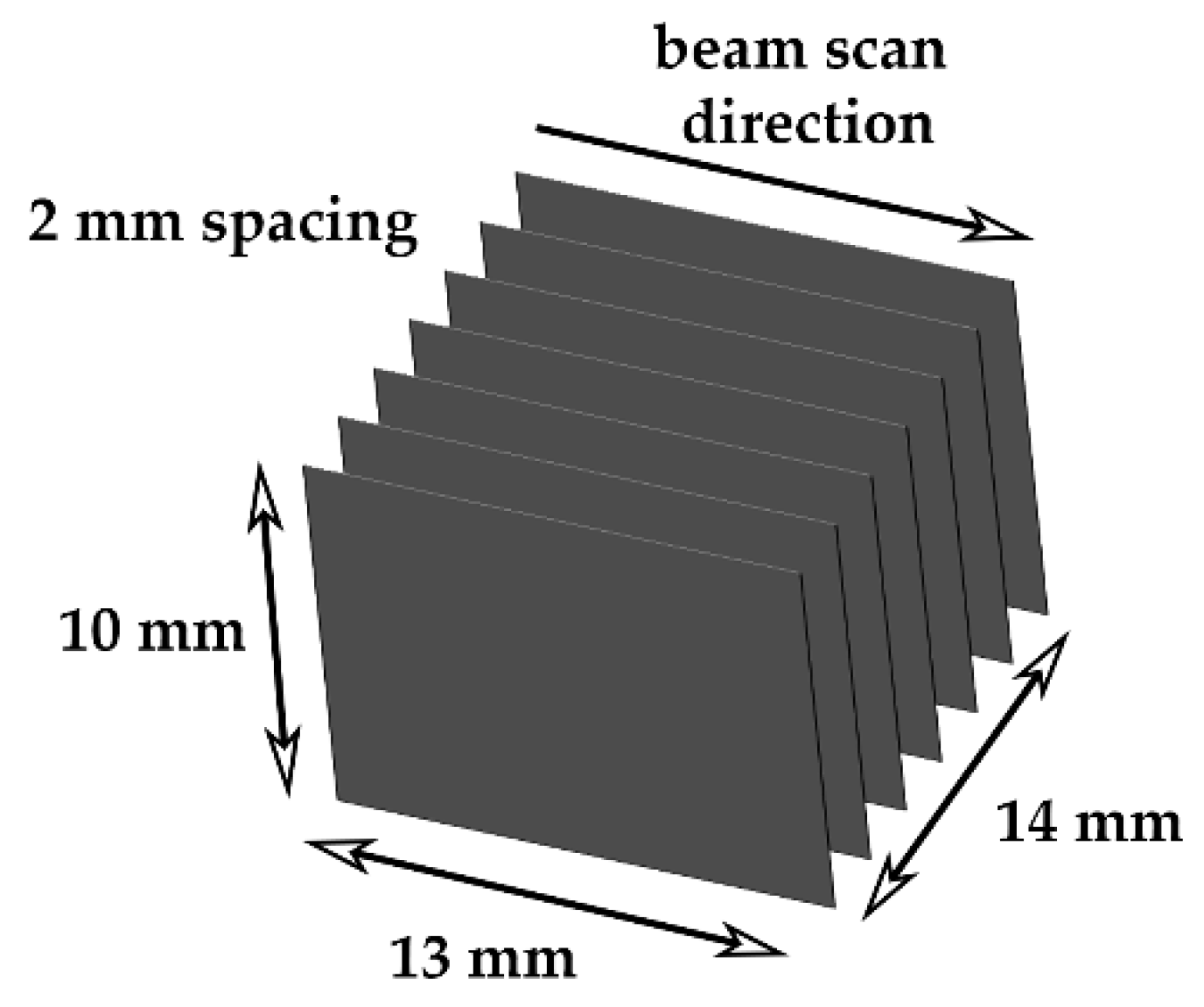
Based on the model results, single melt track width wafers 13 mm long and 10 mm tall were fabricated using hydrogen-treated HO-Cu powder with 2.0 mm on center spacings, as shown in
Figure 3. The build substrate was a machined OFE copper substrate that measured 90 mm in diameter and 30 mm in thickness. During the EB-PBF processing, the build temperature was maintained at 270 °C, measured by a thermocouple attached to the bottom of the plate, and the partial pressures of H
2O vapor and other gas species were monitored using the RGA. Each wafer was melted with a line energy of 0.8 J/mm (600 mm/s, 8 mA, 0 mA focus offset) and layer thickness of 40 µm, with the power analyze function (Arcam specific thermal controls) disabled. The beam scan direction was constant (right to left) for each wafer and each layer.
Upon the build completion, each block of wafers was removed from the surrounding sintered powder and build surface using a razor blade. The sintered powder cake between the solid wafers was serially sectioned with a razor blade as a function of the distance from the melt pool, and placed on carbon tape for the SEM analysis. Additionally, the top surface of the intact sintered cake was analyzed on a JOEL 6010LA, in order to observe sintered powder characteristics at specific distances from the melt track.
2.6. EB-PBF Processing of Hydrogen-Treated Copper Powder
To directly observe and quantify the H
2O release during EB-PBF, single layer discs were melted using the various hydrogen-treated and untreated powders.
Figure 4 illustrates the experiment. The fixture upon which the experiments were carried out was an annealed OFE copper cylinder, 100 mm in diameter and 50 mm thick. Six equally spaced cylindrical pockets, measuring 25 mm in diameter by 3 mm deep were milled into the surface at a radial distance of 30 mm from the plate center, such that each pocket is effectively isolated from the others, as seen in
Figure 4. After the machining, the copper substrate was cleaned using a standard ultra-high vacuum (UHV) protocol for machined copper.
Each of the pockets in the substrate were filled and leveled to the top surface of the plate with ~5 grams of LO-Cu, MO-Cu, or HO-Cu powder before or after hydrogen treatment, each having a replicate. The oxygen and hydrogen content of the powder, as noted previously, was measured by inert gas fusion prior to this experiment. A type K thermocouple was affixed to the bottom of the substrate, and the substrate was placed on top of the build piston, which allows for the thermal isolation from the system.
The vacuum chamber was evacuated to a pressure of 3.75 × e−5 Torr, and the copper substrate was heated by repeatedly scanning the defocused electron beam in a circular area, 25 mm in diameter at the center of the substrate, with a beam current of ~2–4 mA. The substrate temperature was measured by the thermocouple at the bottom, and a two-color pyrometer through a sapphire window positioned in one of the upper viewports.
The substrate heating was manually modulated until both of the temperature readings agreed within 5% of the target value (300 °C), and then this temperature was maintained for a 2 h outgassing cycle. A 200 amu residual gas analyzer (SRS-RGA200) was used to measure the partial pressure of the gas species of interest for this study (O, H, N, NO, NO2, and H2O). During the outgassing cycle, the background H20 partial pressure was observed to decrease and stabilize at ~8 × e−7 Torr. This outgassing period at 300 °C also allowed sufficient time for the initial stages of sintering to take place within the powder. This was observed with the IR camera by a uniform emissivity shift across all six powder pockets.
The focused electron beam was then raster scanned over an area measuring 20 mm in diameter on the surface of each one of the powder pockets. A line energy of 0.8 J/mm (600 mm/s, 8 mA, 0 mA focus offset, 100 µm hatch offset) was sufficient to melt and consolidate a single layer of copper supported underneath by the sintered powder. The melting process was recorded by the thermal imaging camera through a germanium port at 25 Hz, and by in-situ monitoring of the back scattered electron signal. A thermal video of the melting process is available in the
Supplementary Materials. During the melting, the outgassing of the key gas species was monitored by the RGA at 1 Hz. This process was repeated on each powder pocket with approximately 10 min to pass between each raster melt in order to allow for the RGA partial pressures and substrate temperature to stabilize. The solids samples were then cooled under a vacuum to room temperature.
The material in each pocket was removed from the substrate and the sintered powder was mechanically removed from the solid wafer with a wire brush in an inert gas glove box. The oxygen and hydrogen content of both the sintered powder and solid wafer from each pocket were measured by inert gas fusion (LECO Form 203-821-455, LECO OHN836, LECO, St. Joseph, MI, USA). The experiment was repeated several times, varying the position of each powder type and the order in which powders were melted.
2.7. High Speed Footage of Melt Pool
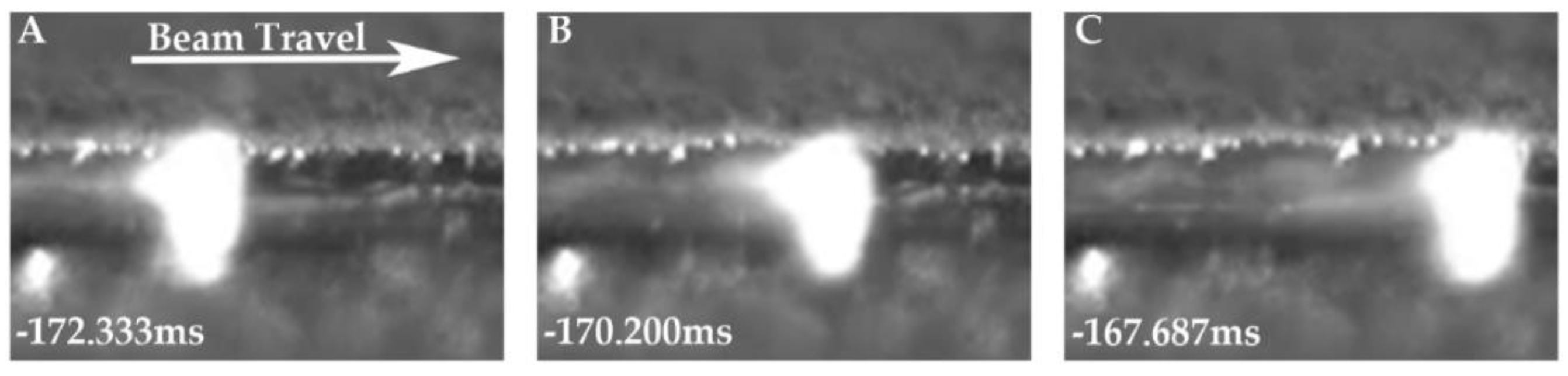
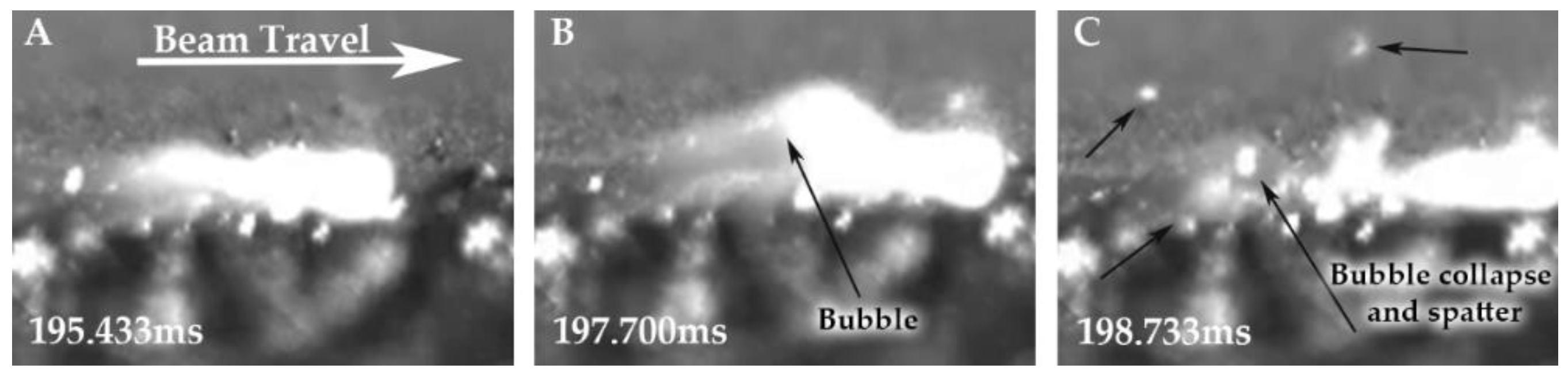
In order to directly observe the H
2O vapor escaping from the melt pool, a Photron SA-X2 high-speed camera was utilized to capture the footage at 30,000 frames per second, at a focal length of 30 cm. The footage was captured for both a hydrogen-treated LO-Cu and HO-Cu melt pool using the same procedure for the EB-PBF processing as described in
Section 2.6.
2.8. Solid Sample Fabrication
To validate the process for fabricating the components of high purity copper with hydrogen heat-treated powers, solid cylindrical specimens measuring 15 mm in diameter were fabricated for the microstructural and chemical analysis, using both untreated and hydrogen-treated MO-Cu powder. The untreated powder had an oxygen content (~600 wt. ppm) similar to what was used in the studies by Frigola et al. [
1], Lodes et al. [
8], and Guschlbauer et al. [
9]. The processing parameters used were 600 mm/s, 8 mA, 18 mA focus offset, and 120 µm hatch offset with a 40 µm layer thickness. This falls within the processing space (~166 J/mm
3) used by both Frigola et al. [
1] and Guschlbauer et al. [
9]. The build temperature was maintained at 300 ± 10 °C for the duration of the fabrication. The gas species in the chamber were monitored during the fabrication process using the RGA, as described earlier.
4. Discussion
The oxygen content of each of the three starting powder conditions results from surface oxide films and oxides along the grain boundaries. The copper oxide will continue to grow when exposed to oxygen, as it does not passivate [
42]. The surface oxide thickness as a function of time at room temperature was empirically derived by White and Germer [
43]. A typical oxide thickness would fall between 3–5 nm, but could be much thicker. The overall contribution of this oxide layer becomes a significant portion of the chemistry for a powder with a high specific surface area [
44].
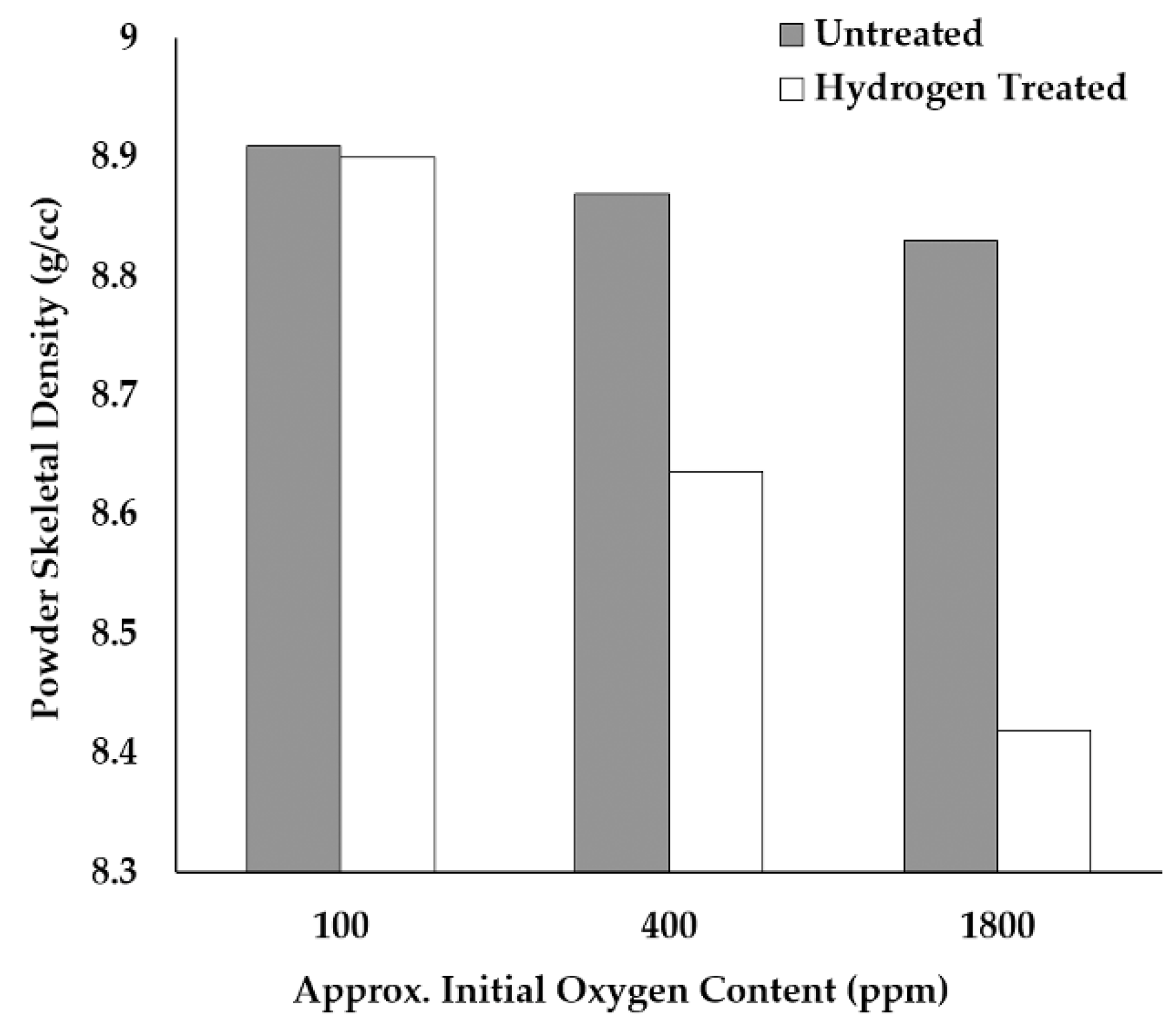
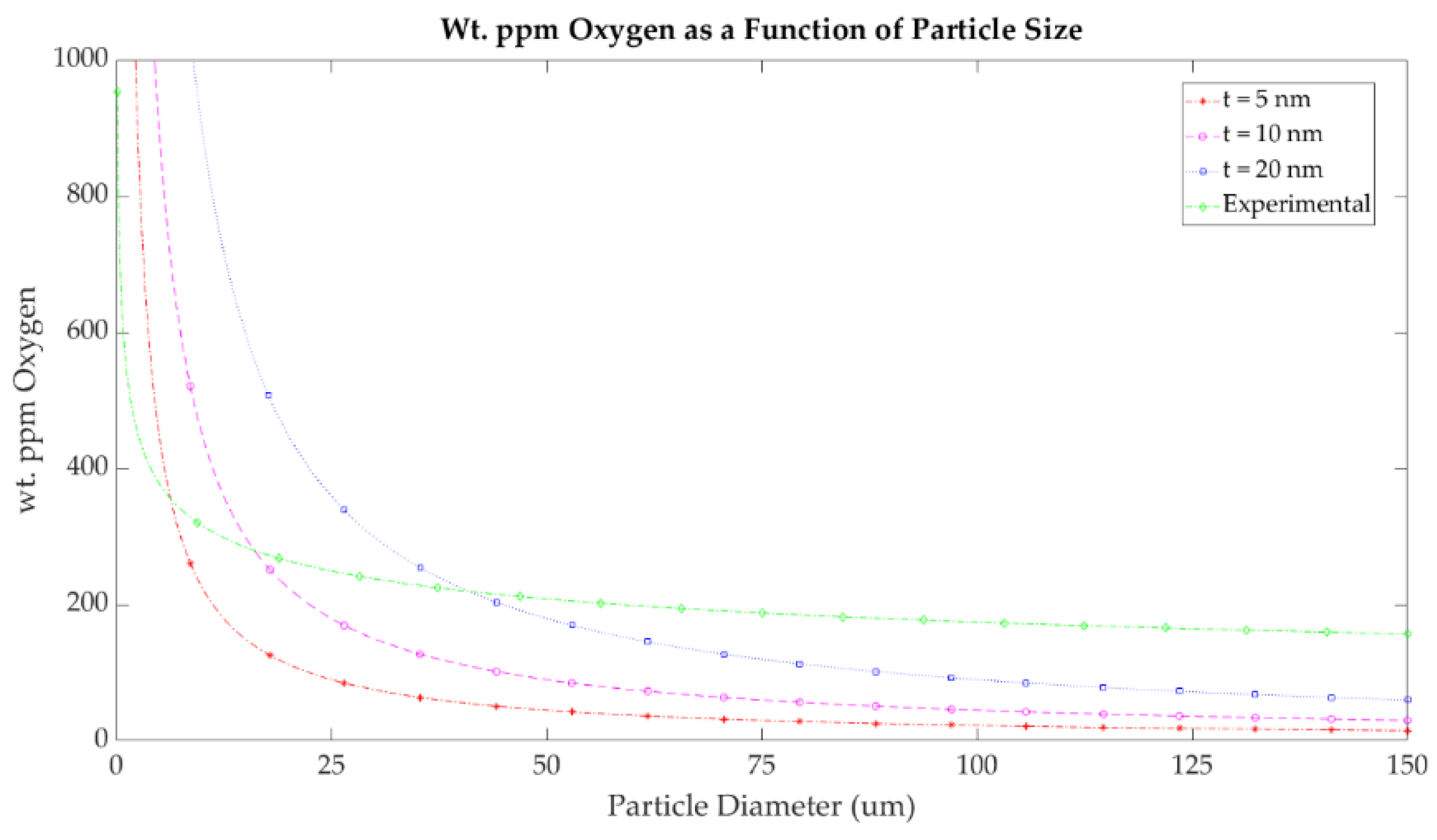
Figure 19 shows the calculated wt. ppm of oxygen as a function of the particle diameter for cuprous oxide surface films of various thicknesses. In the case of a 5 nm coating, and a typical Gaussian powder size distribution centered at a 35 µm diameter, the oxygen content is estimated to be roughly 75 wt. ppm oxygen, assuming all oxygen neglecting the 2 wt. ppm in solid solution at room temperature [
39]. The experimental data gathered in this study suggest that the oxide thickness on the LO-Cu particles used in this study is ~15–20 nm, consistent with our STEM observations. Volumetrically, this accounts for the measured 200 wt. ppm oxygen, which was mainly surface oxides in the LO-Cu material for particles in the range of 15–53 µm.
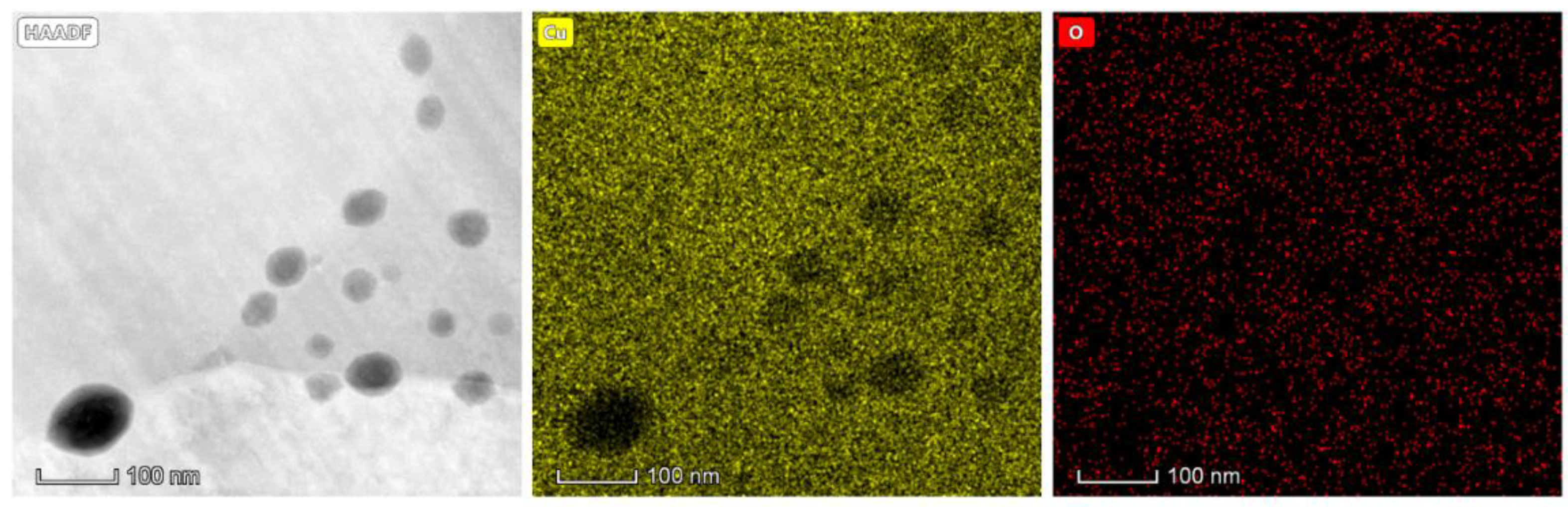
The MO-Cu and HO-Cu particles greatly exceed this oxygen content and show a large number of submicron oxides along the grain boundaries, as seen in
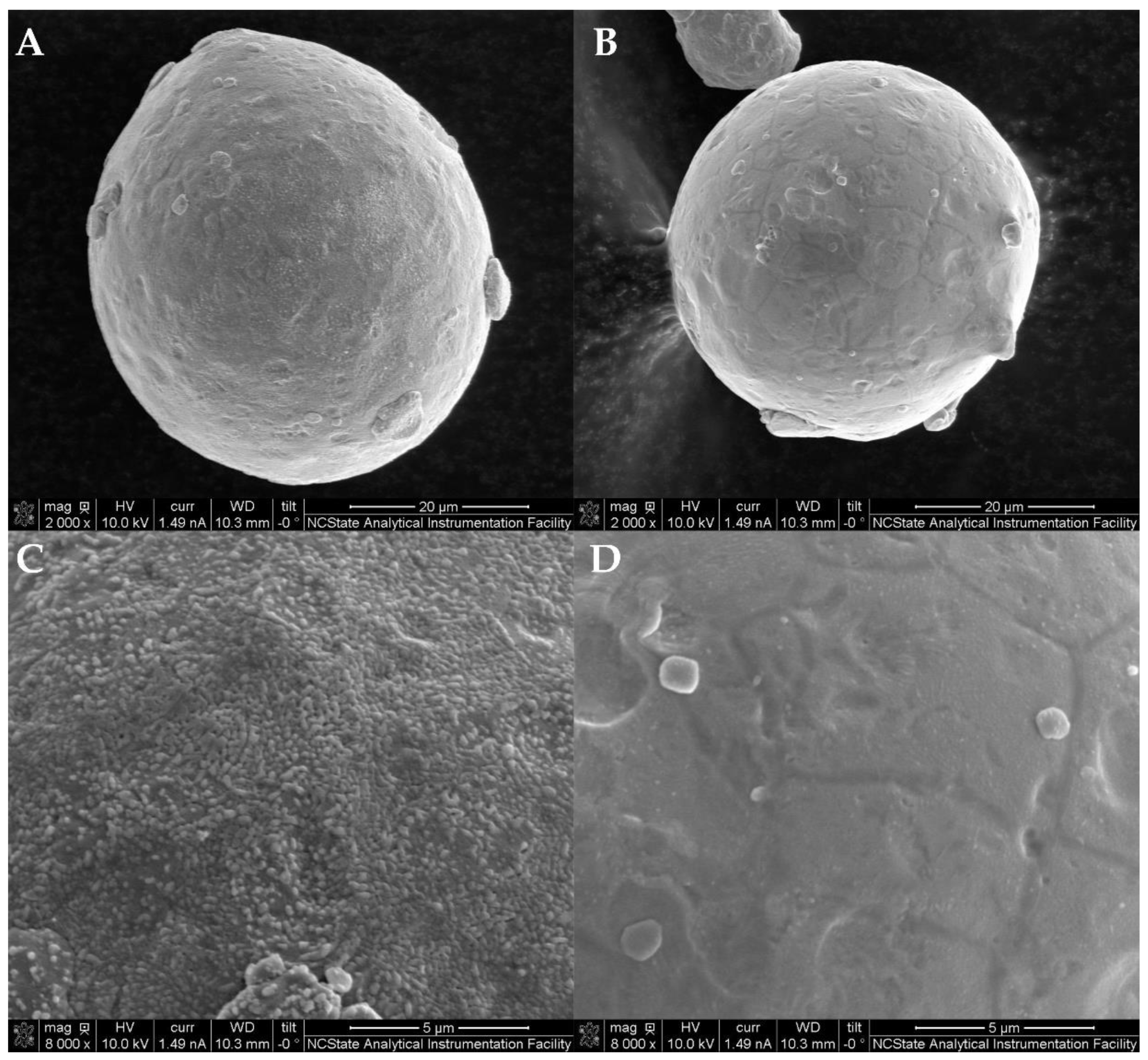
Figure 7B,C. Naturally, the HO-Cu particles show the largest number of submicron oxides along the grain boundaries, with several larger oxides at triple points of the microstructure because of its exposure to air at elevated temperatures.
To remove the oxide particles, a hydrogen atmosphere heat treatment is employed to reduce the copper oxide to copper and H
2O. This was first observed in the MO-Cu powder in
Figure 7C,D, where the surface oxides were removed by hydrogen treatment. As hydrogen diffuses rapidly in copper (900 µm
2/s at 400 °C [
34]), it allows for the reduction of oxides present inside the particles in a relatively short amount of time. The hydrogen treatment temperature and time was chosen to allow for sufficient hydrogen diffusion through the particles, while not reaching sintering temperatures for the fine powder size distribution. Although not directly reported herein, it is worth noting that, not since the completion of this study, we have subsequently demonstrated the hydrogen reduction of copper powder in several furnace types (including batch and continuous) in both pure hydrogen and in forming gas (5% h, 95% Ar), and we have observed consistent results in all scenarios for the equivalent treatment times. After hydrogen treatment, the MO-Cu and HO-Cu powders exhibit a micron scale porosity at the triple points of the grain boundaries, and a smaller submicron porosity along the grain boundaries. These pores have previously been shown in the literature to be trapped H
2O vapor [
28,
30,
35].
According to Ito and Hayashi [
35], the formation of H
2O vapor in closed pores is as a result of the reduction of copper oxide during hydrogen heat treatment. The H
2O vapor pressure inside a closed pore is higher than that of the surface stress of the pore, which inhibits the pore from collapsing. However, the H
2O vapor pressure is not great enough to overcome the mechanical strength of the grain boundaries of the particle at room temperature.
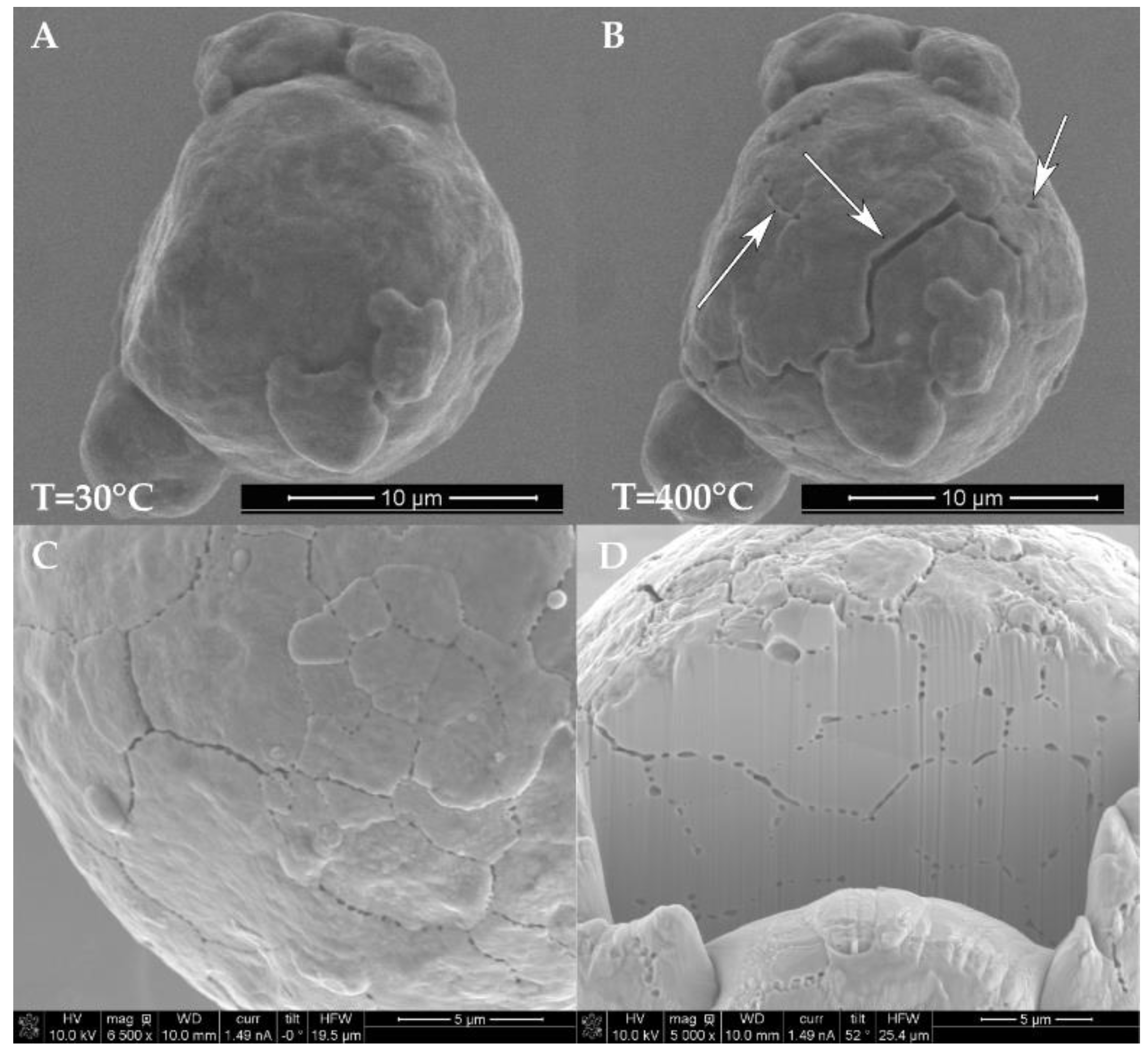
At around 350 °C, the strength of the copper is significantly reduced, and the increasing internal pressure of the H
2O vapor overcomes the mechanical strength of the grain boundary and the pore begins to expand in size. This leads to the fracturing of the particle, seen as an extensive network of interconnected pores along the grain boundaries, and a release of H
2O vapor into the vacuum system, as seen in the SEM images in
Figure 11C,D, and the RGA data from
Figure 14. Notably, our work is presented for a given size distribution of gas atomized powder. While it is apparent that the reduction of internal Cu
2O and the formation of steam pores is, for all intents and purposes, independent of particle size (owing to the rapid diffusion of hydrogen in copper at 400 °C), the practical upper and lower limits on particle size, for which the observed phenomena hold true, is not clear from this research. It is likely that the combined influences of grain boundary strength, grain size, radius of curvature, and oxygen content will contribute to the steam embrittlement/fracture mechanism in different ways.
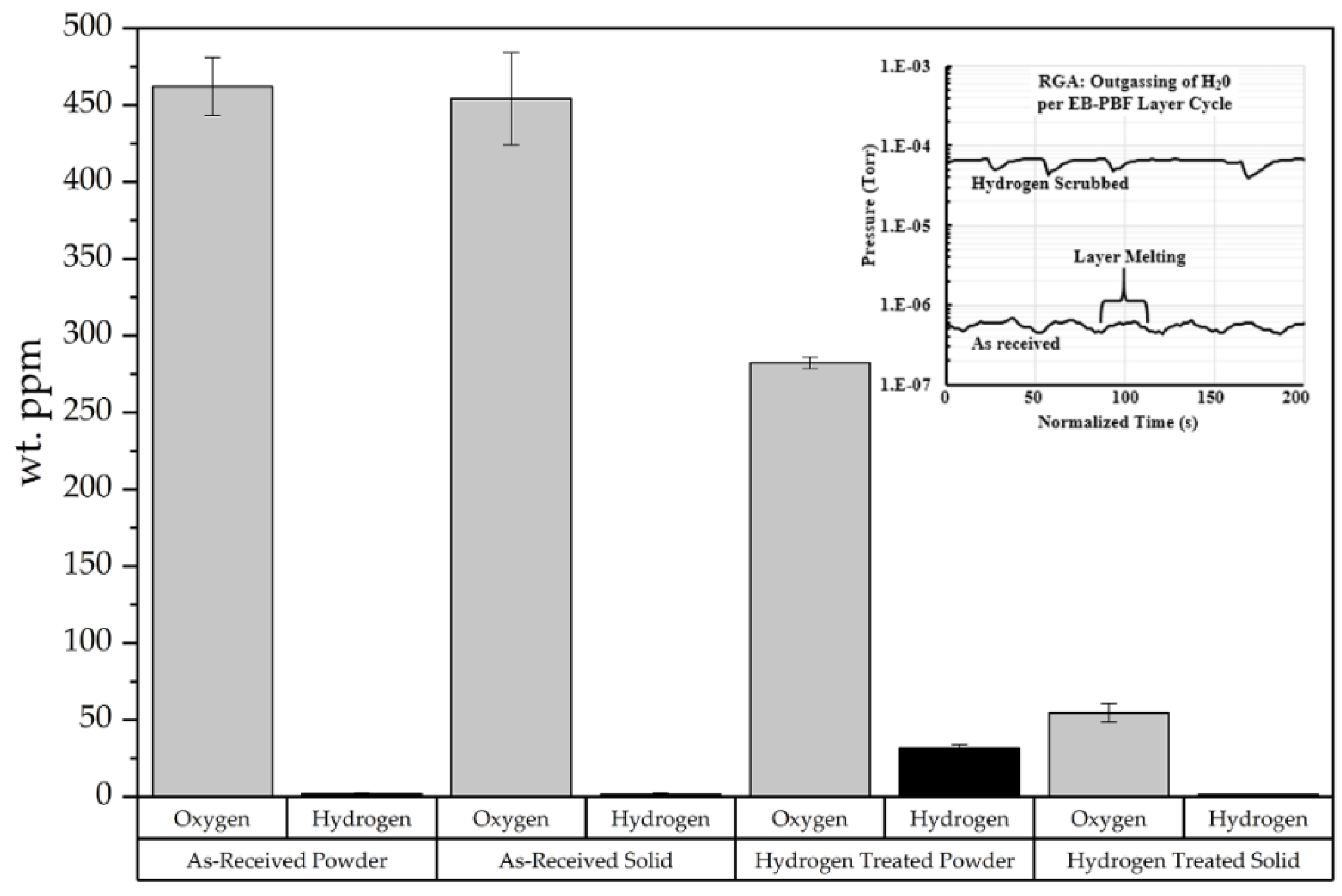
The observations shown here suggest that H
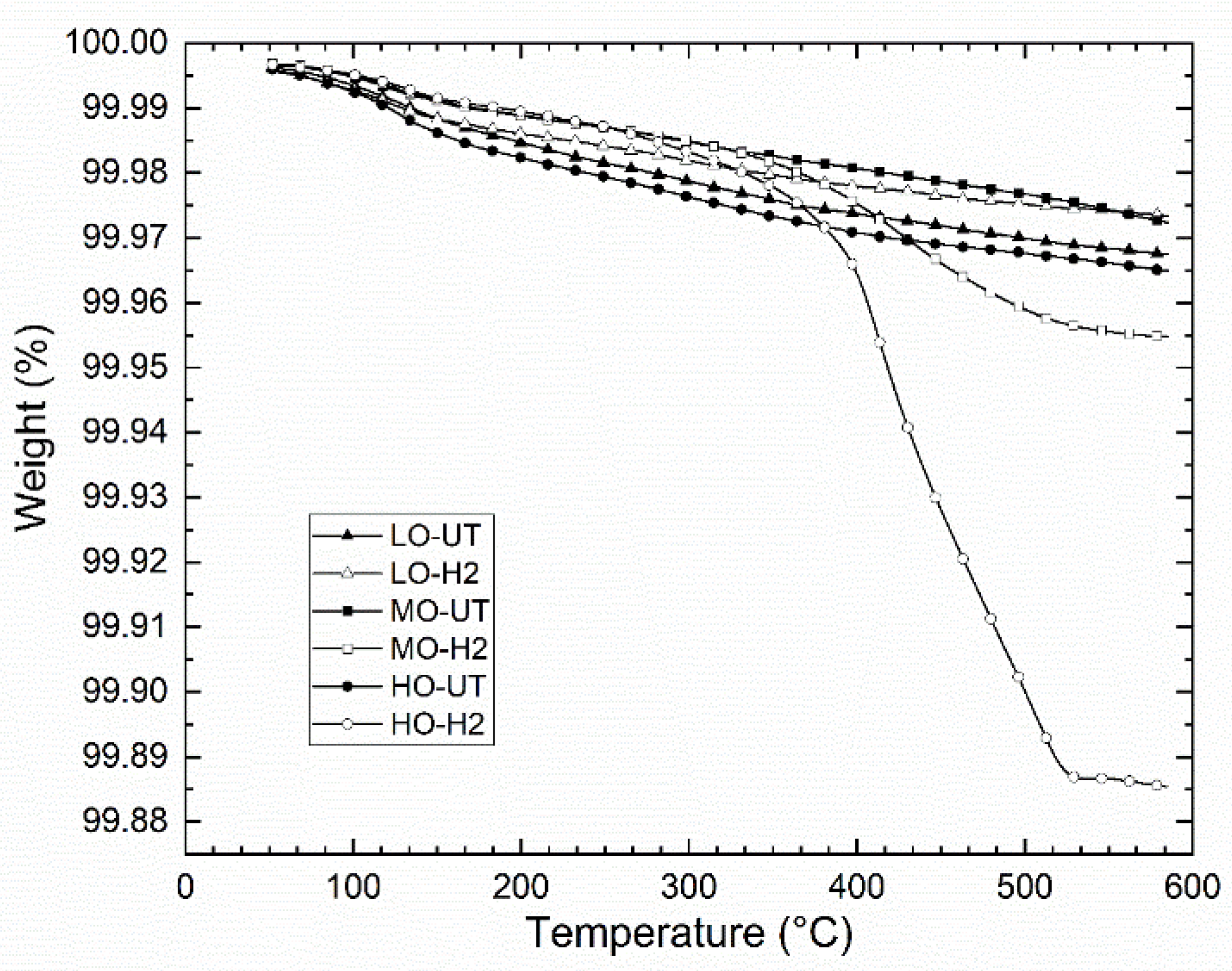
2O is released by two mechanisms during EB-PBF. A chemical analysis of the sintered powder bed shows a similar oxygen and hydrogen content as the starting powder, as seen in
Table 1, and suggests that the H
2O vapor is not released during the preheating step, which is measured in the range of 250–270 °C. While not explored in this study, it is anticipated that higher preheat temperatures, similar to those used by Frigola [
1] and Guschlbauer [
9], would be sufficiently high to elicit the effect observed by the TGA and heated stage SEM experiments. In the present case, the simulation results and experiments of a single electron beam melt line in a copper powder bed show that as the electron beam scans across the sample during the melting step, the highly localized heat in front and to the sides of the beam causes the particles in the heat affected zone to fracture because of the H
2O vapor expansion at 400 °C. The scanning electron microscopy of the particles surrounding the single wafer tracks show fracture surfaces similar to that of
Figure 11C.
The chemical analyses of the single layer melt solid discs of MO-Cu and HO-Cu show that some amount of H
2O vapor is trapped during the solidification of the melt pool, as seen in
Figure 14. We theorize that as the particles become molten, the volume of the H
2O vapor rapidly expands because of the significant pressure drop due to the vacuum environment, and become bubbles of H
2O vapor in the melt pool. According to Boyle’s law, a pressure of 5.7 MPa confined to a sphere with a diameter of 1 um will expand to a sphere with a diameter of 1 mm at a pressure of 5 × e
−9 MPa. This is approximately a 10
9 increase in the volume of the trapped H
2O vapor from within a closed pore. The H
2O vapor bubbles are able to escape the melt pool, which is evident in the decrease in oxygen and hydrogen content of the single melt layer discs. However, because of the rapid solidification of the melt pool (10
−4 to 10
−6 °C/s) and the high viscosity of molten copper, and the large amount of spatter associated with bubble collapse, a portion of these H
2O vapor bubbles may become trapped in the solid single layer discs as pores. An observation of the multiple layer build validation runs suggest that these pores are largely eliminated by the subsequent remelting of the previous layers, which allows repeated opportunities for the H
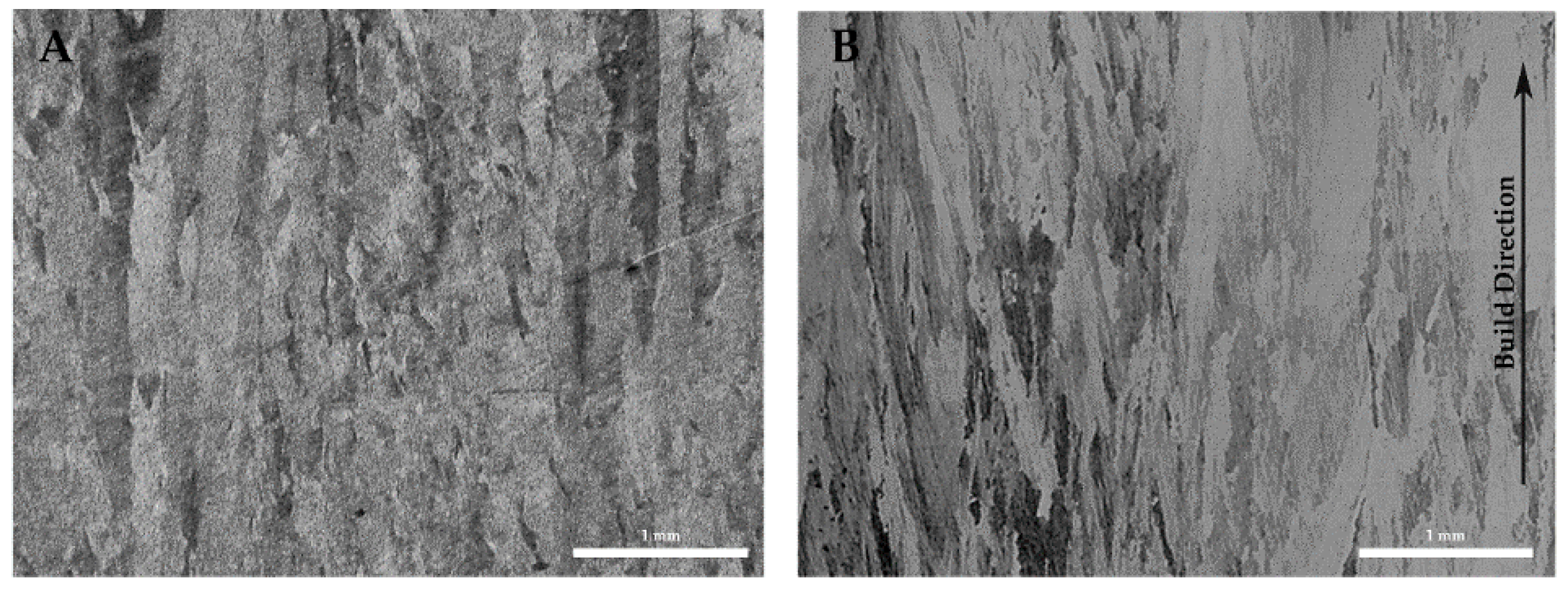
2O vapor to escape to the surface. This is in stark contrast to the traditional copper processing routes (powder metal, sintering, annealing etc.) for which oxygen containing copper powder exposed to a high temperature hydrogen atmosphere results in deleterious embrittlement, cracking, swelling, and porosity. This is clearly shown in the density and microstructures of
Figure 18. Also evident is an apparent difference in grain size. This was not investigated in this study, but it is reasonable that the differences in oxygen content and the turbulent liquid pool/spatter brought about by the escape of water vapor during solidification could lead to the disruption of grain growth observed. Further analyses would be required in order to verify this hypothesis.
While the present study has focused on the EB-PBF processing of copper, it is entirely plausible that a comparable methodology could be repeated for the LPBF processes. However, the poor absorptivity, lower ambient temperatures, and lack of a vacuum atmosphere may contribute to a significantly different outcome. In the work by El-Wardany et al. [
6], a relatively high oxygen copper powder was heat treated in a forming gas environment in order to remove the surface oxides and subsequently be coated with a polydimethylsiloxane (PDMS) polymer. No further data on the chemistry, properties, or microstructure of this material were reported, other than the observation of the formation of unexpectedly large pores in single track welds that were not present in the untreated powders. These results, and the results of the current study suggest that further research is warranted, which also accounts for the potentially important effects of chamber atmosphere and the partial pressures of the relevant gas species during the AM processing of copper.