Upper Limb Robotics in Rehabilitation: An Approach to Select the Devices, Based on Rehabilitation Aims, and Their Evaluation in a Feasibility Study
Abstract
Featured Application
Abstract
1. Introduction
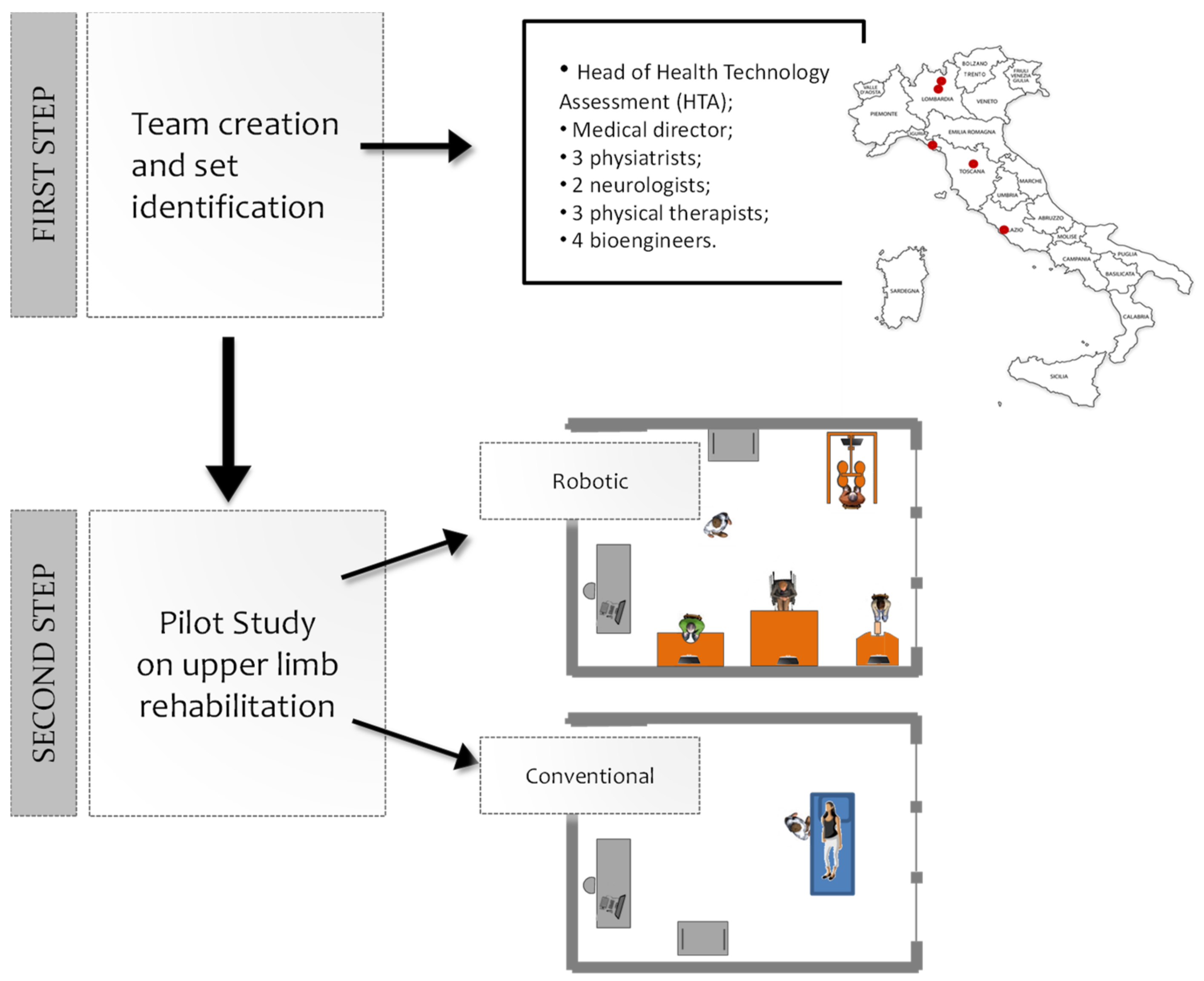
2. Materials and Methods
2.1. The Robotic Device Set’s Identification
2.2. Feasibility Pilot Study
2.2.1. Sample
2.2.2. Clinical Evaluation and Instrumental Assessment
2.2.3. Usability of the Set
2.2.4. Rehabilitation Treatments
2.3. Statistical Analysis
3. Results
3.1. Characteristics of the Set of Robotic Devices
- A robotic device that allows passive, active, and active-assistive planar movements of the shoulder and elbow joints;
- A robotic device that allows passive, active, and active-assistive finger flexion and extension movements;
- A sensorized technological system that allows unassisted three-dimensional movements of the shoulder, elbow, and wrist joints, both unimanual and bimanual;
- An electro-mechanical system that allows three-dimensional, unimanual, and bimanual, movements of the shoulder joint with gravity compensation.
3.2. Feasibility Pilot Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Truelsen, T.; Piechowski-Jóźwiak, B.; Bonita, R.; Mathers, C.; Bogousslavsky, J.; Boysen, G. Stroke incidence and prevalence in Europe: A review of available data. Eur. J. Neurol. 2006, 13, 581–598. [Google Scholar] [CrossRef] [PubMed]
- Gowland, C. Recovery of motor function following stroke: Profile and predictors. Physiother. Can. 1982, 34, 77–84. [Google Scholar] [CrossRef]
- Kwakkel, G.; Wagenaar, R.C.; Kollen, B.J.; Lankhorst, G.J. Predicting disability in stroke—A critical review of the literature. Age Ageing 1996, 25, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Norouzi-Gheidari, N.; Archambault, P.S.; Fung, J. Effects of robot-assisted therapy on stroke rehabilitation in upper limbs: Systematic review and meta-analysis of the literature. J. Rehabil. Res. Dev. 2012, 49, 479–496. [Google Scholar] [CrossRef] [PubMed]
- Pollock, A.; Farmer, S.E.; Brady, M.C.; Langhorne, P.; Mead, G.E.; Mehrholz, J.; van Wijck, F. Interventions for improving upper limb function after stroke. Cochrane Database Syst. Rev. 2014, 11, CD010820. [Google Scholar] [CrossRef] [PubMed]
- Mehrholz, J.; Pohl, M.; Platz, T.; Kugler, J.; Elsner, B. Electromechanical and robot-assisted arm training for improving activities of daily living, arm function, and arm muscle strength after stroke. Cochrane Database Syst. Rev. 2018, 9, CD006876. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, R.S.; Naro, A.; Russo, M.; Bramanti, P.; Carioti, L.; Balletta, T.; Buda, A.; Manuli, A.; Filoni, S.; Bramanti, A. Shaping neuroplasticity by using powered exoskeletons in patients with stroke: A randomized clinical trial. J. Neuroeng. Rehabil. 2018, 15, 35. [Google Scholar] [CrossRef]
- Calabrò, R.S.; Russo, M.; Naro, A.; Milardi, D.; Balletta, T.; Leo, A.; Filoni, S.; Bramanti, P. Who May Benefit From Armeo Power Treatment? A Neurophysiological Approach to Predict Neurorehabilitation Outcomes. PM&R 2016, 8, 971–978. [Google Scholar]
- Calabrò, R.S.; Cacciola, A.; Bertè, F.; Manuli, A.; Leo, A.; Bramanti, A.; Naro, A.; Milardi, D.; Bramanti, P. Robotic gait rehabilitation and substitution devices in neurological disorders: Where are we now? Neurol. Sci. 2016, 37, 503–514. [Google Scholar] [CrossRef]
- Molteni, F.; Gasperini, G.; Cannaviello, G.; Guanziroli, E. Exoskeleton and End-Effector Robots for Upper and Lower Limbs Rehabilitation: Narrative Review. PM&R 2018, 10, S174–S188. [Google Scholar]
- Fugl-Meyer, A.R.; Jääskö, L.; Leyman, I.; Olsson, S.; Steglind, S. The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand. J. Rehabil. Med. 1975, 7, 13–31. [Google Scholar] [PubMed]
- Demeurisse, G.; Demol, O.; Robaye, E. Motor evaluation in vascular hemiplegia. Eur. Neurol. 1980, 19, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, R.W.; Smith, M.B. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys. Ther. 1987, 67, 206–207. [Google Scholar] [CrossRef] [PubMed]
- Aprile, I.; Tonali, P.; Caliandro, P.; Pazzaglia, C.; Foschini, M.; Di Stasio, E.; Mondelli, M.; Padua, L.; Italian CTS and other entrapments Study Group. Italian multicentre study of peroneal mononeuropathy: Multiperspective follow-up. Neurol. Sci. 2009, 30, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Vanclay, F.; Cooper, B. Improving the sensitivity of the Barthel Index for stroke rehabilitation. J. Clin. Epidemiol. 1989, 42, 703–709. [Google Scholar] [CrossRef]
- Dixon, J.S.; Bird, H.A. Reproducibility along a 10 cm vertical visual analogue scale. Annu. Rheum. Dis. 1981, 40, 87–89. [Google Scholar] [CrossRef] [PubMed]
- Brooke, J. SUS-A quick and dirty usability scale. Usability Eval. Ind. 1996, 189, 4–7. [Google Scholar]
- Radder, B.; Prange-Lasonder, G.B.; Kottink, A.I.R.; Holmberg, J.; Sletta, K.; van Dijk, M.; Meyer, T.; Melendez-Calderon, A.; Buurke, J.H.; Rietman, J.S. Home rehabilitation supported by a wearable soft-robotic device for improving hand function in older adults: A pilot randomized controlled trial. PLoS ONE 2019, 14, e0220544. [Google Scholar] [CrossRef]
- Aprile, I.; Germanotta, M.; Cruciani, A.; Loreti, S.; Pecchioli, C.; Cecchi, F.; Montesano, A.; Galeri, S.; Diverio, M.; Falsini, C.; et al. Upper Limb Robotic Rehabilitation after Stroke: A Multicenter, Randomized Clinical Trial. J. Neurol. Phys. Ther. (in press).
- Maciejasz, P.; Eschweiler, J.; Gerlach-Hahn, K.; Jansen-Troy, A.; Leonhardt, S. A survey on robotic devices for upper limb rehabilitation. J. Neuroeng. Rehabil. 2014, 11, 3. [Google Scholar] [CrossRef]
- Jakob, I.; Kollreider, A.; Germanotta, M.; Benetti, F.; Cruciani, A.; Luca, P.; Aprile, I. Robotic and Sensor Technology for Upper Limb Rehabilitation. PM&R 2018, 10, S189–S197. [Google Scholar]
- Langhorne, P.; Coupar, F.; Pollock, A. Motor recovery after stroke: A systematic review. Lancet Neurol. 2009, 8, 741–754. [Google Scholar] [CrossRef]
- Kaleshtari, M.H.; Ciobanu, I.; Seiciu, P.L.; Marin, A.G.; Berteanu, M. Towards a Model of Rehabilitation Technology Acceptance and Usability. Int. J. Soc. Sci. Humanit. 2016, 6, 612. [Google Scholar] [CrossRef]
- Langhorne, P.; Sandercock, P.; Prasad, K. Evidence-based practice for stroke. Lancet Neurol. 2009, 8, 308–309. [Google Scholar] [CrossRef]
- Bertani, R.; Melegari, C.; De Cola, M.C.; Bramanti, A.; Bramanti, P.; Calabrò, R.S. Effects of robot-assisted upper limb rehabilitation in stroke patients: A systematic review with meta-analysis. Neurol. Sci. 2017, 38, 1561–1569. [Google Scholar] [CrossRef]
- Buschfort, R.; Brocke, J.; Heß, A.; Werner, C.; Waldner, A.; Hesse, S. The arm studio to intensify the upper limb rehabilitation after stroke: Concept, acceptance, utilization and preliminary clinical results. J. Rehabil. Med. 2010, 42, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Hesse, S.; Heß, A.; Werner, C.C.; Kabbert, N.; Buschfort, R. Effect on arm function and cost of robot-assisted group therapy in subacute patients with stroke and a moderately to severely affected arm: A randomized controlled trial. Clin. Rehabil. 2014, 28, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Bustamante Valles, K.; Montes, S.; de Jesus Madrigal, M.; Burciaga, A.; Martínez, M.E.; Johnson, M.J. Technology-assisted stroke rehabilitation in Mexico: A pilot randomized trial comparing traditional therapy to circuit training in a Robot/technology-assisted therapy gym. J. Neuroeng. Rehabil. 2016, 13, 83. [Google Scholar] [CrossRef] [PubMed]
- Wagner, T.H.; Lo, A.C.; Peduzzi, P.; Bravata, D.M.; Huang, G.D.; Krebs, H.I.; Ringer, R.J.; Federman, D.G.; Richards, L.G.; Haselkorn, J.K.; et al. An economic analysis of robot-assisted therapy for long-term upper-limb impairment after stroke. Stroke 2011, 42, 2630–2632. [Google Scholar] [CrossRef]
| Commercial Name | Type of System | Treated Segment(s) | Type of Movement | Description and Assisted Movements | Certification | Evaluation Period | Price Range |
|---|---|---|---|---|---|---|---|
| Gloreha—Idrogenet srl., Lumezzane (BS), Italy | Exoskeleton | Hand | Spatial | Actuated glove for fingers flexion-extension | Medical Device | June–July 2015 | Low |
| Armeo Power—Hocoma AG, Volketswil, Switzerland | Exoskeleton | Upper limb and Hand (grip only) | Spatial | Actuated exoskeleton with 6 degrees of freedom for: - shoulder flex/ext, horizontal abduction/adduction, and internal/external rotation; - elbow flex/ext - forearm prono/supination; - wrist flex/ext | Medical Device | June 2015 | High |
| Amadeo—Tyromotion GmbH, Graz, Austria | End-effector | Hand | Linear | Device with 5 degrees of freedom. Sliders to be attached to fingertips for fingers flexion-extension | Medical Device | June 2015 | Medium |
| Motore—Humanware srl, Pisa, Italy | End-effector | Upper limb | Planar | 2 Degrees of freedom robot with a handgrip moving on wheels on top of a desk for shoulder horizontal abduction/adduction, and elbow flex/ext | Medical Device | January–June 2015 | Medium |
| Physioassistant “braccio di ferro”—Celin srl, Follo (SP), Italy | End-effector | Upper limb | Planar | 2 Degrees of freedom robotic arm with handgrip for shoulder horizontal abduction/adduction, and elbow flex/ext | Prototype (not yet certified as Medical Device) | June 2015 | Price not available (prototype) |
| ReoGo—Motorika Inc., Mount Laurel (NJ), USA | End-effector | Upper limb | Spatial | Robotic arm with 6 degrees of freedom, with forearm-hand support allowing movements of elbow and shoulder. | Medical Device | June 2015 | Medium |
| Diego—Tyromotion GmbH, Graz, Austria | Electro-mechanical unweighting system | Upper limb | Spatial | Motorized slings to be applied at elbow and wrist level for upper limb unweighting. Allows movements of shoulder, elbow and wrist | Medical Device | June 2015 | Medium |
| Armeo Spring—Hocoma AG, Volketswil, Switzerland | Spring based unweighting system | Upper limb | Spatial | Spring based exoskeleton with six degrees of freedomfor upper limb unweighting. Allows movements of shoulder and elbow | Medical Device | June 2015 | Medium |
| Pablo—Tyromotion GmbH, Graz, Austria | Sensorized technological system | Upper limb | Spatial | Sensorized handgip (inertial measurement unit) able to record the hand movements in the space. No assistance to movement provided. | Medical Device | June 2015 | Low |
| Ultra—Humanware srl, Pisa, Italy | Sensorized technological system | Upper limb | Spatial | Articulated arm with seven degrees of freedom able to track position and speed of the hand during movements in 3D space | Medical Device | June 2015 | Low |
| Item in the Evaluation Form | Transformation of Text Based Values into Quantitative Values | |
|---|---|---|
| Product maturity | Prototype | 0 |
| Commercial product | 1 | |
| Provides outcome measures | Yes | 1 |
| No | 0 | |
| Empty | 0 | |
| Provides normative values | Yes | 1 |
| No | 0 | |
| Empty | 0 | |
| Possible safety issues | Yes | 0 |
| No | 1 | |
| Empty | 1 | |
| Contraindications | Yes | 0 |
| No | 1 | |
| Empty | 1 | |
| Literature supporting efficacy | Not-published data | 0 |
| Conference proceedings/not peer reviewed journals | 1 | |
| Scientific paper on peer reviewed journals | 2 | |
| Empty | 0 | |
| Purchase priority according to compiler | Low | 1 |
| Low-medium | 1.5 | |
| Medium | 2 | |
| Medium-high | 2.5 | |
| High | 3 | |
| Confidence level of evaluator | Low | 0.5 |
| Low-medium | 0.6 | |
| Medium | 0.7 | |
| Medium-high | 0.8 | |
| High | 1 | |
| Maximum level of impairment | Low | 1 |
| Medium | 2 | |
| High | 3 | |
| Customizable exercises | Yes | 1.5 |
| No | 0.5 | |
| Empty | 0.5 | |
| Autonomous use by the patient | The patient can use the device in autonomy | 1 |
| The patient can use the device under physiotherapist supervision | 1 | |
| The physiotherapist must control continuously the patient’s robot training | 0 | |
| Empty | 0 | |
| Possibility of using the solution in group therapies | Yes | 1 |
| No | 0 | |
| Empty | 0 | |
| Number of clinicians involved in the treatment | 1 | 1 |
| 2 | 2 | |
| 3 | 3 | |
| Parameter | Weight in the Ranking Formula |
|---|---|
| Provides outcome measures | 3 |
| Provides normative values | 1 |
| Safety issues | 1 |
| Contraindications | 1 |
| Literature supporting efficacy | 3 |
| Purchase priority | 3 |
| Level of impairment | 2 |
| Customizable exercises | 2 |
| Efficiency level | 4 |
| Conventional Group (n = 14) | Robotic Group (n = 16) | p (between Groups) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T0 | T1 | p (within Group) | T0 | T1 | p (within Group) | ||||||
| Median | Range | Median | Range | Median | Range | Median | Range | ||||
| Barthel Index | 51 | (8–84) | 51 | (12–81) | 0.074 | 44 | (8–90) | 54.5 | (21–94) | 0.001 | 0.002 |
| Deambulation Index | 2 | (0–5) | 2 | (0–5) | 1 | 1 | (0–5) | 2 | (0–7) | 0.009 | 0.019 |
| Dynamometer (affected side) | 3 | (0–12) | 8 | (0–10) | 0.113 | 0 | (0–16) | 2 | (0–20) | 0.021 | 0.667 |
| Dynamometer (not affected side) | 14 | (2–38) | 12 | (2–38) | 0.66 | 24 | (4–46) | 25 | (4–50) | 0.918 | 0.918 |
| Pinch Test (affected side) | 2 | (0–3) | 2 | (0–3) | 0.157 | 0 | (0–6) | 0.5 | (0–7) | 0.034 | 0.423 |
| Pinch Test (not affected side) | 3 | (2–7) | 3 | (3–7) | 0.107 | 4.5 | (2–10) | 5 | (3–12) | 0.034 | 0.854 |
| NRS | 5 | (0–10) | 5 | (0–10) | 0.458 | 3.5 | (0–8) | 2.5 | (0–7) | 0.796 | 0.728 |
| Fugl–Meyer | 17 | (2–56) | 23 | (2–56) | 0.063 | 10 | (2–49) | 16 | (2–57) | 0.006 | 0.046 |
| Flexor synergy | 2 | (0–12) | 4 | (0–12) | 0.063 | 3 | (0–12) | 5.5 | (0–12) | 0.070 | 0.400 |
| Extensor synergy | 2 | (0–6) | 2 | (0–6) | 0.157 | 1.5 | (0–6) | 2.5 | (0–6) | 0.457 | 0.697 |
| Volitional movement mixing synergies | 1 | (0–6) | 3 | (0–6) | 0.157 | 0.5 | (0–6) | 1.5 | (0–6) | 0.034 | 0.355 |
| Volitional movement with little or no synergy | 1 | (0–5) | 2 | (0–5) | 0.157 | 1 | (0–5) | 1.5 | (0–6) | 0.149 | 0.473 |
| Wrist | 2 | (0–9) | 3 | (0–9) | 0.157 | 0 | (0–7) | 1 | (0–10) | 0.141 | 0.498 |
| Hand | 7 | (0–14) | 7 | (0–14) | 0.157 | 2 | (0–14) | 4.5 | (0–13) | 0.065 | 0.046 |
| Coordination/speed | 2 | (2–5) | 2 | (2–5) | 1 | 2 | (0–5) | 2 | (0–6) | 0.131 | 0.400 |
| Ashworth | |||||||||||
| Shoulder | 2 | (0–3) | 1 | (0–2) | 0.046 | 0 | (0–2) | 0.5 | (0–2) | 0.317 | 0.101 |
| Elbow | 2 | (0–3) | 1 | (0–3) | 0.046 | 1 | (0–2) | 0 | (0–3) | 0.058 | 0.79 |
| Wrist | 2 | (0–3) | 1 | (0–3) | 0.046 | 1 | (0–3) | 0 | (0–3) | 0.915 | 0.637 |
| Motricity | |||||||||||
| Shoulder | 14 | (0–33) | 19 | (0–33) | 0.157 | 14 | (9–25) | 14 | (0–25) | 1 | 0.790 |
| Elbow | 19 | (9–33) | 19 | (9–33) | 0.157 | 9 | (0–25) | 14 | (0–25) | 0.034 | 0.423 |
| Hand | 19 | (0–33) | 19 | (0–33) | 1 | 11 | (0–26) | 19 | (0–26) | 0.066 | 0.257 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aprile, I.; Cruciani, A.; Germanotta, M.; Gower, V.; Pecchioli, C.; Cattaneo, D.; Vannetti, F.; Padua, L.; Gramatica, F. Upper Limb Robotics in Rehabilitation: An Approach to Select the Devices, Based on Rehabilitation Aims, and Their Evaluation in a Feasibility Study. Appl. Sci. 2019, 9, 3920. https://doi.org/10.3390/app9183920
Aprile I, Cruciani A, Germanotta M, Gower V, Pecchioli C, Cattaneo D, Vannetti F, Padua L, Gramatica F. Upper Limb Robotics in Rehabilitation: An Approach to Select the Devices, Based on Rehabilitation Aims, and Their Evaluation in a Feasibility Study. Applied Sciences. 2019; 9(18):3920. https://doi.org/10.3390/app9183920
Chicago/Turabian StyleAprile, Irene, Arianna Cruciani, Marco Germanotta, Valerio Gower, Cristiano Pecchioli, Davide Cattaneo, Federica Vannetti, Luca Padua, and Furio Gramatica. 2019. "Upper Limb Robotics in Rehabilitation: An Approach to Select the Devices, Based on Rehabilitation Aims, and Their Evaluation in a Feasibility Study" Applied Sciences 9, no. 18: 3920. https://doi.org/10.3390/app9183920
APA StyleAprile, I., Cruciani, A., Germanotta, M., Gower, V., Pecchioli, C., Cattaneo, D., Vannetti, F., Padua, L., & Gramatica, F. (2019). Upper Limb Robotics in Rehabilitation: An Approach to Select the Devices, Based on Rehabilitation Aims, and Their Evaluation in a Feasibility Study. Applied Sciences, 9(18), 3920. https://doi.org/10.3390/app9183920








