Investigation of the Broadband Microwave Absorption of Citric Acid Coated Fe3O4/PVDF Composite Using Finite Element Method
Abstract
:1. Introduction
2. Methodology
2.1. Synthesis of C-Fe3O4 Nanoparticles
2.2. Preparation of C-Fe3O4/PVDF Nanocomposites
2.3. Electromagnetic Properties Measurement
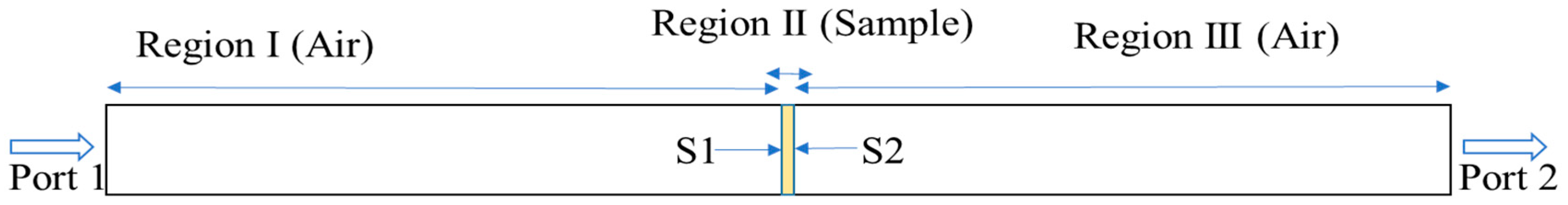
2.4. The Finite Element Method
2.5. Characterizations
3. Results and Discussion
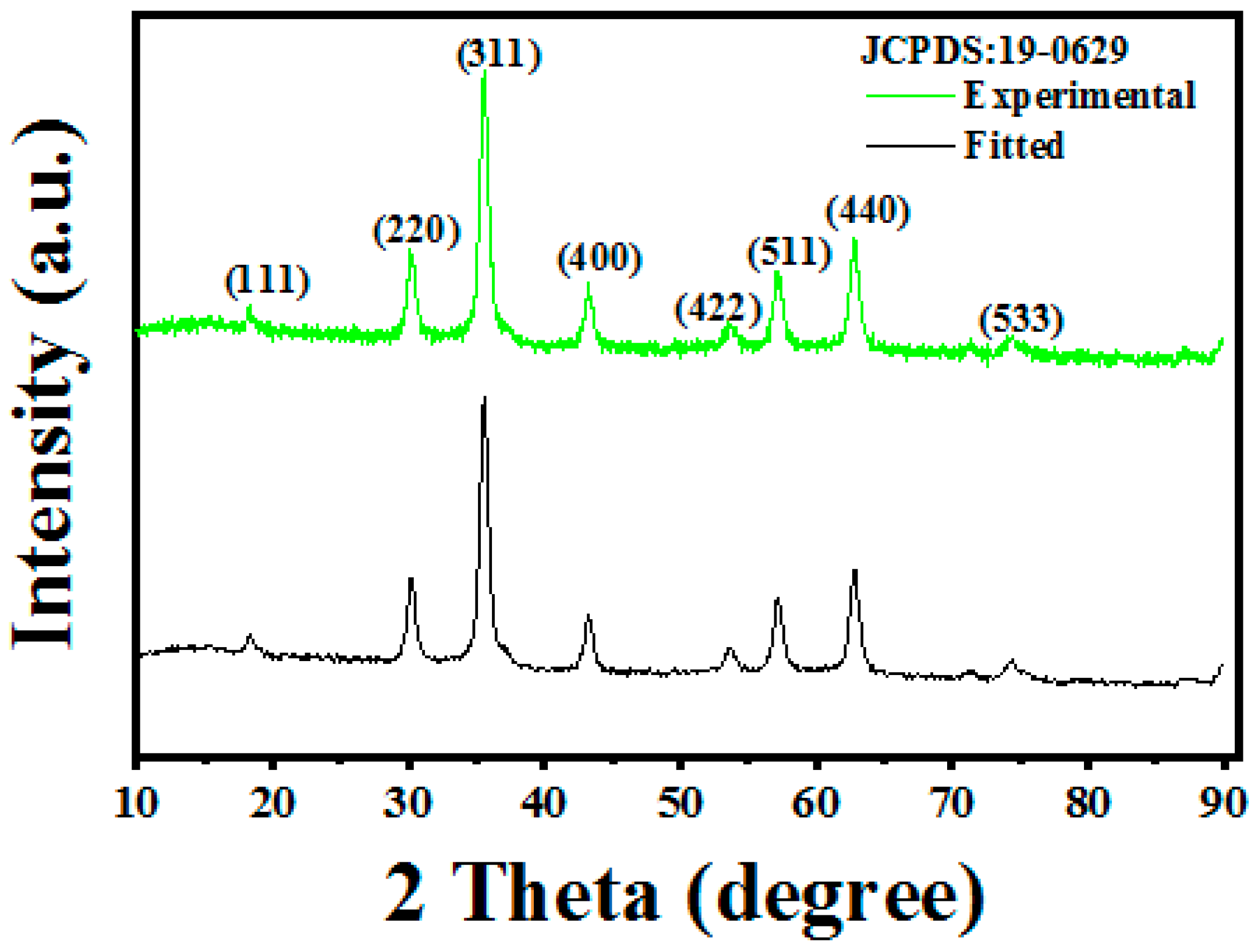
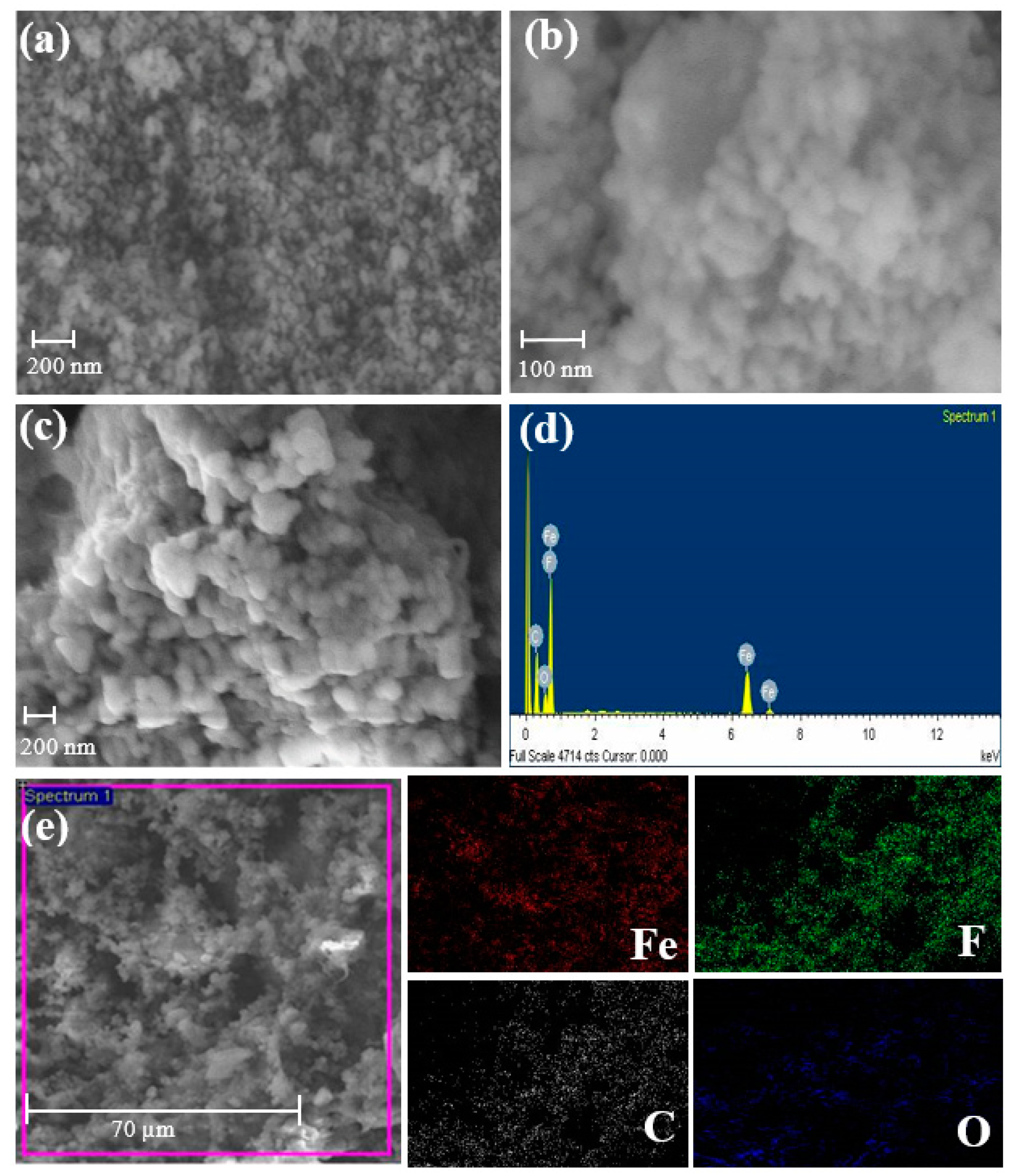
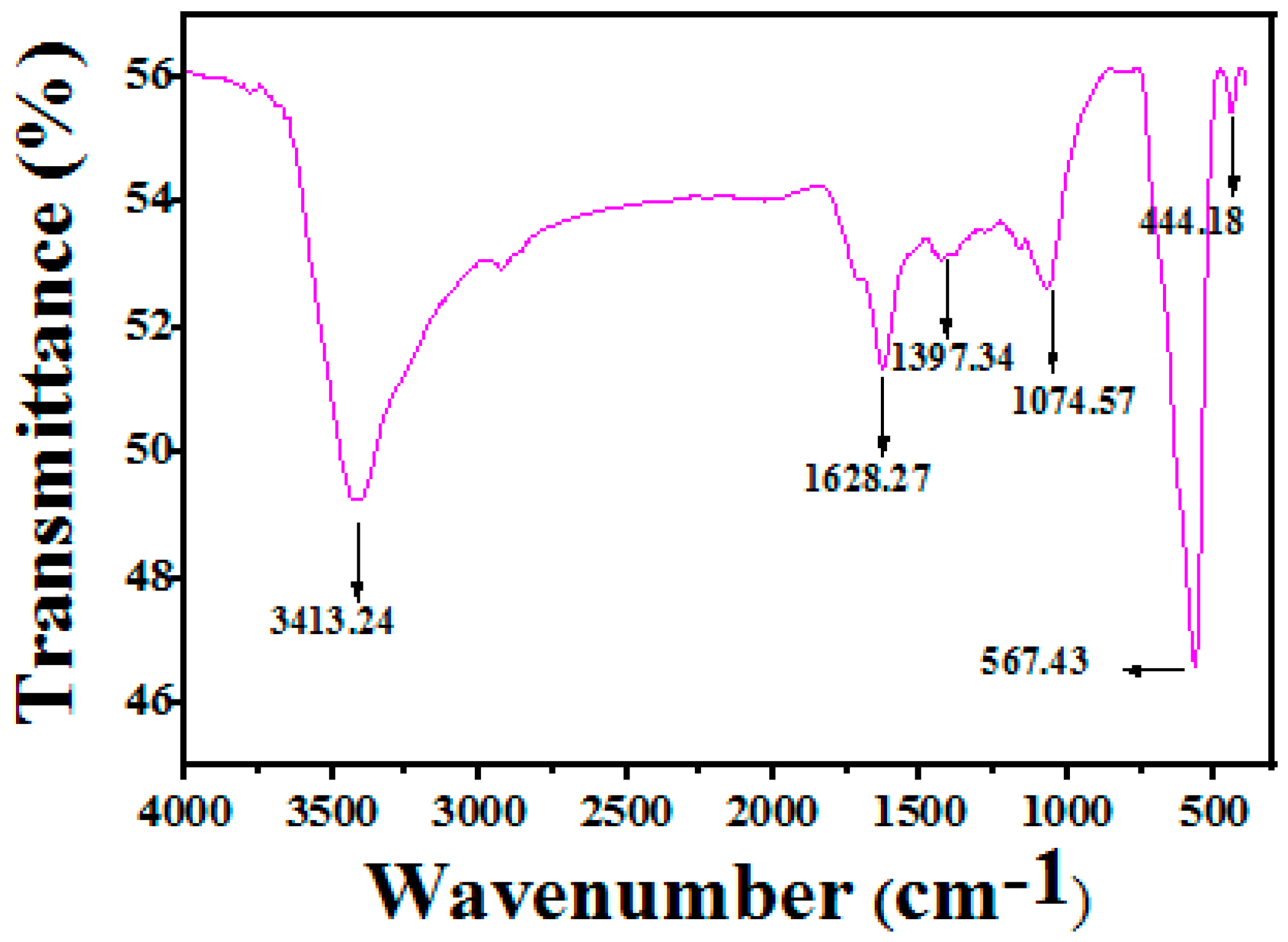
3.1. Structure and Morphology Analysis
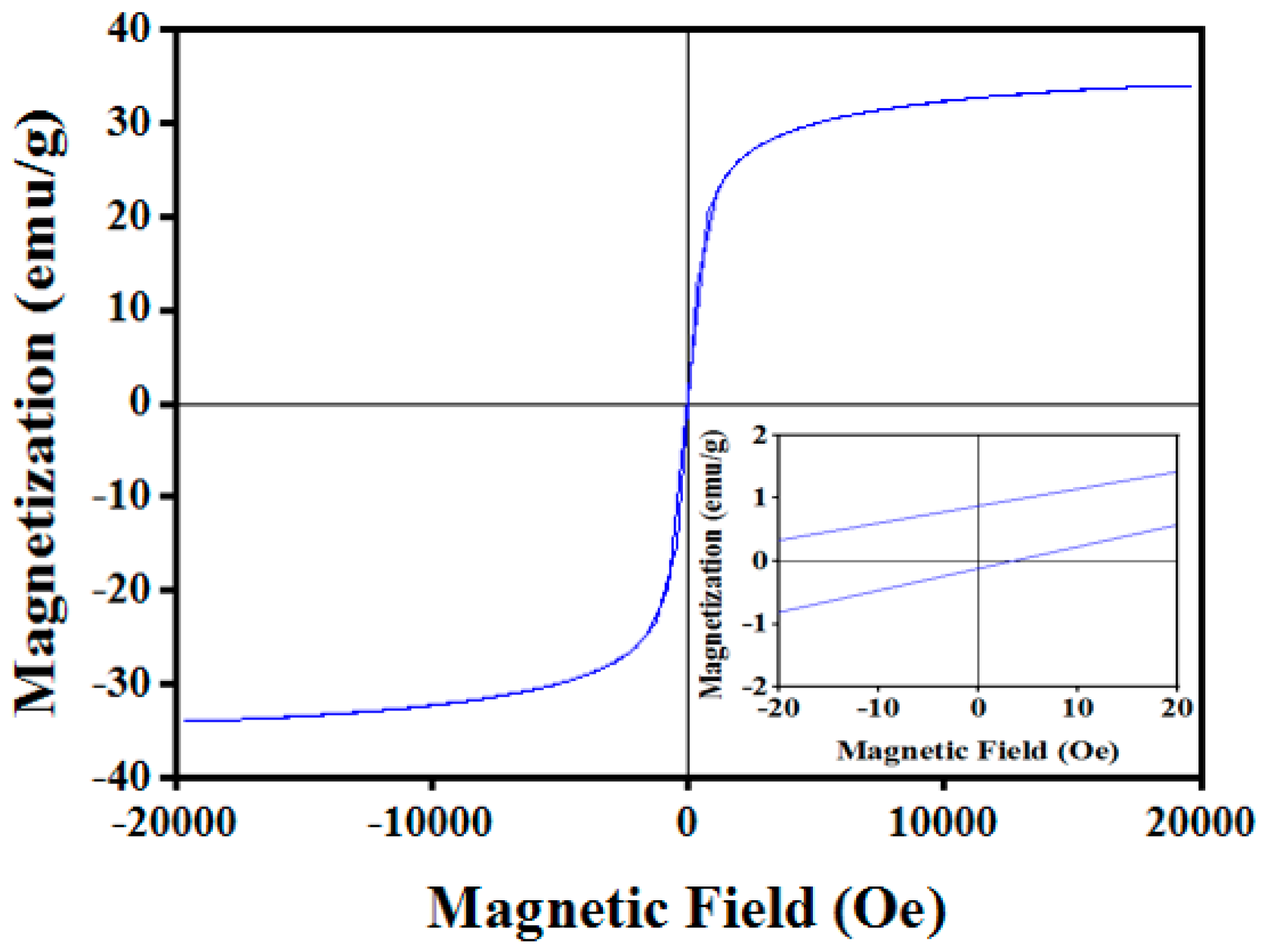
3.2. Magnetic Properties of C-Fe3O4
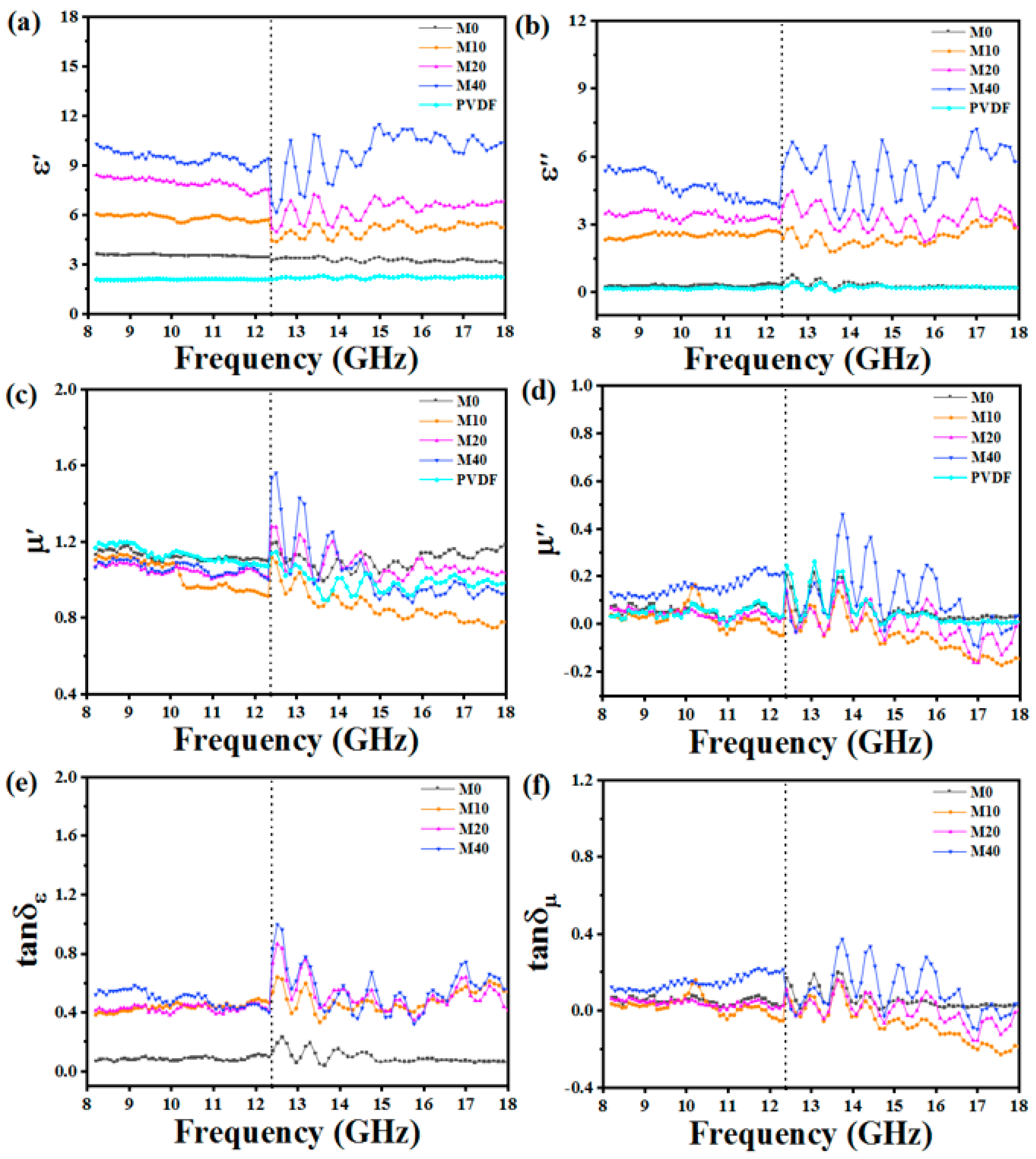
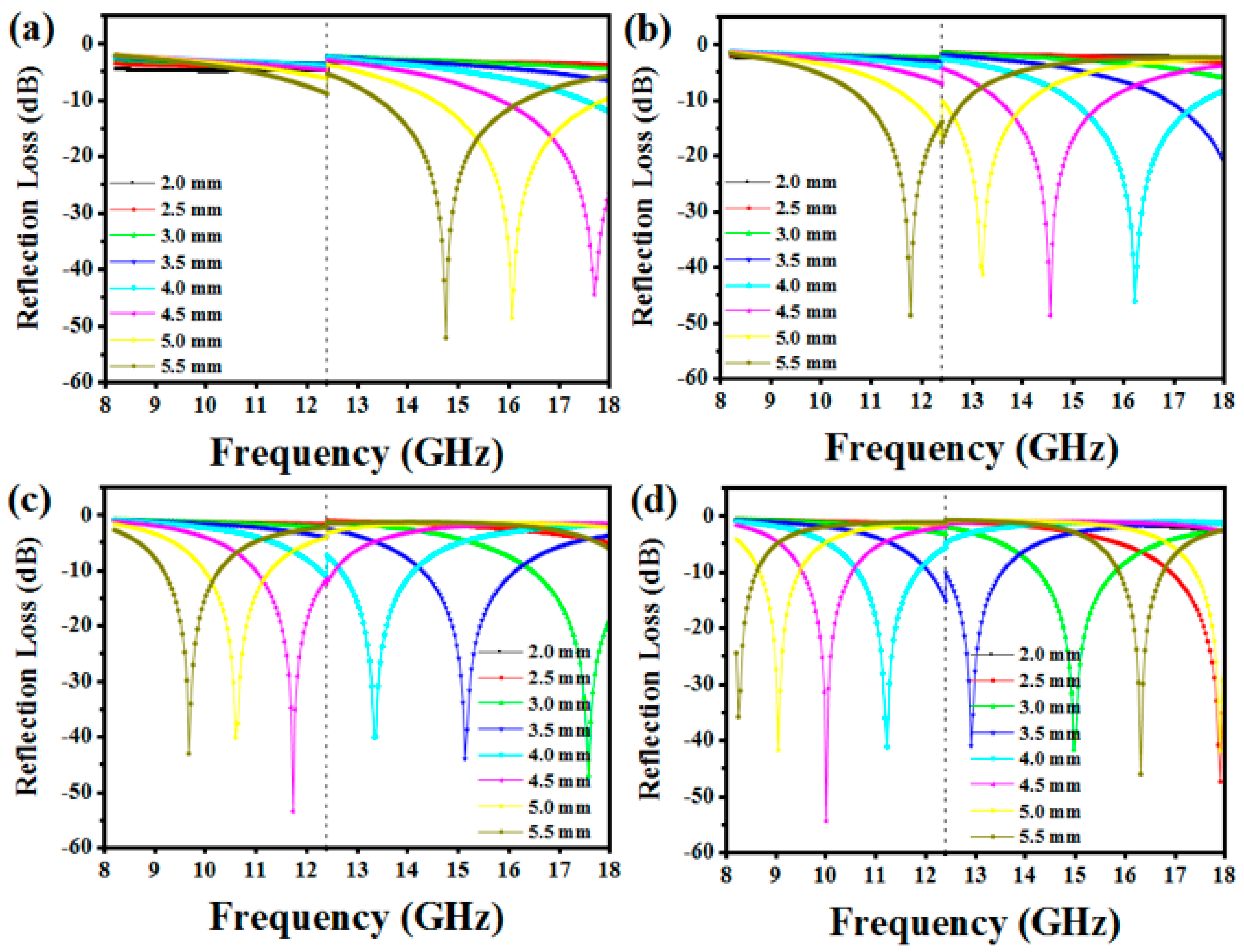
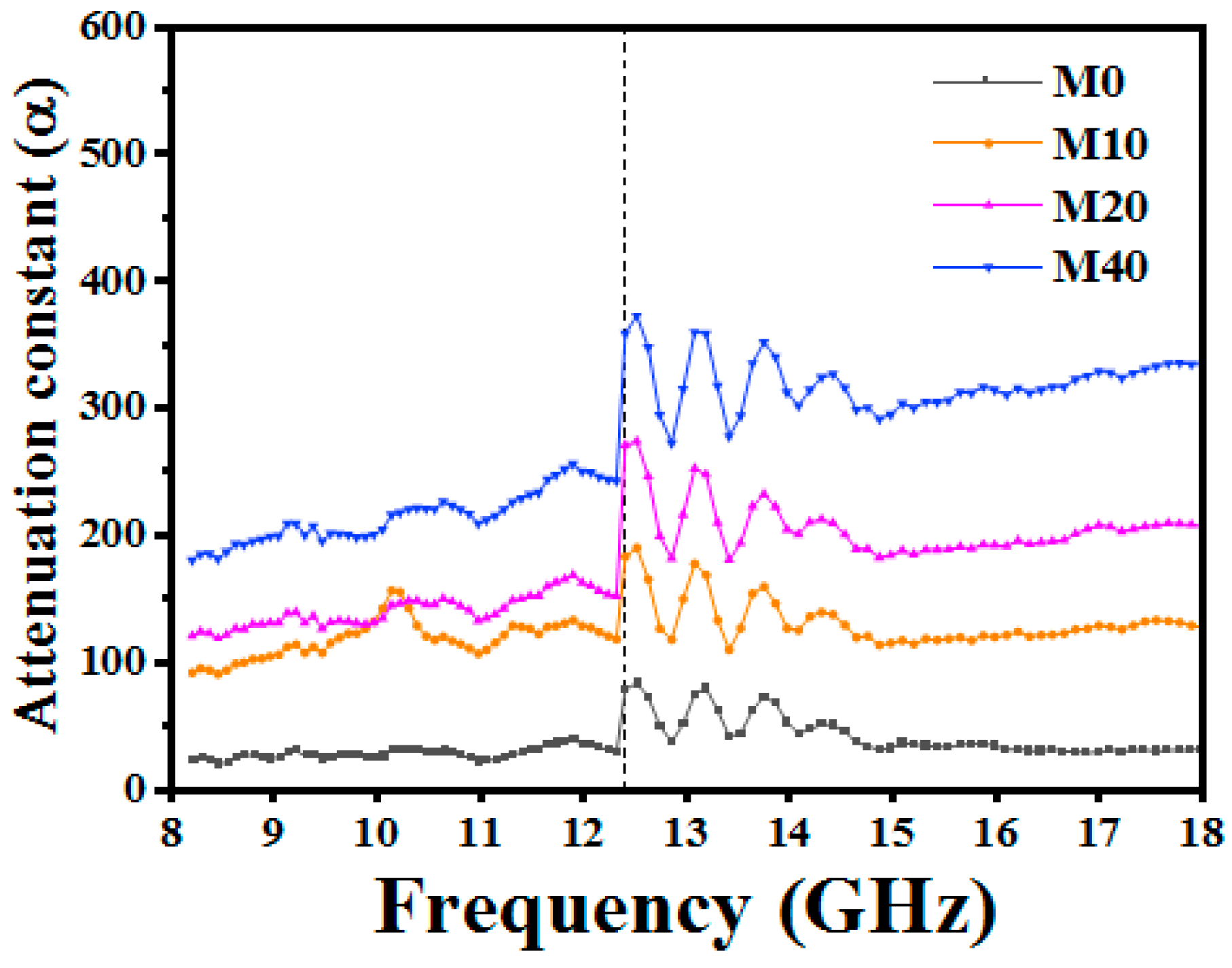
3.3. EM Wave Absorption Properties
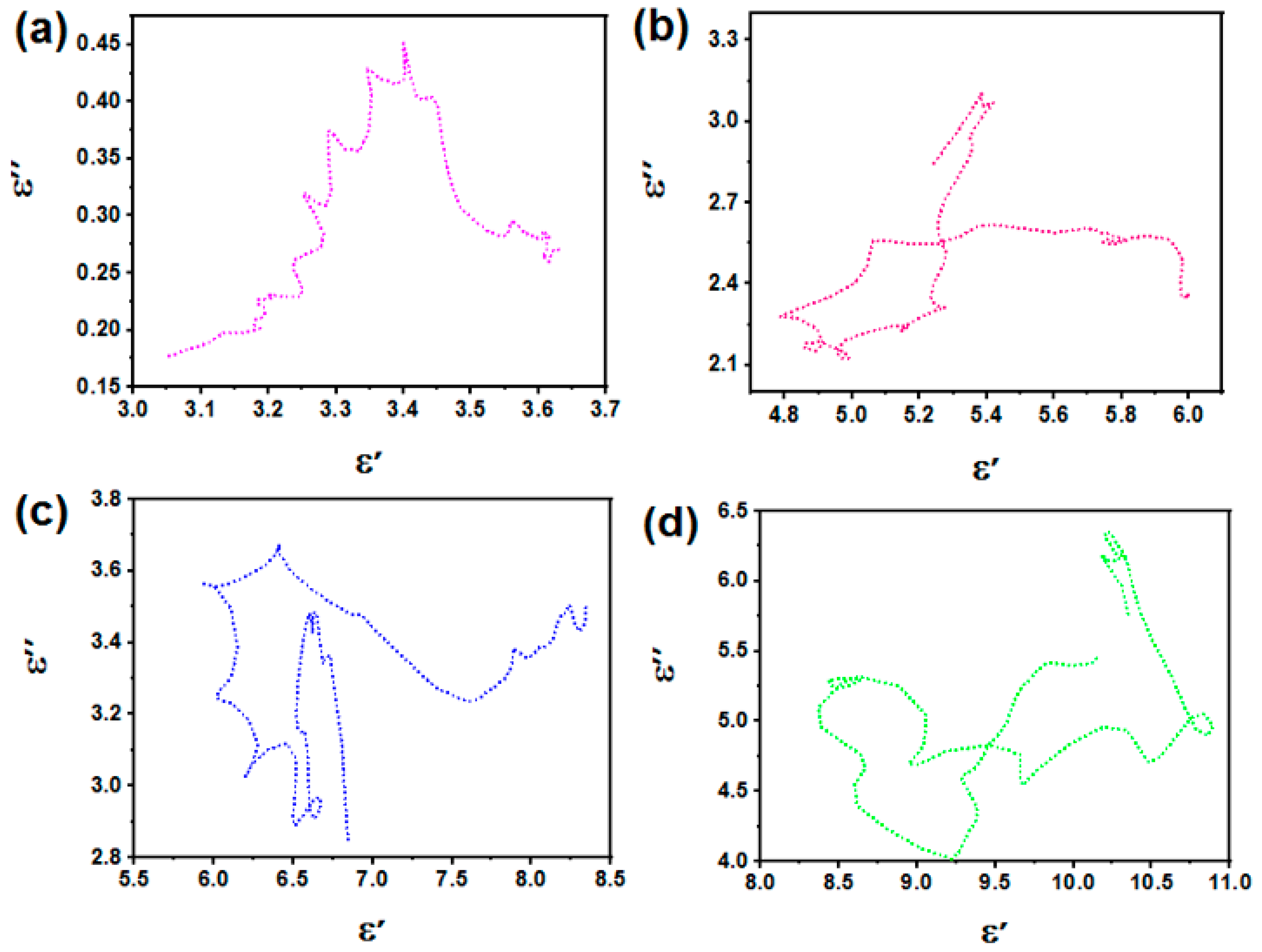
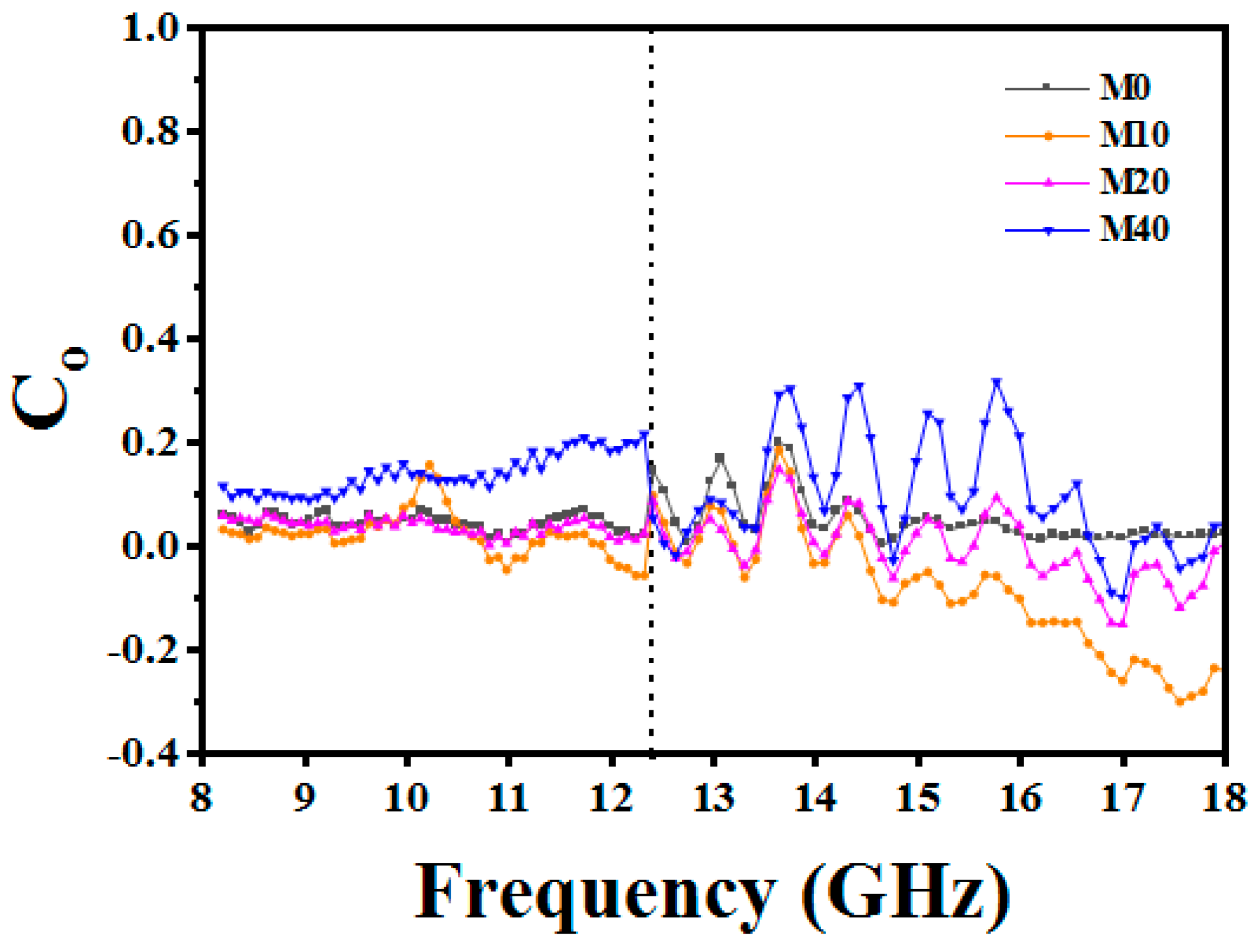
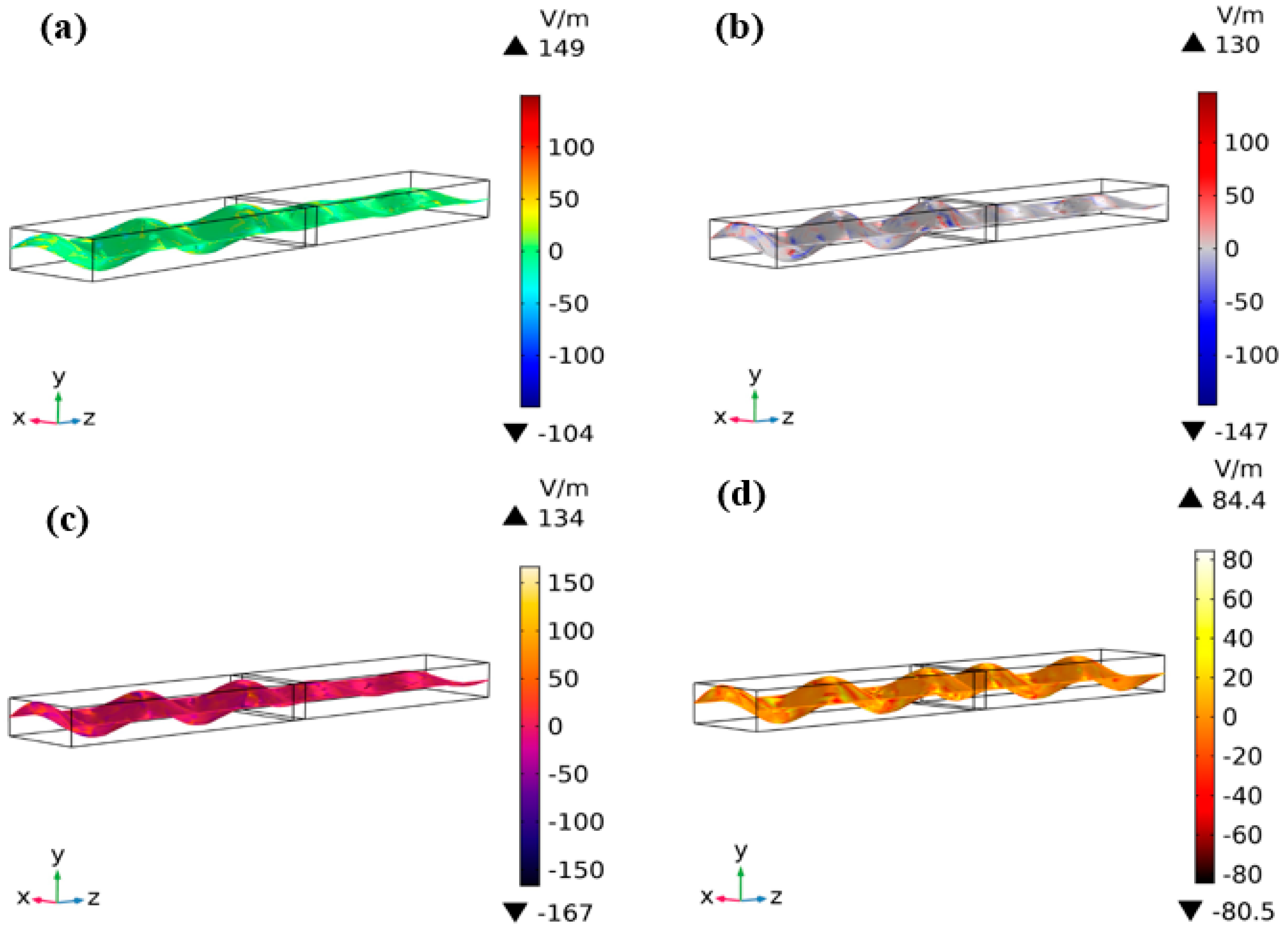
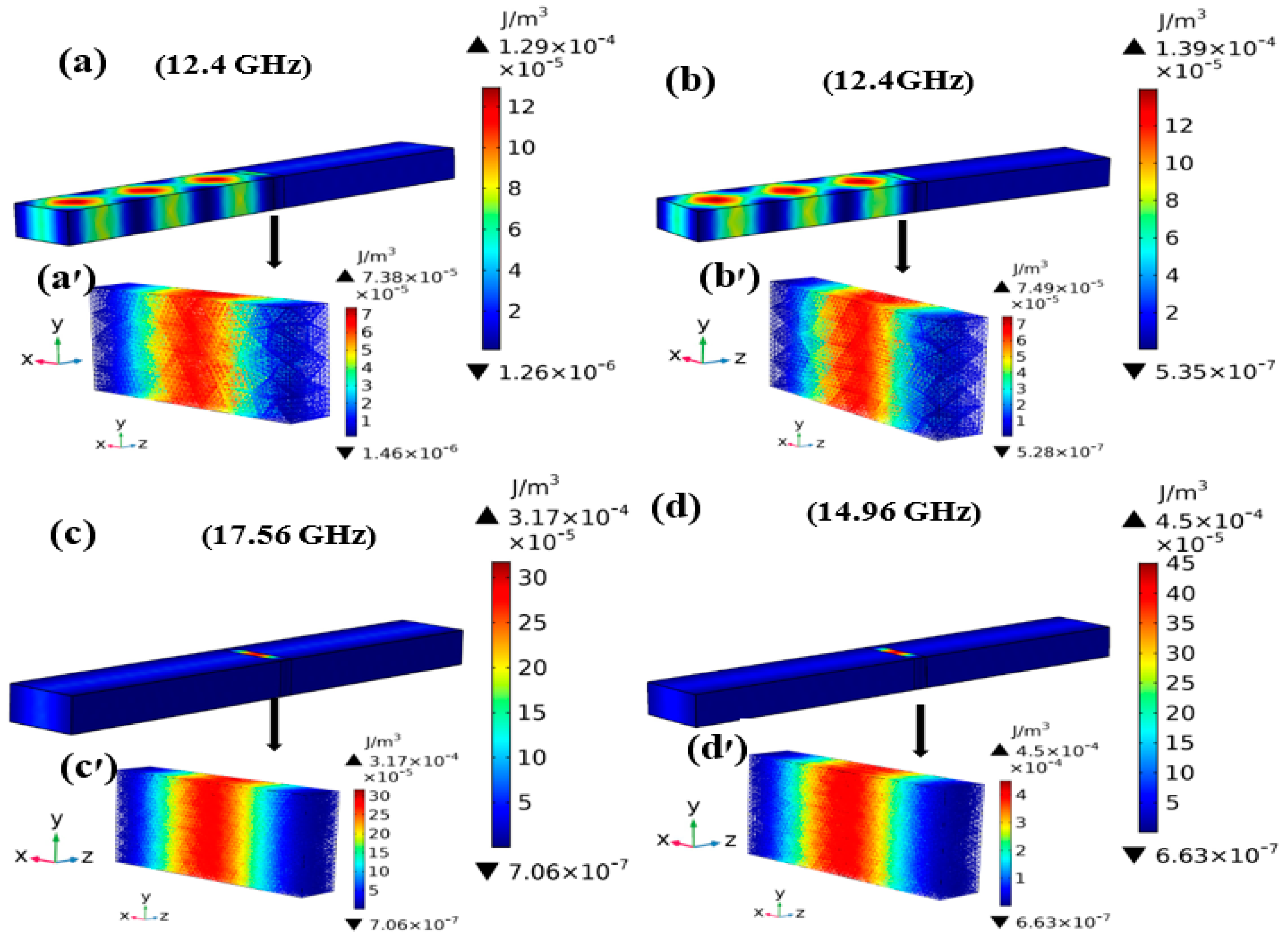
3.4. E-Field Distribution and EM Energy Density Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yadav, P.; Rattan, S.; Tripathi, A.; Kumar, S. Swift heavy-ions irradiated Nano-magnetite/Exfoliated-nanographite/Polymethylmethacrylate nanocomposites with excellent microwave-absorption performance. Mater. Lett. 2019, 253, 222–225. [Google Scholar] [CrossRef]
- Melvin, G.J.H.; Zhu, Y.; Ni, Q.Q. Nanomaterials: Electromagnetic Wave Energy Loss. In Nanotechnology: Applications in Energy, Drug and Food; Springer International Publishing: Cham, Switzerland, 2019; pp. 73–79. [Google Scholar]
- Zou, T.; Wu, Y.; Li, H. Electromagnetic and microwave absorbing properties of carbon-encapsulated cobalt nanoparticles. Mater. Lett. 2018, 214, 280–282. [Google Scholar] [CrossRef]
- Shu, R.; Zhang, G.; Zhang, J.; Wang, X.; Wang, M.; Gan, Y.; Shi, J.; He, J. Synthesis and high-performance microwave absorption of reduced graphene oxide/zinc ferrite hybrid nanocomposite. Mater. Lett. 2018, 215, 229–232. [Google Scholar] [CrossRef]
- Lv, L.; Liu, J.; Liu, H.; Liu, C.; Lu, Y.; Sun, K.; Fan, R.; Wang, N.; Lu, N.; Guo, Z. An overview of electrically conductive polymer nanocomposites toward electromagnetic interference shielding. Eng. Sci. 2018, 2, 26–42. [Google Scholar] [CrossRef]
- Shanenkov, I.; Sivkov, A.; Ivashutenko, A.; Zhuravlev, V.; Guo, Q.; Li, L.; Li, G.; Wei, G.; Han, W. Magnetite hollow microspheres with a broad absorption bandwidth of 11.9 GHz: Toward promising lightweight electromagnetic microwave absorption. Phys. Chem. Phys. Chem. 2017, 19, 19975–19983. [Google Scholar] [CrossRef]
- Wu, N.; Liu, C.; Xu, D.; Liu, J.; Liu, W.; Shao, Q.; Guo, Z. Enhanced Electromagnetic Wave Absorption of Three-Dimensional Porous Fe3O4/C Composite Flowers. ACS Sustain. Chem. Eng. 2018, 6, 12471–12480. [Google Scholar] [CrossRef]
- Sui, M.; Sun, X.; Lou, H.; Li, X.; Lv, X.; Li, L.; Gu, G. Synthesis of hollow Fe3O4 particles via one-step solvothermal approach for microwave absorption materials: Effect of reactant concentration, reaction temperature and reaction time. J. Mater. Sci. 2018, 29, 7539–7550. [Google Scholar] [CrossRef]
- Wang, G.; Chang, Y.; Wang, L.; Liu, L.; Liu, C. Facilely preparation and microwave absorption properties of Fe3O4 nanoparticles. Mater. Res. Bull. 2013, 48, 1007–1012. [Google Scholar] [CrossRef]
- Shang, C.; Ji, G.; Liu, W.; Zhang, X.; Lv, H.; Du, Y. One-pot in situ molten salt synthesis of octahedral Fe3O4 for efficient microwave absorption application. RSC Adv. 2015, 5, 80450–80456. [Google Scholar] [CrossRef]
- Li, X.; Zhang, B.; Ju, C.; Han, X.; Du, Y.; Xu, P. Morphology-controlled synthesis and electromagnetic properties of porous Fe3O4 nanostructures from iron alkoxide precursors. J. Phys. Chem. C 2011, 115, 12350–12357. [Google Scholar] [CrossRef]
- Li, C.; Ge, Y.; Jiang, X.; Waterhouse, G.I.N.; Zhang, Z.; Yu, L. Porous Fe3O4/C microspheres for efficient broadband electromagnetic wave absorption. Ceram. Int. 2018, 44, 19171–19183. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Y.; Cui, X.; Zeng, M.; Yu, R.; Wang, G.S. Flexible nanocomposites with enhanced microwave absorption properties based on Fe3O4/SiO2 nanorods and polyvinylidene fluoride. J. Mater. Chem. A 2015, 3, 12197–12204. [Google Scholar] [CrossRef]
- Liu, J.; Dai, C.; Hu, Y. Aqueous aggregation behavior of citric acid coated magnetite nanoparticles: Effects of pH, cations, anions, and humic acid. Environ. Res. 2018, 161, 49–60. [Google Scholar] [CrossRef]
- Micheli, D.; Pastore, R.; Vricella, A.; Delfini, A.; Marchetti, M.; Santoni, F. Electromagnetic Characterization of Materials by Vector Network Analyzer Experimental Setup. In Spectroscopic Methods for Nanomaterials Characterization; Elsevier: Amsterdam, The Netherlands, 2017; pp. 195–236. [Google Scholar]
- Soleimani, H.; Yahya, N.; Sabet, M.; Abbas, Z.; Soleimani, H.; Kozlowski, G.; Ghotbi, M.Y. Electromagnetic properties of lanthanum iron garnet filled PVDF-polymer composite at microwave frequencies using finite element method (FEM) and Nicholson–Ross–Weir (NRW) method. Dig. J. Nanomater. Biostruct. 2014, 9, 53–60. [Google Scholar]
- Shahid, M.K.; Kim, Y.; Choi, Y.G. Magnetite synthesis using iron oxide waste and its application for phosphate adsorption with column and batch reactors. Chem. Eng. Res. Des. 2019, 148, 169–179. [Google Scholar] [CrossRef]
- Talbot, D.; Abramson, S.; Griffete, N.; Bee, A. pH-sensitive magnetic alginate/γ-Fe2O3 nanoparticles for adsorption/desorption of a cationic dye from water. J. Water Process Eng. 2018, 25, 301–308. [Google Scholar] [CrossRef]
- Du, Y.; Liu, W.; Qiang, R.; Wang, Y.; Han, X.; Ma, J.; Xu, P. Shell thickness-dependent microwave absorption of core–shell Fe3O4@C composites. ACS Appl. Mater. Interfaces 2014, 6, 12997–13006. [Google Scholar] [CrossRef]
- Wang, C.; Murugadoss, V.; Kong, J.; He, Z.; Mai, X.; Shao, Q.; Chen, Y.; Guo, L.; Liu, C.; Angaiah, S.; et al. Overview of carbon nanostructures and nanocomposites for electromagnetic wave shielding. Carbon 2018, 140, 696–733. [Google Scholar] [CrossRef]
- Jiang, J.; Li, D.; Geng, D.; An, J.; He, J.; Liu, W.; Zhang, Z. Microwave absorption properties of core double-shell FeCo/C/BaTiO3 nanocomposites. Nanoscale 2014, 6, 3967–3971. [Google Scholar] [CrossRef]
- Xiang, Z.; Song, Y.; Xiong, J.; Pan, Z.; Wang, X.; Liu, L.; Liu, R.; Yang, H.; Lu, W. Enhanced electromagnetic wave absorption of nanoporous Fe3O4 @ carbon composites derived from metal-organic frameworks. Carbon 2019, 142, 20–31. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, Q.; Gao, X.; Chen, X.; Wang, Y.; Li, W.; Wu, J. Effect of absorbers’ composition on the microwave absorbing performance of hollow Fe3O4 nanoparticles decorated CNTs/graphene/C composites. J. Alloy. Compd. 2018, 748, 706–716. [Google Scholar] [CrossRef]
- Meng, F.; Wang, H.; Huang, F.; Guo, Y.; Wang, Z.; Hui, D.; Zhou, Z. Graphene-based microwave absorbing composites: A review and prospective. Compos. Part B Eng. 2018, 137, 260–277. [Google Scholar] [CrossRef]
- Zhao, B.; Deng, J.; Zhang, R.; Liang, L.; Fan, B.; Bai, Z.; Shao, G.; Park, C.B. Recent advances on the electromagnetic wave absorption properties of Ni based materials. Eng. Sci. 2018, 3, 5–40. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhao, H.; Yang, Z.; Lv, J.; Cao, J.; Qi, X.; Ji, G.; Du, Y. An unusual route to grow carbon shell on Fe3O4 microspheres with enhanced microwave absorption. J. Alloy. Compd. 2018, 762, 463–472. [Google Scholar] [CrossRef]
- Liu, P.; Huang, Y.; Yan, J.; Zhao, Y. Magnetic graphene@PANI@porous TiO2 ternary composites for high-performance electromagnetic wave absorption. J. Mater. Chem. C 2016, 4, 6362–6370. [Google Scholar] [CrossRef]
- Zhang, T.; Huang, D.; Yang, Y.; Kang, F.; Gu, J. Fe3O4/carbon composite nanofiber absorber with enhanced microwave absorption performance. Mater. Sci. Eng. B 2013, 178, 1–9. [Google Scholar] [CrossRef]
- Li, Z.; Li, X.; Zong, Y.; Tan, G.; Sun, Y.; Lan, Y.; He, M.; Ren, Z.; Zheng, X. Solvothermal synthesis of nitrogen-doped graphene decorated by superparamagnetic Fe3O4 nanoparticles and their applications as enhanced synergistic microwave absorbers. Carbon 2017, 115, 493–502. [Google Scholar] [CrossRef]
- Movassagh-Alanagh, F.; Bordbar Khiabani, A.; Salimkhani, H. Improvement in magnetic and microwave absorption properties of nano-Fe3O4@CFs composites using a modified multi-step EPD process. Appl. Surf. Sci. 2017, 420, 726–739. [Google Scholar] [CrossRef]
- Kittel, C. Theory of the dispersion of magnetic permeability in ferromagnetic materials at microwave frequencies. Phys. Rev. 1946, 70, 281. [Google Scholar] [CrossRef]
- Micheli, D.; Pastore, R.; Delfini, A.; Giusti, A.; Vricella, A.; Santoni, F.; Marchetti, M.; Tolochko, O.; Vasilyeva, E. Electromagnetic characterization of advanced nanostructured materials and multilayer design optimization for metrological and low radar observability applications. Acta Astronaut. 2017, 134, 33–40. [Google Scholar] [CrossRef]
- Liu, X.; Hao, C.; Jiang, H.; Zeng, M.; Yu, R. Hierarchical NiCo2O4/Co3O4/NiO porous composite: A lightweight electromagnetic wave absorber with tunable absorbing performance. J. Mater. Chem. C 2017, 5, 3770–3778. [Google Scholar] [CrossRef]
- Kong, L.; Wang, C.; Yin, X.; Fan, X.; Wang, W.; Huang, J. Electromagnetic wave absorption properties of a carbon nanotube modified by a tetrapyridinoporphyrazine interface layer. J. Mater. Chem. C 2017, 5, 7479–7488. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, L.; Jiang, K.; Fan, M.; Jin, C.; Han, J.; Wu, W.; Tong, G. Distinctly enhanced permeability and excellent microwave absorption of expanded graphite/Fe3O4 nanoring composites. RSC Adv. 2017, 7, 11561–11567. [Google Scholar] [CrossRef]
- Idris, F.M.; Hashim, M.; Abbas, Z.; Ismail, I.; Nazlan, R.; Ibrahim, I.R. Recent developments of smart electromagnetic absorbers based polymer-composites at gigahertz frequencies. J. Magn. Magn. Mater. 2016, 405, 197–208. [Google Scholar] [CrossRef]
- Zhou, W.; Hu, X.; Bai, X.; Zhou, S.; Sun, C.; Yan, J.; Chen, P. Synthesis and electromagnetic, microwave absorbing properties of core-shell Fe3O4-poly(3, 4-ethylenedioxythiophene) microspheres. ACS Appl. Mater. Interfaces 2011, 3, 3839–3845. [Google Scholar] [CrossRef]
- Liu, X.; Cui, X.; Chen, Y.; Zhang, X.J.; Yu, R.; Wang, G.S.; Ma, H. Modulation of electromagnetic wave absorption by carbon shell thickness in carbon encapsulated magnetite nanospindles–poly (vinylidene fluoride) composites. Carbon 2015, 95, 870–878. [Google Scholar] [CrossRef]
- Hou, C.L.; Li, T.H.; Zhao, T.K.; Liu, H.G.; Liu, L.H.; Zhang, W.J. Electromagnetic wave absorbing properties of multi-wall carbon nanotube/Fe3O4 hybrid materials. New Carbon Mater. 2013, 28, 184–190. [Google Scholar] [CrossRef]
- Osouli-Bostanabad, K.; Hosseinzade, E.; Kianvash, A.; Entezami, A. Modified nano-magnetite coated carbon fibers magnetic and microwave properties. Appl. Surf. Sci. 2015, 356, 1086–1095. [Google Scholar] [CrossRef]
- Osouli-Bostanabad, K.; Aghajani, H.; Hosseinzade, E.; Maleki-Ghaleh, H.; Shakeri, M. High microwave absorption of nano-Fe3O4 deposited electrophoretically on carbon fiber. Mater. Manuf. Process. 2016, 31, 1351–1356. [Google Scholar] [CrossRef]
- Liu, X.; Guo, H.; Xie, Q.; Luo, Q.; Wang, L.S.; Peng, D.L. Enhanced microwave absorption properties in GHz range of Fe3O4/C composite materials. J. Alloy. Compd. 2015, 649, 537–543. [Google Scholar] [CrossRef]

| Absorbers | Filling Ratio (wt.%) | Optimal RL (dB) (Corresponding Frequency GHz) | Thickness (mm) | Bandwidth (RL < −dB) | References |
|---|---|---|---|---|---|
| Fe3O4 nanoparticles | 60 | −21.2 (5.7) | 2.0 | 3.6 | [9] |
| Fe3O4@ PEDOT | 20 | −30.0 (9.5) | 4.0 | 4.2 | [37] |
| Fe3O4/C/PVDF | 60 | −36.5 (11.1) | 5.0 | 2.3 | [38] |
| MWCNT/Fe3O4 | 70 | −18.2 (12.04) | 2.0 | 1.04 | [39] |
| Fe3O4/CF | 50 | −8.0 (9.2) | 2.0 | 0.7 | [40] |
| Fe3O4/C flowers | 50 | −54.6 (5.7) | 4.27 | 6.0 | [7] |
| Fe3O4/SiO2/PVDF | 40 | −28.6 (8.10) | 2.5 | 2.11 | [13] |
| Fe3O4/CF | 50 | −11.0 (10.37) | 1.70 | 1.03 | [41] |
| Fe3O4/C | 60 | −46.0 (15.50) | 2.5 | 6.80 | [42] |
| C-Fe3O4/PVDF | 20 | −43.9 (15.13) | 3.5 | 2.45 | This work |
| C-Fe3O4/PVDF | 40 | −41.75 (14.96) | 3.0 | 2.01 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adebayo, L.L.; Soleimani, H.; Yahya, N.; Abbas, Z.; Ridwan, A.T.; Wahaab, F.A. Investigation of the Broadband Microwave Absorption of Citric Acid Coated Fe3O4/PVDF Composite Using Finite Element Method. Appl. Sci. 2019, 9, 3877. https://doi.org/10.3390/app9183877
Adebayo LL, Soleimani H, Yahya N, Abbas Z, Ridwan AT, Wahaab FA. Investigation of the Broadband Microwave Absorption of Citric Acid Coated Fe3O4/PVDF Composite Using Finite Element Method. Applied Sciences. 2019; 9(18):3877. https://doi.org/10.3390/app9183877
Chicago/Turabian StyleAdebayo, Lawal Lanre, Hassan Soleimani, Noorhana Yahya, Zulkifly Abbas, Ayinla Tobi Ridwan, and Fatai Adisa Wahaab. 2019. "Investigation of the Broadband Microwave Absorption of Citric Acid Coated Fe3O4/PVDF Composite Using Finite Element Method" Applied Sciences 9, no. 18: 3877. https://doi.org/10.3390/app9183877
APA StyleAdebayo, L. L., Soleimani, H., Yahya, N., Abbas, Z., Ridwan, A. T., & Wahaab, F. A. (2019). Investigation of the Broadband Microwave Absorption of Citric Acid Coated Fe3O4/PVDF Composite Using Finite Element Method. Applied Sciences, 9(18), 3877. https://doi.org/10.3390/app9183877





