Ionization and Attachment Coefficients in C4F7N Gas Measured by the Steady-State Townsend Method
Abstract
1. Introduction
2. Experiments
3. Results and Discussion
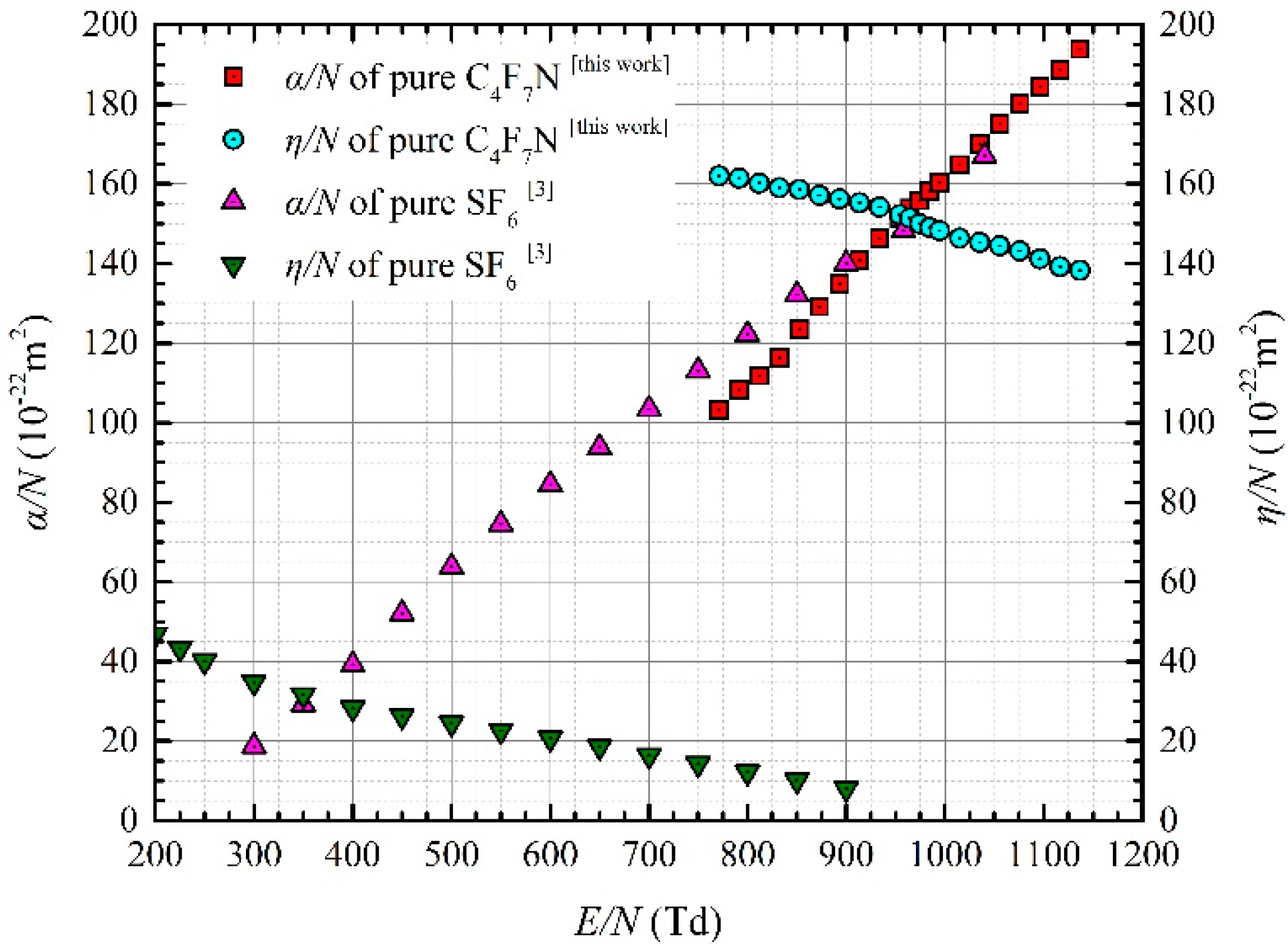
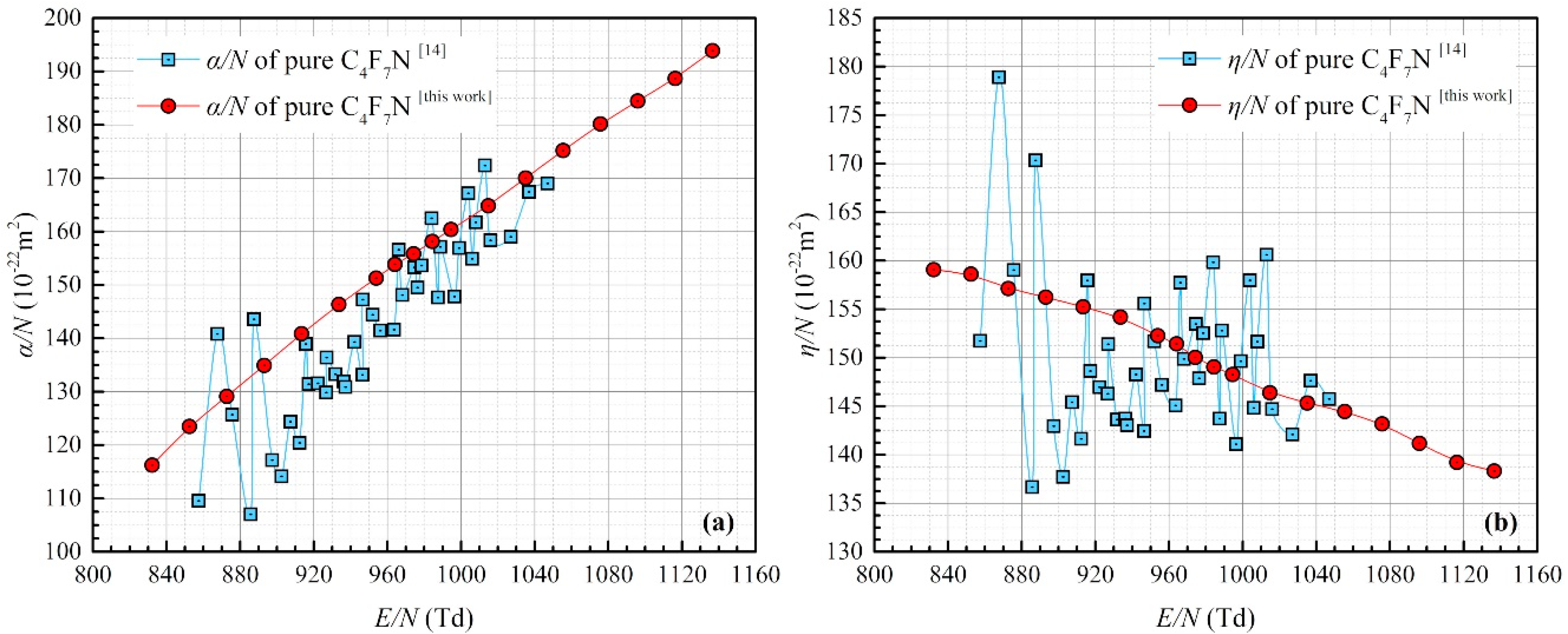
3.1. Ionization and Attachment Coefficients
3.2. Effective Ionization Coefficients
3.3. Critical Electric Fields
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Christophorou, L.G.; Van Brunt, R.J. SF6/N2 Mixtures Basic and HV Insulation Properties. IEEE Trans. Dielectr. Electr. Insul. 1995, 2, 952–1003. [Google Scholar] [CrossRef]
- Dincer, M.S.; Raju, G.G. Ionization and Attachment Coefficients in SF6+N2 Mixtures. IEEE Trans. Electr. Insul. 1984, EI-19, 40–44. [Google Scholar] [CrossRef]
- Christophorou, L.G.; Olthoff, J.K. Electron Interactions with SF6. J. Phys. Chem. Ref. Data 2000, 29, 267–330. [Google Scholar] [CrossRef]
- Yamaji, M.; Nakamura, Y.; Morokuma, Y. Measurements of ionization and attachment coefficients in 0.468% and 4.910% c-CC4F8/Ar mixtures and pure c-C4F8. J. Phys. D Appl. Phys. 2004, 37, 432–437. [Google Scholar] [CrossRef]
- de Urquijo, J.; Basurto, E. Electron attachment, ionization and drift in c-C4F8. J. Phys. D Appl. Phys. 2001, 34, 1352–1354. [Google Scholar] [CrossRef]
- Naidu, M.S.; Prasad, A.N.; Craggs, J.D. Electron transport, attachment and ionization in c-C4F8 and iso-C4F8. J. Phys. D Appl. Phys. 1972, 5, 741–746. [Google Scholar] [CrossRef]
- Li, X.; Zhao, H.; Wu, J.; Jia, S. Analysis of the insulation characteristics of CF3I mixtures with CF4, CO2, N2, O2 and air. J. Phys. D Appl. Phys. 2013, 46, 345203–345210. [Google Scholar] [CrossRef]
- Hasegawa, H.; Date, H.; Shimozuma, M.; Itoh, H. Properties of Electron Swarms in CF3I. Appl. Phys. Lett. 2009, 95, 101504–101506. [Google Scholar] [CrossRef]
- Raju, G.G. Gaseous Electronics: Tables, Atoms, and Molecules; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2012. [Google Scholar]
- Kieffel, Y.; Irwin, T.; Ponchon, P.; Owens, J. Green Gas to Replace SF6 in Electrical Grids. IEEE Power Energy Mag. 2016, 14, 32–39. [Google Scholar] [CrossRef]
- Kieffel, Y.; Biquez, F.; Poncho, P. Alternative gas to SF6 for use in high voltage switchgears: g3. In Proceedings of the 23rd International Conference on Electricity Distribution (CIRED), Lyon, France, 15–18 June 2015; p. 230. [Google Scholar]
- Townsend, J.S. The Conductivity produced in Gases by the Motion of Negatively-charged Ions. Nature 1900, 62, 340–341. [Google Scholar] [CrossRef]
- Nechmi, H.E.; Beroual, A.; Giodet, A.; Vinson, P. Effective Ionization Coefficient and Limiting Field Strength of Fluoronitriles-CO2 Mixtures. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 886–892. [Google Scholar] [CrossRef]
- Chachereau, A.; Hösl, A.; Franck, C.M. Electrical insulation properties of the perfluoronitrile C4F7N. J. Phys. D Appl. Phys. 2018, 51, 495201–495210. [Google Scholar] [CrossRef]
- Bhalla, M.S.; Craggs, J.D. Measurement of Ionization and Attachment Coefficients in Sulphur Hexafluoride in Uniform Fields. Proc. Phys. Soc. 1962, 80, 151–160. [Google Scholar] [CrossRef]
- Raju, G.G.; Dincer, M.S. Measurement of ionization and attachment coefficients in SF6 and SF6+N2. J. Appl. Phys. 1982, 53, 8562–8567. [Google Scholar] [CrossRef]
- Fréchette, M.F. Experimental study of SF6/N2 and SF6/CCl2F2 mixtures by the steady-state Townsend method. J. Appl. Phys. 1986, 59, 3684–3693. [Google Scholar] [CrossRef]
- Long, Y.; Guo, L.; Shen, Z.; Chen, C.; Chen, Y.; Li, F. Ionization and Attachment Coefficients in C4F7N/N2 Gas Mixtures for Use as a Replacement to SF6. IEEE Trans. Dielectr. Electr. Insul. 2019, 26, 1358–1362. [Google Scholar] [CrossRef]
- Long, Y.; Guo, L.; Shen, Z.; Chen, C.; Zhou, W.; Liu, W. Investigation of Ionization Characteristics of C4F7N-N2 Gas Mixtures as Alternative Gas to SF6 by Steady-state Townsend Method. High Volt. Eng. 2019, 45, 1064–1070. [Google Scholar]
- Christophorou, L.G.; Olthoff, J.K. Electron Interactions with CF6I. J. Phys. Chem. Ref. Data 2000, 29, 553–569. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Xiao, S.; Chen, Q.; Tang, J.; Chen, D.; Wang, D. Decomposition Properties of C4F7N/N2 Gas Mixture: An Environmentally Friendly Gas to Replace SF6. Ind. Eng. Chem. Res. 2018, 57, 5173–5182. [Google Scholar] [CrossRef]
- Wang, C.; Wu, Y.; Sun, H.; Duan, J.; Niu, C.; Yang, F. Thermophysical Properties Calculation of C4F7N/CO2 mixture Based on Computational Chemistry—A Theoretical Study of SF6 Alternative. In Proceedings of the 4th International Conference on Electric Power Equipment-Switching Technology (ICEPE-ST), Xi’an, China, 22–25 October 2017; pp. 255–258. [Google Scholar]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, Z.; Long, Y.; Shen, Z.; Chen, C.; Guo, L.; Zhou, W. Ionization and Attachment Coefficients in C4F7N Gas Measured by the Steady-State Townsend Method. Appl. Sci. 2019, 9, 3686. https://doi.org/10.3390/app9183686
Qin Z, Long Y, Shen Z, Chen C, Guo L, Zhou W. Ionization and Attachment Coefficients in C4F7N Gas Measured by the Steady-State Townsend Method. Applied Sciences. 2019; 9(18):3686. https://doi.org/10.3390/app9183686
Chicago/Turabian StyleQin, Zhaoyu, Yunxiang Long, Zhenyu Shen, Cheng Chen, Liping Guo, and Wenjun Zhou. 2019. "Ionization and Attachment Coefficients in C4F7N Gas Measured by the Steady-State Townsend Method" Applied Sciences 9, no. 18: 3686. https://doi.org/10.3390/app9183686
APA StyleQin, Z., Long, Y., Shen, Z., Chen, C., Guo, L., & Zhou, W. (2019). Ionization and Attachment Coefficients in C4F7N Gas Measured by the Steady-State Townsend Method. Applied Sciences, 9(18), 3686. https://doi.org/10.3390/app9183686





