Continuum Modeling and Simulation in Bone Tissue Engineering
Abstract
Featured Application
Abstract
1. Introduction
2. Mechanics Modeling
2.1. Constitutive Behavior Modeling and Scaffold Design
2.2. Simulation of Applications of Interest
3. Transport and Flow Modeling
4. Modeling of Physical Phenomena
5. Perspectives
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef]
- Hing, K.A. Bone repair in the twenty-first century: Biology, chemistry or engineering? Philos. Trans. R. Soc. A 2004, 362, 2821–2850. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.; Aoki, H.; Tabata, T.; Ogiso, M. Biocompatibility of apatite ceramics mandibles. Biomater. Med. Devices. Artif. Organs 1979, 7, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Jarcho, M. Calcium phosphate ceramics as hard tissue prosthetics. Clin. Ortho. 1981, 157, 259–278. [Google Scholar] [CrossRef]
- Eggli, P.S.; Muller, W.; Schenk, P.K. Porous hydroxyapatite and tricalcium phosphate cylinders with two different pore size ranges implanted in the cancellous bone of rabbits. A comparative histomorphometric and histology study of bony in-growth and implant substitution. Clin. Ortho. 1988, 232, 127–138. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Calcium phosphate materials in restorative dentistry. Adv. Dent. Res. 1988, 2, 164–180. [Google Scholar] [CrossRef] [PubMed]
- Ohgushi, H.; Goldberg, V.M.; Caplan, A.I. Heterotopic osteogenesis in porous ceramics induced by marrow cells. J. Ortho. Res. 1989, 7, 568–578. [Google Scholar] [CrossRef]
- Ohgushi, H.; Okumura, M.M.; Tamai, S.; Shors, E.C.; Caplan, A.I. Marrow cell induced osteogenesis in porous hydroxyapatite and tricalcium phosphate. J. Biomed. Mat. Res. 1990, 24, 1563–1570. [Google Scholar] [CrossRef]
- Okumura, M.; Ohgushi, H.; Tamai, S. Bonding osteogenesis in coralline hydroxyapatite combined with bone marrow cells. Biomaterials 1991, 12, 411–416. [Google Scholar] [CrossRef]
- Ohgushi, H.; Dohi, Y.; Yoshikawa, T.; Tamai, S.; Tabata, S.; Okunaga, K.; Shibuya, T. Osteogenic differentiation of cultured marrow stromal stem cells on the surface of bioactive glass ceramic. J. Biomed. Mater. Res. 1996, 32, 341–348. [Google Scholar] [CrossRef]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L.; Splinter, R.J.; Allen, W.C. Bonding mechanisms at the interface of ceramic prosthetic materials. J. Biomed. Mater. Res. Symp. 1971, 2, 117–141. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics. J. Am. Ceram. Soc. 1998, 81, 1705–1728. [Google Scholar] [CrossRef]
- Chen, Q.Z.; Boccaccini, A.R. Poly(d,l-lactic acid) coated 45S5 Bioglass®-based R-based scaffolds: Processing and characterization. J. Biomed. Mat. Res. A 2006, 77, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Mastrogiacomo, M.; Muraglia, A.; Komlev, V.; Peyrin, F.; Rustichelli, F.; Crovace, A.; Cancedda, R. Tissue engineering of bone: Search for a better scaffold. Orthod. Craniofac. Res. 2005, 8, 277–284. [Google Scholar] [CrossRef]
- Mastrogiacomo, M.; Papadimitropoulos, A.; Cedola, A.; Peyrin, F.; Giannoni, P.; Pearce, S.G.; Alini, M.; Giannini, C.; Guagliardi, A.; Cancedda, R. Engineering of bone using bone marrow stromal cells and a silicon-stabilized tricalcium phosphate bioceramic: Evidence for a coupling between bone formation and scaffold resorption. Biomaterials 2007, 28, 1376–1384. [Google Scholar] [CrossRef] [PubMed]
- Fialkov, J.A.; Holy, C.E.; Shoichet, M.S.; Davies, J.E. In vivo bone engineering in a rabbit femur. J. Craniofac. Surg. 2003, 14, 324–332. [Google Scholar] [CrossRef]
- Holy, C.E.; Fialkov, J.A.; Davies, J.E.; Shoichet, M.S. Use of a biomimetic strategy to engineer bone. J. Biomed. Mater. Res. A 2003, 65, 447–453. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, F.; Ho, S.T.; Woodruff, M.A.; Lim, T.M.; Hutmacher, D.W. Combined marrow stromal cell-sheet techniques and high-strength biodegradable composite scaffolds for engineered functional bone grafts. Biomaterials 2007, 28, 814–824. [Google Scholar] [CrossRef]
- Savarino, L.; Baldini, N.; Greco, M.; Capitani, O.; Pinna, S.; Valentini, S.; Lombardo, B.; Esposito, M.T.; Pastore, L.; Ambrosio, L.; et al. The performance of poly-e-caprolactone scaffolds in a rabbit femur model with and without autologous stromal cells and BMP4. Biomaterials 2007, 28, 3101–3109. [Google Scholar] [CrossRef]
- Bassett, C.A.L. Biologic significance of piezoelectricity. Calcif. Tissue Int. 1967, 1, 252–272. [Google Scholar] [CrossRef]
- Rajabi, A.H.; Jaffe, M.; Arinzeh, T.L. Piezoelectric materials for tissue regeneration: A review. Acta Biomater. 2015, 24, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Beaupre, G.S.; Orr, T.E.; Carter, D.R. An approach for time-dependent bone modelling and remodelling: Theoretical development. J. Orthop. Res. 1990, 8, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Hollister, S.J.; Maddox, R.D.; Taboas, J.M. Optimal design and fabrication of scaffolds to mimic tissue properties and satisfy biological constraints. Biomaterials 2002, 23, 4095–4103. [Google Scholar] [CrossRef]
- Lin, C.Y.; Kikuchi, N.; Hollister, S.J. A novel method for biomaterial scaffold internal architecture design to match bone elastic properties with desired porosity. J. Biomech. 2004, 37, 623–636. [Google Scholar] [CrossRef]
- Taboas, J.M.; Maddox, R.D.; Krebsbach, P.H.; Hollister, S.J. Indirect solid free form fabrication of local and global porous, biomimetic and composite 3D polymer-ceramic scaffolds. Biomaterials 2003, 24, 181–194. [Google Scholar] [CrossRef]
- Sanz-Herrera, J.A.; Kasper, C.; van Griensven, M.; Garcia-Aznar, J.M.; Ochoa, I.; Doblare, M. Mechanical and flow characterization of Sponceram® carriers: Evaluation by homogenization theory and experimental validation. J. Biomed. Mater. Res. B 2008, 87, 42–48. [Google Scholar] [CrossRef]
- Sturm, S.; Zhou, S.; Mai, Y.W.; Li, Q. On stiffness of scaffolds for bone tissue engineering-a numerical study. J. Biomech. 2010, 43, 1738–1744. [Google Scholar] [CrossRef]
- Coelho, P.G.; Hollister, S.J.; Flanagan, C.L.; Fernandes, P.R. Bioresorbable scaffolds for bone tiss engineering: Optimal design, fabrication, mechanicaltesting and scale-size effects analysis. Med. Eng. Phys. 2015, 37, 287–296. [Google Scholar] [CrossRef]
- Ranganathan, S.I.; Yoon, D.M.; Henslee, A.M.; Nair, M.B.; Smid, C.; Kasper, F.K.; Tasciotti, E.; Mikos, A.G.; Decuzzi, P.; Ferrari, M. Shaping the micromechanical behavior of multi-phase composites for bone tissue engineering. Acta Biomater. 2010, 6, 3448–3456. [Google Scholar] [CrossRef]
- Scheiner, S.; Sinibaldi, R.; Pichler, B.; Komlev, V.; Renghini, C.; Vitale-Brovarone, C.; Rustichelli, F.; Hellmich, C. Micromechanics of bone tissue-engineering scaffolds, based on resolution error-cleared computer tomography. Biomaterials 2009, 30, 2411–2419. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, A.; Dormieux, L.; Hellmich, C.; Sanahuja, J. Mechanical behavior of hydroxyapatite biomaterials: An experimentally validated micromechanical model for elasticity and strength. J. Biomed. Mater. Res. A 2009, 88, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Kariem, H.; Pastrama, M.I.; Roohani-Esfahani, S.I.; Pivonka, P.; Zreiqat, H.; Hellmich, C. Micro-poro-elasticity of baghdadite-based bone tissue engineering scaffolds: A unifying approach based on ultrasonics, nanoindentation, and homogenization theory. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 46, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, S.; Komlev, V.S.; Hellmich, C. Computational methods for the predictive design of bone tissue engineering scaffolds. In 3D Printing and Biofabrication; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Chen, Y.; Schellekens, M.; Zhou, S.; Cadman, J.; Li, W.; Appleyard, R.; Li, Q. Design optimization of scaffold microstructures using shear stress criterion towards regulated flow-induced erosion. J. Biomech. Eng. 2011, 133, 081008. [Google Scholar] [CrossRef] [PubMed]
- Boccaccio, A.; Uva, A.E.; Fiorentino, M.; Monno, G.; Ballini, A.; Desiate, A. Optimal load for bone tissue scaffolds with an assigned geometry. Int. J. Med. Sci. 2018, 15, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Egan, P.F.; Gonella, V.C.; Engensperger, M.; Ferguson, S.J.; Shea, K. Computationally designed lattices with tuned properties for tissue engineering using 3D printing. PLoS ONE 2017, 12, e0182902. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.P.G.; Lacroix, D. Micromechanical study of the load transfer in a polycaprolactone-collagen hybrid scaffold when subjected to unconfined and confined compression. Biomech. Model. Mechanobiol. 2018, 17, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Prendergast, P.J.; Huiskes, R.; Søballe, K. Biophysical stimuli on cells during tissue ifferentiation at implant interfaces. J. Biomech. 1997, 30, 539–548. [Google Scholar] [CrossRef]
- Kelly, D.J.; Prendergast, P.J. Prediction of the optimal mechanical properties for a scaffold used in osteochondral defect repair. Tissue Eng. 2006, 12, 2509–2519. [Google Scholar] [CrossRef]
- Olivares, A.L.; Marsal, E.; Planell, J.A.; Lacroix, D. Finite element study of scaffold architecture design and culture conditions for tissue engineering. Biomaterials 2009, 30, 6142–6149. [Google Scholar] [CrossRef] [PubMed]
- Khayyeri, H.; Checa, S.; Tägil, M.; Prendergast, P.J. Corroboration of mechanobiological simulations of tissue differentiation in an in vivo bone chamber using a lattice-modeling approach. J. Orthop. Res. 2009, 27, 1659–1666. [Google Scholar] [CrossRef] [PubMed]
- Sandino, C.; Lacroix, D. A dynamical study of the mechanical stimuli and tissue differentiation within a CaP scaffold based on micro-CT finite element models. Biomech. Model. Mechanobiol. 2011, 10, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Sandino, C.; Checa, S.; Prendergast, P.J.; Lacroix, D. Simulation of angiogenesis and cell differentiation in a CaP scaffold subjected to compressive strains using a lattice modeling approach. Biomaterials 2010, 31, 2446–2452. [Google Scholar] [CrossRef] [PubMed]
- Checa, S.; Prendergast, P.J. Effect of cell seeding and mechanical loading on vascularization and tissue formation inside a scaffold: A mechanobiological model using a lattice approach to simulate cell activity. J. Biomech. 2010, 43, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Milan, J.L.; Planell, J.A.; Lacroix, D. Computational modelling of the mechanical environment of osteogenesis within a polylactic acid–calcium phosphate glass scaffold. Biomaterials 2009, 30, 4219–4226. [Google Scholar] [CrossRef] [PubMed]
- Milan, J.L.; Planell, J.A.; Lacroix, D. Simulation of bone tissue formation within a porous scaffold under dynamic compression. Biomech. Model Mechanobiol. 2010, 9, 583–596. [Google Scholar] [CrossRef]
- Hendrikson, W.J.; van Blitterswijk, C.A.; Verdonschot, N.; Moroni, L.; Rouwkema, J. Modeling mechanical signals on the surface of microCT and CAD based rapid prototype scaffold models to predict (early stage) tissue development. Biotechnol. Bioeng. 2014, 111, 1864–1875. [Google Scholar] [CrossRef] [PubMed]
- Hutmacher, D.W.; Singh, H. Computational fluid dynamics for improved bioreactor design and 3D culture. Trends Biotechnol. 2008, 26, 166–172. [Google Scholar] [CrossRef]
- Hossain, M.S.; Chen, X.B.; Bergstrom, D.J. Investigation of the in vitro culture process for skeletal-tissue-engineered constructs using computational fluid dynamics and experimental methods. J. Biomech. Eng. 2012, 134, 121003. [Google Scholar] [CrossRef]
- Patrachari, A.R.; Podichetty, J.T.; Madihally, S.V. Application of computational fluid dynamics in tissue engineering. J. Biosci. Bioeng. 2012, 114, 123–132. [Google Scholar] [CrossRef]
- Voronov, R.; VanGordon, S.; Sikavitsas, V.I.; Papavassiliou, D.V. Computational modeling of flow-induced shear stresses within 3D salt-leached porous scaffolds imaged via micro-CT. J. Biomech. 2010, 43, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Hendrikson, W.J.; Deegan, A.J.; Yang, Y.; van Blitterswijk, C.A.; Verdonschot, N.; Moroni, L.; Rouwkema, J. Influence of additive manufactured scaffold architecture on the distribution of surface strains and fluid flow shear stresses and expected osteochondral cell differentiation. Front. Bioeng. Biotechnol. 2017, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, M.T.; Bridgen, D.T.; Laganà, M.; Cioffi, M.; Boschetti, F. Integration of experimental and computational microfluidics in 3D tissue engineering. In Methods in Bioengineering: 3D Tissue Engineering. Boston: Artech House; Berthiaume, F., Morgan, J., Eds.; Artech House: Norwood, MA, USA, 2010; pp. 237–252. [Google Scholar]
- Sadir, S.; Kadir, M.R.A.; Harun, M.N. Simulation of direct perfusión through 3D cellular scaffolds with different porosity. Bioinformatics 2011, 5, 123–126. [Google Scholar]
- Sikavitsas, V.I.; Bancroft, G.N.; Holtorf, H.L.; Jansen, J.A.; Mikos, A.G. Mineralized matrix deposition by marrow stromal osteoblasts in 3D perfusion culture increases with increasing fluid shear forces. Proc. Natl. Acad. Sci. USA 2003, 100, 14683–14688. [Google Scholar] [CrossRef] [PubMed]
- Holtorf, H.L.; Jansen, J.A.; Mikos, A.G. Flow perfusion culture induces the osteoblastic differentiation of marrow stromal cell-scaffold constructs in the absence of dexamethasone. J. Biomed. Mater. Res. A 2005, 72, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Stolberg, S.; McCloskey, K.E. Can shear stress direct stem cell fate? Biotechnol. Prog. 2009, 25, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Kreke, M.R.; Sharp, L.A.; Lee, Y.W.; Goldstein, A.S. Effect of intermittent shear stress on mechanotransductive signaling and osteoblastic differentiation of bone marrow stromal cells. Tissue Eng. Part. A 2008, 14, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Sonnaert, M.; Papantoniou, I.; Bloemen, V.; Kerckhofs, G.; Luyten, F.P.; Schrooten, J. Human periosteal-derived cell expansion in a perfusion bioreactor system: Proliferation, differentiation and extracellular matrix formation. J. Tissue Eng. Regen. Med. 2017, 11, 519–530. [Google Scholar] [CrossRef]
- Nava, M.M.; Raimondi, M.T.; Pietrabissa, R. A multiphysics 3D model of tissue growth under interstitial perfusion in a tissue-engineering bioreactor. Biomech. Model. Mechanobiol. 2013, 12, 1169–1179. [Google Scholar] [CrossRef]
- Zhao, F.; van Rietbergen, B.; Ito, K.; Hofmann, S. Flow rates in perfusion bioreactors to maximise mineralisation in bone tissue engineering in vitro. J. Biomech. 2018, 79, 232–237. [Google Scholar] [CrossRef]
- Byrne, D.P.; Lacroix, D.; Planell, J.A.; Kelly, D.J.; Prendergast, P.J. Simulation of tissue differentiation in a scaffold as a function of porosity, Young’s modulus and dissolution rate: Application of mechanobiological models in tissue engineering. Biomaterials 2007, 28, 5544–5554. [Google Scholar] [CrossRef] [PubMed]
- Adachi, T.; Osako, Y.; Tanaka, M.; Hojo, M.; Hollister, S.J. Framework for optimal design of porous scaffold microstructure by computational simulation of bone regeneration. Biomaterials 2006, 27, 3964–3972. [Google Scholar] [CrossRef] [PubMed]
- Adachi, T.; Tsubota, K.I.; Tomita, Y.; Hollister, S.J. Trabecular surface remodeling simulation for cancellous bone using microstructural voxel finite element models. J. Biomech. Eng. T ASME 2001, 123, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Comisar, W.A.; Kazmers, N.H.; Mooney, D.J.; Linderman, J.J. Engineering RGD nanopatterned hydrogels to control preosteoblast behavior: A combined computational and experimental approach. Biomaterials 2007, 28, 4409–4417. [Google Scholar] [CrossRef] [PubMed]
- Chen, V.J.; Smith, L.A.; Ma, P.X. Bone regeneration on computer-designed nano-fibrous scaffolds. Biomaterials 2006, 27, 3973–3979. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Herrera, J.A.; García-Aznar, J.M.; Doblaré, M. Micro–macro numerical modelling of bone regeneration in tissue engineering. Comput. Methods Appl. Mech. Eng. 2008, 197, 3092–3107. [Google Scholar] [CrossRef]
- Sanz-Herrera, J.A.; García-Aznar, J.M.; Doblaré, M. On scaffold designing for bone regeneration: A computational multiscale approach. Acta Biomater. 2009, 5, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Shipley, R.J.; Jones, G.W.; Dyson, R.J.; Sengers, B.G.; Bailey, C.L.; Catt, C.J.; Please, C.P.; Malda, J. Design criteria for a printed tissue engineering construct: A mathematical homogenization approach. J. Theor. Biol. 2009, 259, 489–502. [Google Scholar] [CrossRef]
- Chan, K.S.; Liang, W.; Francis, W.L.; Nicolella, D.P. A multiscale modeling approach to scaffold design and property prediction. J. Mech. Behav. Biomed. Mat. 2010, 3, 584–593. [Google Scholar] [CrossRef]
- Sanz-Herrera, J.A.; García-Aznar, J.M.; Doblaré, M. Scaffold microarchitecture determines internal bone directional growth structure: A numerical study. J. Biomech. 2010, 43, 2480–2486. [Google Scholar] [CrossRef]
- Sanz-Herrera, J.A.; García-Aznar, J.M.; Doblaré, M. A mathematical approach to bone tissue engineering. Proc. R. Soc. A 2009, 367, 2055–2078. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.K.; Carpentier, O.; Monchau, F.; Chai, F.; Hornez, J.C.; Hivart, P. Numerical optimization of cell colonization modelling inside scaffold for perfusion bioreactor: A multiscale model. Med. Eng. Phys. 2018, 57, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chan, J.K.; Teoh, S.H. Review of vascularised bone tissue-engineering strategies with a focus on co-culture systems. J. Tissue Eng. Regen. Med. 2015, 9, 85–105. [Google Scholar] [CrossRef] [PubMed]
- Shimko, D.A.; Nauman, E.A. Development and characterization of a porous poly(methyl methracrylate) scaffold with controllable modulus and permeability. J. Biomed. Mater. Res. 2007, 80, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Swider, P.; Conroy, M.; Pedrono, A.; Ambard, D.; Mantell, S.; Søballe, K.; Bechtold, J.E. Use of high-resolution MRI for investigation of fluid flow and global permeability in a material with interconnected porosity. J. Biomech. 2007, 40, 2112–2118. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; de Wijn, J.R.; Li, J.; Layrolle, P.; de Groot, K. Macroporous biphasic calcium phosphate scaffold with high permeability/porosity ratio. Tissue Eng. 2003, 9, 535–548. [Google Scholar] [CrossRef]
- Shimko, D.A.; Shimko, V.F.; Sander, E.A.; Dickson, K.F.; Nauman, E.A. Effect of porosity on the fluid flow characteristics and mechanical properties of tantalum scaffolds. J. Biomed. Mater. Res. 2005, 73, 315–324. [Google Scholar] [CrossRef]
- Ochoa, I.; Sanz-Herrera, J.A.; García-Aznar, J.M.; Doblaré, M.; Yunos, D.M.; Boccaccini, A.R. Permeability evaluation of 45S5 Bioglass®-based scaffolds for bone tissue engineering. J. Biomech. 2009, 42, 257–260. [Google Scholar] [CrossRef]
- Chor, M.V.; Li, W. A permeability measurement system for tissue engineering scaffolds. Meas. Sci. Technol. 2007, 18, 208–216. [Google Scholar] [CrossRef]
- Truscello, S.; Kerckhofs, G.; Van Bael, S.; Pyka, G.; Schrooten, J.; Van Oosterwyck, H. Prediction of permeability of regular scaffolds for skeletal tissue engineering: A combined computational and experimental study. Acta Biomater. 2012, 8, 1648–1658. [Google Scholar] [CrossRef]
- Syahrom, A.; Abdul Kadir, M.R.; Abdullah, J.; Öchsner, A. Permeability studies of artificial and natural cancellous bone structures. Med. Eng. Phys. 2013, 35, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Acosta Santamaría, V.A.; Malvè, M.; Duizabo, A.; Mena Tobar, A.; Gallego Ferrer, G.; García Aznar, J.M.; Doblaré, M.; Ochoa, I. Computational methodology to determine fluid related parameters of non regular three-dimensional scaffolds. Ann. Biomed. Eng. 2013, 41, 2367–2380. [Google Scholar] [CrossRef] [PubMed]
- Olivares, A.L.; Lacroix, D. Computational methods in the modeling of scaffolds for tissue engineering. In Computational Modeling in Tissue Engineering. Studies in Mechanobiology, Tissue Engineering and Biomaterials; Geris, L., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; Volume 10. [Google Scholar]
- Li, E.; Chang, C.C.; Zhang, Z.; Li, Q. Characterization of tissue scaffolds for time dependent biotransport criteria—A novel computational procedure. Comput. Methods Biomech. Biomed. Eng. 2016, 19, 210–224. [Google Scholar] [CrossRef] [PubMed]
- Lemon, G.; King, J.R.; Byrne, M.H.; Jensen, O.E.; Shakesheff, K.M. Mathematical modelling of engineered tissue growth using a multiphase porous flow mixture theory. J. Math. Biol. 2006, 52, 571–594. [Google Scholar] [CrossRef] [PubMed]
- Lemon, G.; King, J.R. Multiphase modelling of cell behaviour on artificial scaffolds: Effects of nutrient depletion and spatially nonuniform porosity. Math. Med. Biol. 2007, 24, 57–83. [Google Scholar] [CrossRef] [PubMed]
- Mohebbi-Kalhori, D.; Behzadmehr, A.; Doillon, C.J.; Hadjizadeh, A. Computational modeling of adherent cell growth in a hollow-fiber membrane bioreactor for large-scale 3-D bone tissue engineering. J. Artif. Organs 2012, 15, 250–265. [Google Scholar] [CrossRef] [PubMed]
- Pego, A.P.; Siebum, B.; Van Luyn, M.J.; Gallego Van Seijen, X.J.; Poot, A.A.; Grijpma, D.W.; Feijen, J. Preparation of degradable porous structures based on 1,3-trimethylene carbonate and d,l-lactide (co)polymers for heart tissue engineering. Tissue Eng. 2003, 9, 981–994. [Google Scholar] [CrossRef]
- Shin, M.; Ishii, O.; Sueda, T.; Vacanti, J.P. Contractile cardiac grafts using a novel nanofibrous mesh. Biomaterials 2004, 25, 3717–3723. [Google Scholar] [CrossRef] [PubMed]
- Ishii, O.; Shin, M.; Sueda, T.; Vacanti, J.P. In vitro tissue engineering of a cardiac graft using a degradable scaffold with an extracellular matrix-like topography. J. Thorac. Cardiovasc. Surg. 2005, 130, 1358–1363. [Google Scholar] [CrossRef]
- Zong, X.; Bien, H.; Chung, C.Y.; Yin, L.; Fang, D.; Hsiao, B.S.; Chu, B.; Entcheva, E. Electrospun fine-textured scaffolds for heart tissue constructs. Biomaterials 2005, 26, 5330–5338. [Google Scholar] [CrossRef]
- Carrier, R.L.; Rupnick, M.; Langer, R.; Schoen, F.J.; Freed, L.E.; Vunjak Novakovic, G. Perfusion improves tissue architecture of engineered cardiac muscle. Tissue Eng. 2002, 8, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Carrier, R.L.; Rupnick, M.; Langer, R.; Schoen, F.J.; Freed, L.E.; Vunjak Novakovic, G. Effects of oxygen on engineered cardiac muscle. Biotechnol. Bioeng. 2002, 78, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Schrumpf, M.A.; Lee, A.T.; Weiland, A.J. Foreign-body reaction and osteolysis induced by an intraosseous poly-l-lactic Acid suture anchor in the wrist: Case report. J. Hand Surg. Am. 2011, 36, 1769–1773. [Google Scholar] [CrossRef]
- Gopferich, A. Polymer bulk erosion. Macromolecules 1997, 30, 2598–2604. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, J.; Han, X.; Sinka, C.; Ding, L. A phenomenological model for the degradation of biodegradable polymers. Biomaterials 2009, 29, 3393–3401. [Google Scholar] [CrossRef]
- Han, X.; Pan, J. A model for simultaneous crystallisation and biodegradation of biodegradable polymers. Biomaterials 2009, 30, 423–430. [Google Scholar] [CrossRef]
- Dhote, V.; Vernerey, F.J. Mathematical model of the role of degradation on matrix development in hydrogel scaffold. Biomech. Model. Mechanobiol. 2004, 13, 167–183. [Google Scholar] [CrossRef]
- Akalp, U.; Bryant, S.J.; Vernerey, F.J. Tuning tissue growth with scaffold degradation in enzyme-sensitive hydrogels: A mathematical model. Soft Matter 2016, 12, 7505–7520. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, S.; Li, Q. Mathematical modeling of degradation for bulk-erosive polymers: Applications in tissue engineering scaffolds and drug delivery systems. Acta Biomater. 2011, 7, 1140–1149. [Google Scholar] [CrossRef]
- Hench, L.L.; Paschall, H.A. Direct chemical bond of bioactive glass ceramic materials to bone and muscle. J. Biomed. Mater. Res. Symp. 1973, 4, 25–42. [Google Scholar] [CrossRef]
- Wilson, J.; Low, S.B. Bioactive ceramics for periodontal treatment: Comparative studies in the Patus monkey. J. Appl. Biomater. 1992, 3, 123–169. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L.; West, J.K. Biological applications of bioactive glasses. Life Chem. Rep. 1996, 13, 187–241. [Google Scholar]
- Roether, J.A.; Boccaccini, A.R.; Hench, L.L.; Maquet, V.; Gautier, S.; Jerome, R. Development and in vitro characterisation of novel bioresorbable and bioactive composite materials based on polylactide foams and bioglasss for tissue engineering applications. Biomaterials 2002, 23, 3871–3878. [Google Scholar] [CrossRef]
- Chen, Q.Z.; Thompson, I.D.; Boccaccini, A.R. 45S5 Bioglass derived glass-ceramic scaffolds for bone tissue engineering. Biomaterials 2006, 27, 2414–2425. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Herrera, J.A.; Boccaccini, A.R. Modelling bioactivity and degradation of bioactive glass based tissue engineering scaffolds. Int. J. Solids Struct. 2011, 48, 257–268. [Google Scholar] [CrossRef]
- Manhas, V.; Guyot, Y.; Kerckhofs, G.; Chai, Y.C.; Geris, L. Computational modelling of local calcium ions release from calcium phosphate-based scaffolds. Biomech. Model. Mechanobiol. 2017, 16, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Herrera, J.A.; Soria, L.; Reina-Romo, E.; Torres, Y.; Boccaccini, A.R. Model of dissolution in the framework of tissue engineering and drug delivery. Biomech. Model. Mechanobiol. 2018, 17, 1331–1341. [Google Scholar] [CrossRef]
- Kraus, T.; Fischerauer, S.F.; Hänzi, A.C.; Uggowitzer, P.J.; Löffler, J.F.; Weinberg, A.M. Magnesium alloys for temporary implants in osteosynthesis: In vivo studies of their degradation and interaction with bone. Acta Biomater. 2012, 8, 1230–1238. [Google Scholar] [CrossRef]
- Brar, H.S.; Platt, M.O.; Sarntinoranont, M.; Martin, P.I.; Manuel, M.V. Magnesium as a biodegradable and bioabsorbable material for medical implants. JOM 2009, 61, 31–34. [Google Scholar] [CrossRef]
- Castellani, C.; Lindtner, R.A.; Hausbrandt, P.; Tschegg, E.; Stanzl-Tschegg, S.E.; Zanoni, G.; Beck, S.; Weinberg, A.M. Bone-implant interface strength and osseointegration: Biodegradable magnesium alloy versus standard titanium control. Acta Biomater. 2011, 7, 432–440. [Google Scholar] [CrossRef]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Grogan, J.A.; Brien, B.J.O.; Leen, S.B.; McHugh, P.E. A corrosion model for bioabsorbable metallic stents. Acta Biomater. 2011, 7, 3523–3533. [Google Scholar] [CrossRef] [PubMed]
- Grogan, J.A.; Leen, S.B.; McHugh, P.E. A physical corrosion model for bioabsorbable metal stents. Acta Biomater. 2014, 10, 2313–2322. [Google Scholar] [CrossRef] [PubMed]
- Bajger, P.; Ashbourn, J.M.A.; Manhas, V.; Guyot, Y.; Lietaert, K.; Geris, L. Mathematical modelling of the degradation behaviour of biodegradable metals. Biomech. Model. Mechanobiol. 2017, 16, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Herrera, J.A.; Reina-Romo, E.; Boccaccini, A.R. In silico design of magnesium implants: Macroscopic modeling. J. Mech. Behav. Biomed. Mater. 2018, 79, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Sun, S.; Chen, M.; Fahlman, B.D.; Liu, D.; Bi, H. In vitro and in vivo corrosion, mechanical properties and biocompatibility evaluation of MgF2-coated MgZn-Zr alloy as cancellous screws. Mater. Sci. Eng. C 2017, 75, 1268–1280. [Google Scholar] [CrossRef] [PubMed]
- Frenning, G.; Brohede, U.; Stromme, M. Finite element analysis of the release of slowly dissolving drugs from cylindrical matrix systems. J. Control. Release 2005, 107, 320–329. [Google Scholar] [CrossRef]
- Peppas, N.A.; Narasimhan, B. Mathematical models in drug delivery: How modeling has shaped the way we design new drug delivery systems. J. Control. Release 2014, 190, 75–81. [Google Scholar] [CrossRef]
- Mozafari, M.; Sefat, F.; Atala, A. Handbook of Tissue Engineering Scaffolds; Elsevier: Amsterdam, The Netherlands, 2019; Volume 1, ISBN 9780081025642. [Google Scholar]
- Visconti, R.P.; Kasyanov, V.; Gentile, C.; Zhang, J.; Markwald, R.R.; Mironov, V. Towards organ printing: Engineering an intra-organ branched vascular tree. Expert. Opin. Biol. Ther. 2010, 10, 409–420. [Google Scholar] [CrossRef]
- Derby, B. Printing and prototyping of tissues and scaffolds. Science 2012, 338, 921–992. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Dababneh, A.B.; Ozbolat, I.T. Bioprinting technology: A current state-of-the-art review. J. Manuf. Sci. Eng. 2014, 136, 061016. [Google Scholar] [CrossRef]
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.H. 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 2016, 34, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Tian, X.; Schreyer, D.J.; Chen, X. Effect of needle geometry on flow rate and cell damage in the dispensing-based biofabrication process. Biotechnol. Prog. 2011, 27, 1777–1784. [Google Scholar] [CrossRef]
- Guyot, Y.; Papantoniou, I.; Chai, Y.C.; Van Bael, S.; Schrooten, J.; Geris, L. A computational model for cell/ECM growth on 3D surfaces using the level set method: A bone tissue engineering case study. Biomech. Model. Mechanobiol. 2014, 13, 1361–1371. [Google Scholar] [CrossRef] [PubMed]
- Guyot, Y.; Luyten, F.P.; Schrooten, J.; Papantoniou, I.; Geris, L. A three dimensional computational fluid dynamics model of shear stress distribution during neotissue growth in a perfusion bioreactor. Biotechnol. Bioeng. 2015, 112, 2591–2600. [Google Scholar] [CrossRef]
- Guyot, Y.; Papantoniou, I.; Luyten, F.P.; Geris, L. Coupling curvature dependent and shear stress-stimulated neotissue growth in dynamic bioreactor cultures: A 3D computational model of a complete scaffold. Biomech. Model. Mechanobiol. 2016, 15, 169–180. [Google Scholar] [CrossRef]
- Billiet, T.; Gevaert, E.; De Schryver, T.; Cornelissen, M.; Dubruel, P. The 3D printing of gelatin methacrylamide cell-laden tissue-engineered constructs with high cell viability. Biomaterials 2014, 35, 49–62. [Google Scholar] [CrossRef]
- Reina-Romo, E.; Papantoniou, I.; Bloemen, V.; Geris, L. Computational design of tissue engineering scaffolds. In Handbook of Tissue Engineering Scaffolds; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
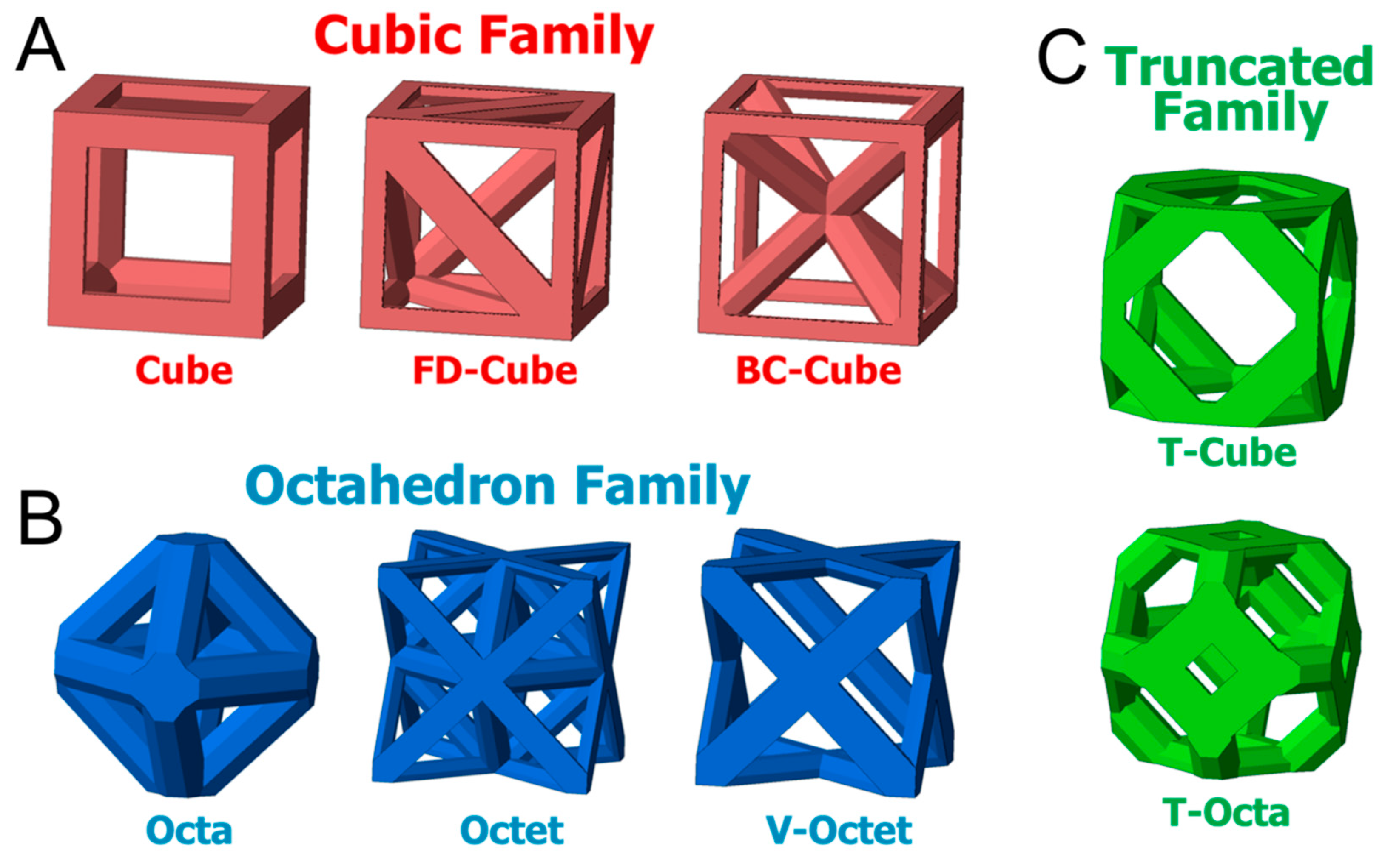
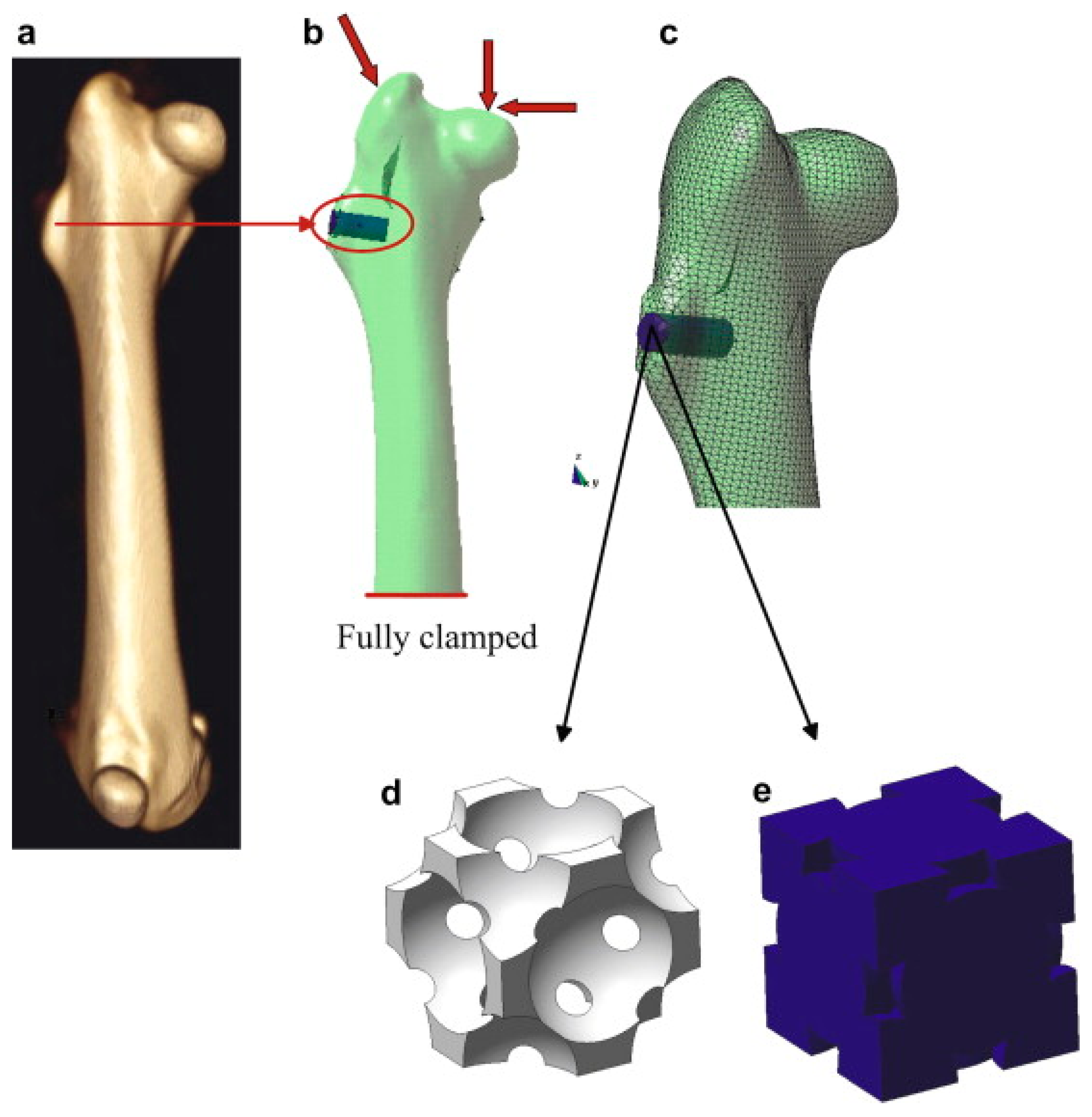
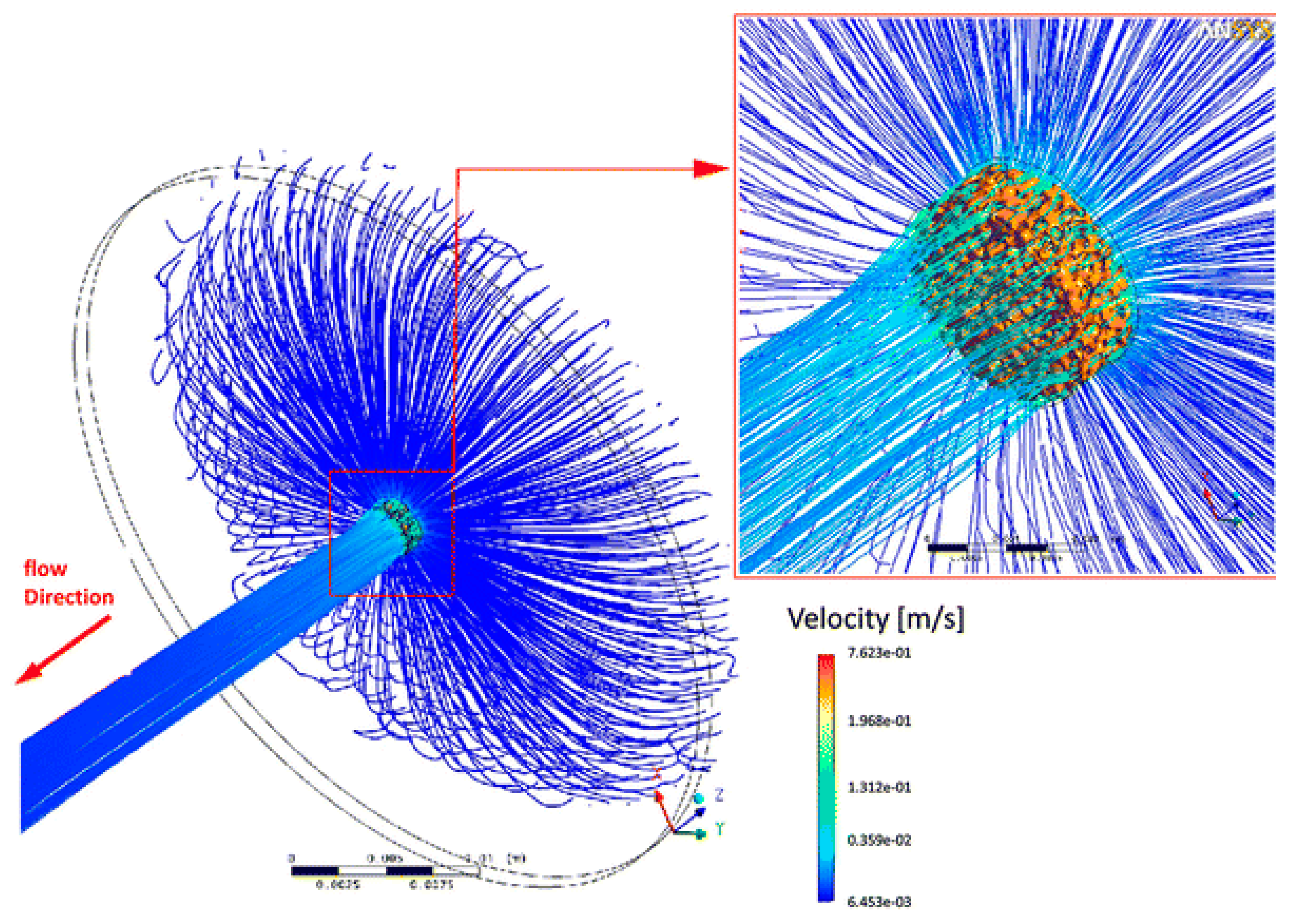
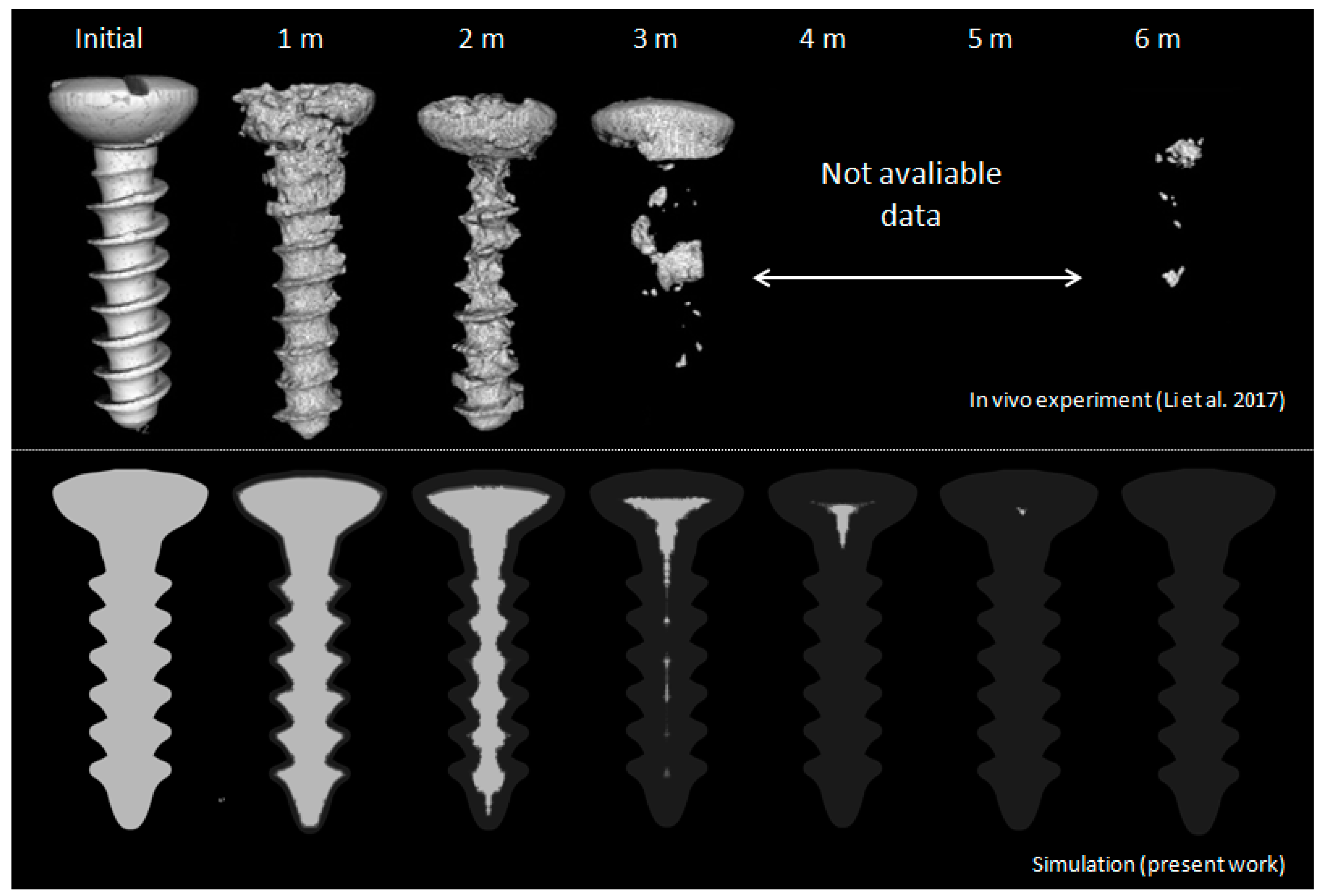
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanz-Herrera, J.A.; Reina-Romo, E. Continuum Modeling and Simulation in Bone Tissue Engineering. Appl. Sci. 2019, 9, 3674. https://doi.org/10.3390/app9183674
Sanz-Herrera JA, Reina-Romo E. Continuum Modeling and Simulation in Bone Tissue Engineering. Applied Sciences. 2019; 9(18):3674. https://doi.org/10.3390/app9183674
Chicago/Turabian StyleSanz-Herrera, Jose A., and Esther Reina-Romo. 2019. "Continuum Modeling and Simulation in Bone Tissue Engineering" Applied Sciences 9, no. 18: 3674. https://doi.org/10.3390/app9183674
APA StyleSanz-Herrera, J. A., & Reina-Romo, E. (2019). Continuum Modeling and Simulation in Bone Tissue Engineering. Applied Sciences, 9(18), 3674. https://doi.org/10.3390/app9183674