Abstract
Allotropic 2D materials are the new frontier of materials science, due to their unique strategic properties and application within several sciences. Allotropic 2D materials have shown tunable physical, chemical, biochemical, and optical characteristics, and among the allotropic materials, graphene has been widely investigated for its interesting properties, which are highly required in biomedical applications. Recently, the synthesis of thin 2D boron sheets, developed on Ag(111) substrates, was able to create a 2D triangular structure called borophene (BO). Borophene has consistently shown anisotropic behavior similar to graphene. In this topical review, we will describe the main properties and latest applications of borophene. This review will critically describe the most interesting uses of borophene as part of electronic and optical circuits. Moreover, we will report how borophene can be an innovative component of sensors within biomedical devices, and we will discuss its use in nanotechnologies and theranostic applications. The conclusions will provide insight into the latest frontiers of translational medicine involving this novel and strategic 2D allotropic material.
1. Introduction
The recent development of cutting-edge nanotechnologies has allowed researchers to achieve significant steps forward in the study of new materials that are applicable to medical biotechnologies. Recently, growing attention has been paid to an interesting 2D material, graphene. After the discovery of this smart and versatile material, scientific researchers have incorporated it into nanomaterials and nanotechnologies [1] with potential applications for innovative devices and medical disposables.
Generally, 2D materials have remarkable physical properties that are applicable to electronic devices, while recently they have found applications in several new technologies in the field of photonics, energy conversion, and nano-engineering [2].
Recently, the synthesis of thin 2D boron sheets, developed on Ag(111) substrates, has resulted in the creation of a 2D triangular structure [3] called borophene (BO).
Unlike other allotrope materials, borophene has marked anisotropic properties; for instance, Young’s modulus, measured along the smoothest surface of a borophene sheet, is higher than graphene [4]. Some researchers working on the thermic conductivity of borophene have shown that it can be explained because of its anisotropy; nevertheless, compared to other similar materials, the thermal properties of borophene are lower, due to the dispersive effect of its phonons. The Poisson’s ratio and the thermal expansion coefficients are also low [3,4].
On the other hand, the anisotropic optical properties reported on borophene sheets are quite interesting, as they hold high optical transparency [5].
Therefore, borophene certainly has peculiar properties, and can be considered a 2D allotropic material, with several aspects that are applicable to medical devices.
In this topical review, we will describe the main properties and the main applications of borophene, with a specific interest towards the biomedical field. In particular, we will describe the main properties of borophene, taking into consideration its molecular and crystal structures. We will further report how structures of borophene can have an impact on its ability to work in optical circuits, on electronic devices, and in biomedical sensors. We will carefully describe the main studies concerning the most important applications of borophene in the medical field, and try to express our perspective on the future challenges that could be overcome as a result of to this promising material. Borophene certainly has the potential for new healthcare applications, which should be of interest to materials scientists.
2. Main Properties of Borophene
The naïve 3D structure of boron makes it neither a metal nor a non-metal: it can be considered a metalloid, commonly used in the production of semiconductors. On the other hand, boron starts to show interesting metallic properties when it holds a two-dimensional structure, similar to other allotropic materials such as graphene. Nevertheless, borophene is made with a different technique; in fact, it is obtained through the physical vapor deposition (PVD) technique, which works by vaporizing boron on a thin film of silver [2] (Figure 1).
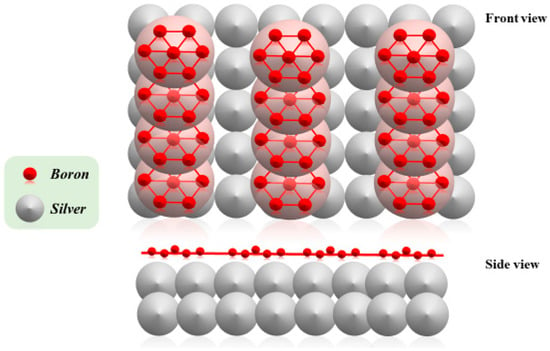
Figure 1.
Atomic geometry of borophene on Ag(111), in front (top) and side (bottom) views.
Borophene surfaces are very different from those observables on graphene; in fact, borophene has ridges with a morphology that strongly depends on how deep the boron atoms bind together. These structural features make borophene a polymorphous and anisotropic material [6].
Some studies carried out using the density functional theory (DFT) have permitted the investigation of three different polymorphic structures of borophene: 2-Pmmn, β12, and χ3. All these structures can show different behaviors under similar compression and torsion forces with deformation energies lower than 100 meV/atom, a peculiarity that could be suitable for the designing of nanometric devices (Figure 2) [7].
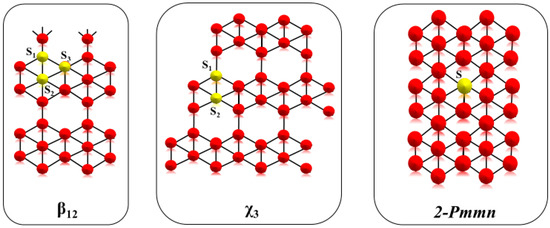
Figure 2.
Crystal structures of β12, χ3 and 2-Pmmn.
In materials science, thermal conductivity plays an important role in defining the performances and lifecycle of a material. Thermal conductivity of borophene has been studied by several research groups [8], and recently thermal conductivity has been investigated by means of the transmission of phonons along the surface, thus allowing verification that thermal conductivity is in fact linked to anisotropy and frequency of phonons.
The properties of borophene have aroused great interest among the scientific community [9,10], and structural and physical experiments have been performed over the years to find the mechanical coefficients of the different forms of borophene (2-Pmmn, β12, χ3) [10].
Chemical reactions could modify some mechanical values of borophene; for instance, electrochemical fluorination is able to decrease the coefficients on the different forms of borophene [10]. In recent studies on fluorinated boron, particular attention has been paid to the structure of 2-Pmmn borophene after a fluorination reaction. In fact, two highly stable anisotropic structures have been obtained during such studies: B4F and B2F [11]. Both these structures were found to be excellent candidates for the production of semiconductors in electronic devices, mainly thanks to their excellent mechanical, thermal, and electrical conductivity properties.
Optical coefficients have also been linked to the anisotropy of the structure of borophene; we can obtain different data based on the different direction of the stimuli on the material’s surface [5].
It should be noted that the B2F structure has excellent optical conductivity in the visible region, and this peculiarity makes it perfect for optical devices (Figure 3).
Figure 3.
Optical coefficients linked to the anisotropy of the structure of borophene make it ideal for optical circuits.
Numerous 2D boron nanostructures have been created by research groups over time. However, monoatomic-thin boron layers have energy that is higher than 3D bulk-state boron, and this fact means that the 2D structure of boron is thermodynamically unstable. In this landscape, a metallic substrate is necessary to counteract the 3D nucleation barrier in order to attract the boron atoms into the 2D monoatomic-thin Boron layer form. Borophene that has been successfully synthesized on the surface of Ag(111) by deposition has also been also defined as an “atomic-thin two-dimensional boron sheet” [12].
The stability of the different forms of borophene is still debated in the scientific community. An experimental study based on first-principles calculations investigated the mechanical, dynamic, and thermodynamic stabilities of striped β12 and χ3 borophene. The results showed that β12 and χ3 borophene have good mechanical, dynamic, and thermodynamic stabilities; on the other hand, striped borophene is not equally stable. The origin of the high stiffness and high instability observed in striped borophene, along the “a” direction, can be attributed both to strong directional bonding and to high stiffness along the “a” direction [13]. Another study conducted by Gao et al. also confirmed the thermodynamic stability of β12 borophene and χ3 borophene [14]. On the contrary, Penev et al. showed that both β12 borophene and χ3 borophene could show naïve phonon-mediated superconductivity [15]. Furthermore, interesting results obtained by first-principles calculations and STM image simulation indicated that β12 and χ3 sheets are thermodynamically unstable [16]. Due to the structural instability of borophene, some researchers have theoretically hypothesized a fully hydrogenated structure of borophene defined as “borophene”, a structure that showed excellent stability in silico [17]. Finally, the good optoelectronic properties and ability to be a superconductor allow borophene-based nanostructures to be strategic for the production of nano-devices that are applicable in different medical fields [18].
2.1. Borophene in Electronic Applications for Medical Devices
The polymorphism showed by 2D boron is quite different from the other 2D materials; the substrate created on its surface plays a key role in the structural stability of borophene. Borophene can have different energy spectra depending on the type of metal substrate [19], and metals with different bases of borophene β12 such as Ag, Cu, Au, and Ni can develop energies ranging from 0.4 to 2.1 meV, or from 15.1 to 28.9 meV. The above-reported behavior allows us to define the degree of polymorphism shown by borophene properly.
Due to the low mass of boron, a strong electron–phonon coupling can amplify the superconductivity of phonons and their propagation. Moreover, the hexagonal molecular geometry that characterizes the structure of borophene and the different critical temperatures, ranging from 10 to over 20 K, are the parameters that make borophene a superconductor [20]. In fact, superconducting transition temperatures have been found to be much higher than theoretically predicted and experimentally observed in other similar materials, such as graphene.
As previously stated, the different forms of borophene hold different properties that allow their application in many different biomedical fields, and the ability of borophene to act as an energy source in combination with other materials is promising for the production of electronic biomedical devices requiring a long-lasting charging, such as implantable or wearable devices. Of course, this application needs to be improved, especially in the charge/discharge processes, in regards to the safety of such high-capacity charging, in terms of charge duration, and so on.
In this context, an important role could be played by borophene in the phase of 2-Pmmn. In a recent study, 2-Pmmn borophene was considered to be a good anode for Li-ion batteries because of the massive oxidative processes that this specific material are able to promote; moreover, 2-Pmmn borophene ensures an elevated conductivity that allows a charge/discharge process without any energy dispersion [21]. Some modifications to 2-Pmmn borophene, such as doping, hydrogenation, or stratifications, may affect the interactions among borophene and other different materials; for example, a lower efficiency is reached between Li and hydrogenated borophene. On the other hand, in Li-Sulfide (LiS) batteries, the use of β12 and χ3 borophene impacts the levels of energy stored. Specifically, the χ3 borophene adapts its structure to absorb energy within LiS batteries, thus reducing the alteration of the structures during the charge/discharge cycles. It is also worth noting that β12 borophene within LiS batteries has shown overall structural stability, even if it seems to ensure a lower adsorption of energy [22].
Excellent results have also been obtained in supercapacitors made with several layers of borophene [23], even after more than 6000 discharge cycles. The use of these multi-layered borophene-based supercapacitors is promising in medical devices, with a special interest towards the rehabilitative robotics, where the storage of energy is fundamental to ensure a correct functionality over time. Furthermore, biomedical implantable devices (IMDs) [24] often require the use of an external charger, and borophene-based technologies can significantly reduce charging time from hours to minutes, thus reducing the limits of these technologies. The use of hybrid power, consisting of LiS and β12 batteries, when applied to IMDs will ensure faster charge/discharge cycles; furthermore, the supercapacitors will allow the storing of a higher quantity of energy, which could be used as a strategic energy reserve, especially in devices that need to last for long periods.
2.2. Borophene in Sensors for Medical Devices
Borophene could be applied for the production of microelectronic sensors and biosensors with high sensibility in signal transmission and detection.
Recent studies have investigated the crystalline structures that characterize B36 borophene, as it can be highly interesting in translational applications related to physics, chemistry, and other fields of microelectronics [25]. Specifically, the encouraging results obtained from B36 borophene in the realization of new biosensors for gas detection have been the first step for further studies promoted within the scientific community. Formaldehyde (HCOH) is known to be highly toxic; however, it is commonly used in many industrial and medical fields because of its high thermal stability and good chemical reactivity. The potential use of B36 borophene as a HCOH biosensor was evaluated by analyzing its consolidated hexagonal hollow structure; specifically, the energy absorption of different HCOH was evaluated by plotting the profiles of LUMO (lowest unoccupied molecular orbital) B36 borophene and HOMO (highest occupied molecular orbital) B36 borophene. In this context, B36 borophene can reliably play the role of a high-conductivity semiconductor, thus generating an electrical signal in the presence of HCOH (Figure 4).
Figure 4.
Borophene can reliably play the role of a high-conductivity conductor, thus generating electrical signals in the presence of samples to analyze.
Finally, the use of borophene for the improvement of 2D nanometric devices has been recently reported [26], in which the hexagonal structure of B36 borophene showed excellent properties, making it ideal for 2D nanostructures to be integrated into biometric sensors.
2.3. Borophene in DNA Sequencing
In a recent study [27], DNA sequencing was performed by defining the order of different bases (thymine (T), adenine (A), cytosine (C), and guanine (G)) as a function of energy absorption and described in turn as a variation of the electric conductivity within B36 borophene nano-wells. Density functional theory (DFT) can explain the level of energy absorption shown by each nitrogenous base observed within the hexagonal structures of the B36 while plotting the HOMO and LUMO profiles. It is well-known that each nitrogenous base, when it comes into contact with B36 borophene, has a different level of energy absorption, with a different electrical conductivity reaction. These variations can be translated with a specific electrical signal. The future challenge is to produce electronic devices based on hybrid nanostructures containing B36 borophene, which will be able to correctly define the DNA sequences by transducing different specific electrical signals.
2.4. Borophene in Nanotechnologies
Researchers are looking for new applications for nanotechnologies by creating hybrid nanomaterials that are able to ensure the highest physical, chemical, and electronic properties.
In regards to physical properties, good flexibility in nanometric structures is highly sought after in the biomedical field. In this context [28], δ6 borophene is considered highly suitable when presented on the thin wearable strips, as its flexibility without any torsional stress can alter its molecular structure. Furthermore, all borophene structures are perfectly functional in both strip and tubular conformations, and this behavior allows the molecular, physical, and mechanical characteristics to be maintained in both 2D and 3D conformations. In nanotechnologies, borophene is often used in combination with other metals such as Ag, Si, Cu, and Au, enhancing their properties without negatively impacting on any biomedical applications.
2.5. Borophene in Radiology for Medical Applications
The applicative principles of the electronic devices are based on one (or more) sources that are able to generate electrical stimuli [24], and one structure, biological or artificial, that is able to detect and amplify such stimuli, mainly arising from tissues located in the human body. Modern microscopic devices are directly implanted into the tissues or organs of patients, such as pacemakers or the automatic defibrillators. Borophene could increase the performances or prolong the lifecycles of biomedical devices, both in nano-strips and integrated with other metals. One example of a ready-to-use device is the portable radiograph [29]. X-rays are produced via a complex process requiring energy. X-ray intensity has been reported to be energy-dependent; in portable disposables, low-intensity X-rays have been linked to the low-power released by batteries. Under some clinical conditions, patients cannot be moved to undergo a traditional X-ray procedure on a fixed radiograph; therefore, portable X-ray machines are crucial in order to acquire diagnoses and monitor up-to-date clinical conditions. In this light, portable devices, including borophene-based supercapacitors with a battery as its power source, could be a breakthrough in medical radiology. Moreover, given the ability of borophene to serve as a supercapacitor, future portable devices will be able to generate X-rays with a tunable power, according to the voltage set on the X-ray tube.
3. Conclusions and Future Insights
Growing scientific evidence has pointed out that borophene has many overlapping attributes with graphene; their mechanical and chemical characteristics are so similar to be easily comparable, even at a nanometric scale. The similarities with graphene, together with the numerous properties and potential applications previously reported, make borophene a promising 2D allotropic material for biomedical devices (Figure 5).
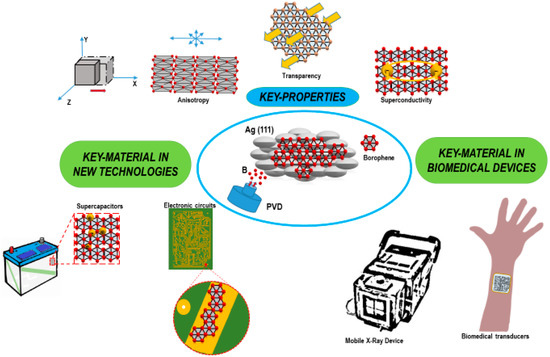
Figure 5.
Strategic applications of borophene (BO) in biomedical fields.
Lately, hydrogel-based carriers, typically consisting of polymeric networks, have moved the challenge forward towards a combination of borophene with graphene for smart and brand-new applications. Song et al. [30] reported how these two biomaterials, if well integrated, may lead to a mutual improvement in their electrical, mechanical, and biocompatibility properties.
The development of new devices based on interactive hybrid materials, involving the combination of graphene, borophene and hydrogel-based polymers, will improve translational research on wearable and implantable biosensors. In fact, hydrogels have already been applied in regenerative medicine, as scaffolds [31,32,33,34,35,36] and adhesive scaffolds for superficial micro-transducers, thanks to their flexibility, biocompatibility, and molecular structure [34]. Borophene has many interesting properties that certainly makes it strategic in the production of electric and optical biosensors; however, graphene-based appliances are still considered the gold standard in these fields. Borophene coupled with hydrogel-based substrates can be considered a smart and highly sensitive way to transform temperature, pH, and other stimuli into electrical, optical, and mechanical signals (the common bio-detectors used to detect analytes in the blood through the variation of electrical impedance). Thanks to borophene, they will be considerably improved if combined with the right metals [19]. Hybrid biosensors, composed of thin-layered hydrogels and borophene-based strips [37], will be useful in the fabrication of supercapacitors that are highly flexible and therefore usable on user-friendly micro-devices.
Furthermore, the high electrical conductivity of borophene will increasingly improve the performances of those new devices, as it is designed to respond to different stimuli simultaneously.
Given that the microbiological contamination of medical devices should be carefully considered, the bacteriostatic potential of graphene oxide has been widely evaluated in recent years [34]. Biologically compatible drug-releasing systems containing chips of borophene have also been considered for their antibacterial and theranostic properties. Although the current literature only reports a few authors describing preliminary information about the antibiotic skills of borophene [2,27,34], this topic represents a crucial step. In fact, this aspect impacts on at least two stages: the stage where the medical devices are built and produced, and the stage during which the clinicians need to translate the transplantation/application of these appliances in vivo.
Future research should investigate the potential issues related to the biomedical applications of borophene in in more depth. A proper approach should primarily consider such protocols that need to take several clinical conditions into consideration, such as syndromic conditions [35,36,37,38] or oncological conditions [39,40,41]. Moreover, to translate such cutting-edge applications of borophene, scientists will need to perform more in-depth studies, especially on the durational stability and cytotoxic effects of borophene, with specific attention to its behavior when in close contact with biological tissues. Finally, interactions with external substances, such as smoking metabolites [42] or metallic appliances [43], should be carefully and preventively considered before clinical use.
In conclusion, borophene is a promising biomaterial. It can be considered “the chameleon” of biomedical materials, as it can behave in different ways and exist in different phases, showing different chemical and physical characteristics that are heterogeneously applicable to medical devices and personalized biomedicine.
Author Contributions
All authors contributed to the conceptualization and the methodology of this article; data curation, M.T., F.G., G.S. and I.M.; writing—original draft preparation, M.T., B.Z., G.S., F.G., I.M., B.C., S.R. and L.F.; supervision, M.T., G.S., B.Z. and L.F.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Novoselov, K.S.; Fal’ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Mannix, A.J.; Zhang, Z.; Guisinger, N.P.; Yakobson, B.I.; Hersam, M.C. Borophene as a prototype for synthetic 2D materials development. Nat. Nanotechnol. 2018, 13, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Mannix, A.J.; Zhou, X.F.; Kiraly, B.; Wood, J.D.; Alducin, D.; Myers, B.D.; Liu, X.; Fisher, B.L.; Santiago, U.; Guest, J.R.; et al. Synthesis of borophenes: Anisotropic, two-dimensional boron polymorphs. Science 2015, 350, 1513–1516. [Google Scholar] [CrossRef]
- Sun, H.; Li, Q.; Wan, X.G. First-principles study of thermal properties of borophene. Phys. Chem. Chem. Phys. 2016, 18, 14927–14932. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Zhang, H.; Shao, H.; Xu, Y.; Zhang, R.; Zhu, H. The electronic, optical, and thermodynamic properties of borophene from first-principles calculations. J. Mater. Chem. C 2016, 4, 3592–3598. [Google Scholar] [CrossRef]
- Khanifaev, J.; Pekoz, R.; Konuk, M.; Durgun, E. The interaction of halogen atoms and molecules with borophene. Phys. Chem. Chem. Phys. 2017, 19, 28963–28969. [Google Scholar] [CrossRef]
- Shukla, V.; Grigoriev, A.; Jena, N.K.; Ahuja, R. Strain controlled electronic and transport anisotropies in two-dimensional borophene sheets. Phys. Chem. Chem. Phys. 2018, 20, 22952–22960. [Google Scholar] [CrossRef]
- Li, D.; He, J.; Ding, G.; Tang, Q.; Ying, Y.; He, J.; Tang, Q.; Ying, Y.; He, J.; Zhong, C.; et al. Stretch-Driven Increase in Ultrahigh Thermal Conductance of Hydrogenated Borophene and Dimensionality Crossover in Phonon Transmission. Adv. Funct. Mater. 2018, 28, 1801685. [Google Scholar] [CrossRef]
- Paduano, F.; Marrelli, M.; Amantea, M.; Rengo, C.; Rengo, S.; Goldberg, M.; Spagnuolo, G.; Tatullo, M. Adipose tissue as a strategic source of mesenchymal stem cells in bone regeneration: A topical review on the most promising craniomaxillofacial applications. Int. J. Mol. Sci. 2017, 18, 2140. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, T.Y.; Wang, H.Q.; Feng, Y.P.; Zheng, J.C. High anisotropy of fully hydrogenated borophene. Phys. Chem. Chem. Phys. 2016, 18, 31424–31430. [Google Scholar] [CrossRef] [PubMed]
- Pekoz, R.; Konuk, M.; Kilic, M.E.; Durgun, E. Two-Dimensional Fluorinated Boron Sheets: Mechanical, Electronic, and Thermal Properties. ACS Omega 2018, 3, 1815–1822. [Google Scholar] [CrossRef]
- Kunstmann, J.; Quandt, A. Broad boron sheets and boron nanotubes: An ab initio study of structural, electronic, and mechanical properties. Phys. Rev. B 2006, 74, 035413. [Google Scholar] [CrossRef]
- Peng, B.; Zhang, H.; Shao, H.; Ning, Z.; Xu, Y.; Ni, G.; Lu, H.; Zhang, D.W.; Zhu, H. Stability and strength of atomically thin borophene from first principles calculations. Mater. Res. Lett. 2017, 5, 399–407. [Google Scholar] [CrossRef]
- Gao, M.; Li, Q.-Z.; Yan, X.-W.; Wang, J. Prediction of phonon-mediated superconductivity in borophene. Phys. Rev. B 2017, 95, 024505. [Google Scholar] [CrossRef]
- Penev, E.S.; Kutana, A.; Yakobson, B.I. Can two-dimensional boron superconduct? Nano Lett. 2016, 16, 2522–2526. [Google Scholar] [CrossRef]
- Luo, Z.; Fan, X.; An, Y. First-principles study on the stability and stm image of borophene. Nanoscale Res. Lett. 2017, 12, 514. [Google Scholar] [CrossRef]
- Xu, L.C.; Du, A.; Kou, L. Hydrogenated borophene as a stable two-dimensional dirac material with an ultrahigh fermi velocity. Phys. Chem. Chem. Phys. 2016, 18, 27284–27289. [Google Scholar] [CrossRef]
- Tian, Y.; Guo, Z.; Zhang, T.; Lin, H.; Li, Z.; Chen, J.; Deng, S.; Liu, F. Inorganic boron-based nanostructures: Synthesis, optoelectronic properties, and prospective applications. Nanomaterials (Basel) 2019, 9, 538. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, Y.; Gao, G.; Yakobson, B.I. Two-Dimensional Boron Monolayers Mediated by Metal Substrates. Angew. Chem. Int. Ed. Engl. 2015, 54, 13022–13026. [Google Scholar] [CrossRef]
- Liao, J.H.; Zhao, Y.C.; Zhao, Y.J.; Xu, H.; Yang, X.B. Phonon-mediated superconductivity in Mg intercalated bilayer borophenes. Phys. Chem. Chem. Phys. 2017, 19, 29237–29243. [Google Scholar] [CrossRef]
- Jiang, H.R.; Lu, Z.; Wu, M.C.; Ciucci, F.; Zhao, T.S. Borophene: A promising anode material offering high specific capacity and high rate capability for lithium-ion batteries. Nano Energy 2016, 23, 97–104. [Google Scholar] [CrossRef]
- Jiang, H.R.; Shy, W.; Liu, M.; Ren, Y.X.; Zhao, T.S. Borophene and defective borophene as potential anchoring materials for lithium–sulfur batteries: A first-principles study. J. Mater. Chem. A 2018, 6, 2107–2114. [Google Scholar] [CrossRef]
- Li, H.; Jing, L.; Liu, W.; Lin, J.; Tay, R.Y.; Tsang, S.H.; Teo, E.H.T. Scalable Production of Few-Layer Boron Sheets by Liquid-Phase Exfoliation and Their Superior Supercapacitive Performance. ACS Nano 2018, 12, 1262–1272. [Google Scholar] [CrossRef]
- Kerativitayanan, P.; Tatullo, M.; Khariton, M.; Joshi, P.; Perniconi, B.; Gaharwar, A.K. Nanoengineered Osteoinductive and Elastomeric Scaffolds for Bone Tissue Engineering. ACS Biomater. Sci. Eng. 2017, 34, 590–600. [Google Scholar] [CrossRef]
- Kootenaei, A.S.; Ansari, G. B36 borophene as an electronic sensor for formaldehyde: Quantum chemical analysis. Phys. Lett. A 2016, 380, 2664–2668. [Google Scholar] [CrossRef]
- Kondo, T. Recent progress in boron nanomaterials. Sci. Technol. Adv. Mater. 2017, 18, 780–804. [Google Scholar] [CrossRef]
- Rastgou, A.; Soleymanabadi, H.; Bodaghi, A. DNA sequencing by borophene nanosheet via an electronic response: A theoretical study. Microelectron. Eng. 2017, 169, 9–15. [Google Scholar] [CrossRef]
- Adamska, L.; Sadasivam, S.; Foley, J.J., IV; Darancet, P.; Sharifzadeh, S. First-principles investigation of borophene as a monolayer transparent conductor. J. Phys. Chem. C 2018, 122, 4037–4045. [Google Scholar] [CrossRef]
- Kim, Y.P.; Park, Y.P.; Cheon, M.W. A study on the characteristics of mobile X-ray device using supercapacitor as internal power. J. Xray Sci. Technol. 2018, 26, 777–784. [Google Scholar] [CrossRef]
- Song, H.S.; Kwon, O.S.; Kim, J.H.; Conde, J.; Artzi, N. 3D hydrogel scaffold doped with 2D graphene materials for biosensors and bioelectronics. Biosens. Bioelectron. 2017, 89, 187–200. [Google Scholar] [CrossRef]
- Aulino, P.; Costa, A.; Chiaravalloti, E.; Perniconi, B.; Adamo, S.; Coletti, D.; Marrelli, M.; Tatullo, M.; Teodori, L. Muscle extracellular matrix scaffold is a multipotent environment. Int. J. Med. Sci. 2015, 12, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Paduano, F.; Marrelli, M.; Alom, N.; Amer, M.; White, L.J.; Shakesheff, K.M.; Tatullo, M. Decellularized bone extracellular matrix and human dental pulp stem cells as a construct for bone regeneration. J. Biomater. Sci. Polym. Ed. 2017, 28, 730–748. [Google Scholar] [CrossRef] [PubMed]
- Di Vito, A.; Giudice, A.; Chiarella, E.; Malara, N.; Bennardo, F.; Fortunato, L. In vitro long-term expansion and high osteogenic potential of periodontal ligament stem cells: More than a mirage. Cell Transpl. 2019, 28, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Patelis, N.; Schizas, D.; Liakakos, T.; Klonaris, C. Aortic Graft Infection: Graphene Shows the Way to an Infection-Resistant Vascular Graft. Front. Surg. 2017, 4, 25. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, M.; Tatullo, M.; Dipalma, G.; Inchingolo, F. Oral infection by staphylococcus aureus in patients affected by white sponge nevus: A description of two cases occurred in the same family. Int. J. Med. Sci. 2012, 9, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Figliuzzi, M.M.; Giudice, A.; Pileggi, S.; Pacifico, D.; Marrelli, M.; Tatullo, M.; Fortunato, L. Implant-prosthetic rehabilitation in bilateral agenesis of maxillary lateral incisors with a mini split crest. Case Rep. Dent. 2016, 2016, 3591321. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Inchingolo, F.; Tatullo, M.; Abenavoli, F.M.; Marrelli, M.; Inchingolo, A.D.; Gentile, M.; Inchingolo, A.M.; Dipalma, G. Non-syndromic multiple supernumerary teeth in a family unit with a normal karyotype: Case report. Int. J. Med. Sci. 2010, 7, 378–384. [Google Scholar] [CrossRef]
- Paduano, S.; Uomo, R.; Amato, M.; Riccitiello, F.; Simeone, M.; Valletta, R. Cyst-like periapical lesion healing in an orthodontic patient: A case report with five-year follow-up. G. Ital. Endod. 2013, 27, 95–104. [Google Scholar] [CrossRef]
- Tatullo, M.; Marrelli, M.; Amantea, M.; Paduano, F.; Santacroce, L.; Gentile, S.; Scacco, S. Bioimpedance Detection of Oral Lichen Planus Used as Preneoplastic Model. J. Cancer 2015, 6, 976–983. [Google Scholar] [CrossRef]
- Inchingolo, F.; Tatullo, M.; Abenavoli, F.M.; Marrelli, M.; Inchingolo, A.D.; Inchingolo, A.M.; Dipalma, G. Non-Hodgkin lymphoma affecting the tongue: Unusual intra-oral location. Head Neck Oncol. 2011, 3, 1. [Google Scholar] [CrossRef]
- Giudice, A.; Bennardo, F.; Barone, S.; Antonelli, A.; Figliuzzi, M.M.; Fortunato, L. Can autofluorescence guide surgeons in the treatment of medication-related osteonecrosis of the jaw? A prospective feasibility study. J. Oral Maxillofac. Surg. 2018, 76, 982–995. [Google Scholar] [CrossRef] [PubMed]
- Tatullo, M.; Gentile, S.; Paduano, F.; Santacroce, L.; Marrelli, M. Crosstalk between oral and general health status in e-smokers. Medicine (Baltimore) 2016, 95, e5589. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, F.; Tatullo, M.; Abenavoli, F.M.; Marrelli, M.; Inchingolo, A.D.; Palladino, A.; Inchingolo, A.M.; Dipalma, G. Oral piercing and oral diseases: A short time retrospective study. Int. J. Med. Sci. 2011, 8, 649–652. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).