Evaluating Litter Yield and Decomposition for Re-Vegetated Mangroves in a Subtropical Mudflat
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
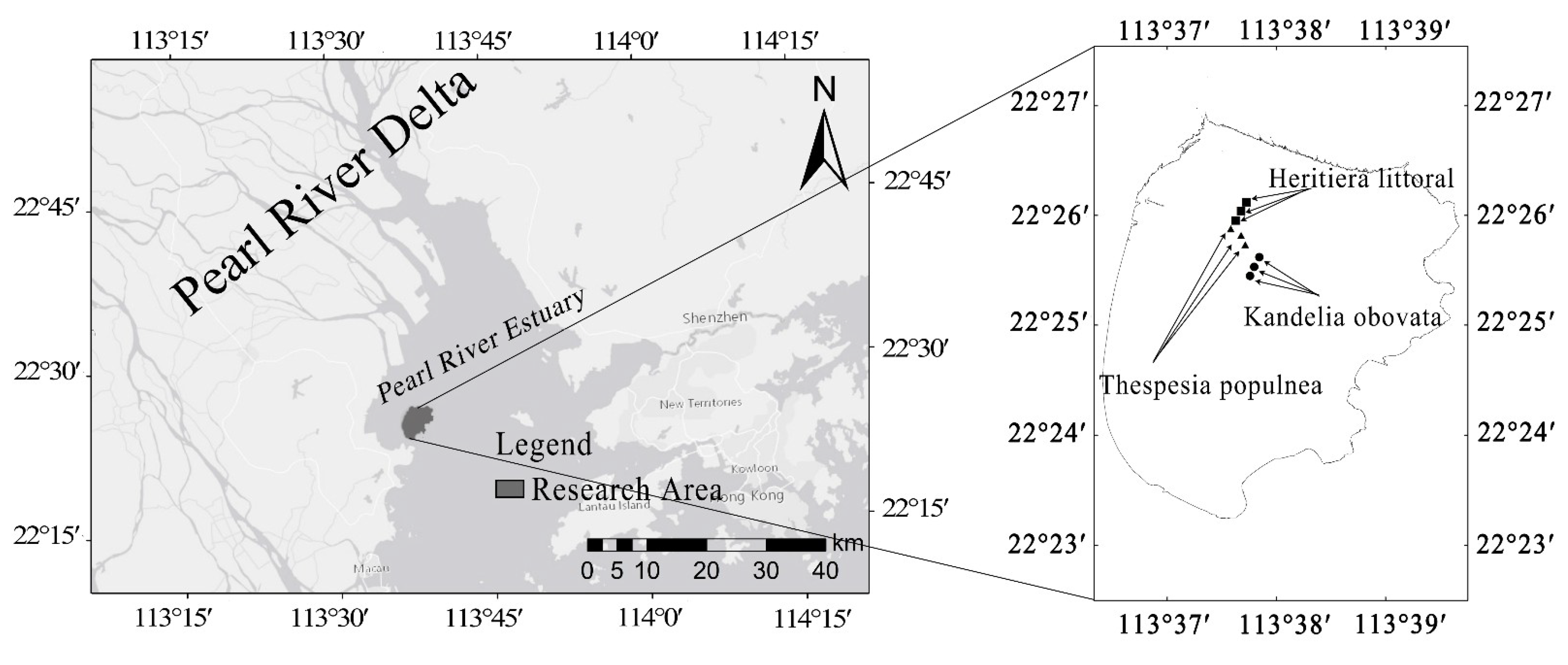
2.1. Selection of Study Site
2.2. Field Investigation and Sample Collection
2.2.1. Monitoring of Litterfall
2.2.2. Litter Decomposition Experiment
2.2.3. Monitoring of Soil Carbon Change
2.3. Laboratory Analysis
2.4. Statistical Analysis Methods
2.5. Calculation of Litter Weight Loss Rate and Soil Carbon Storage
3. Results
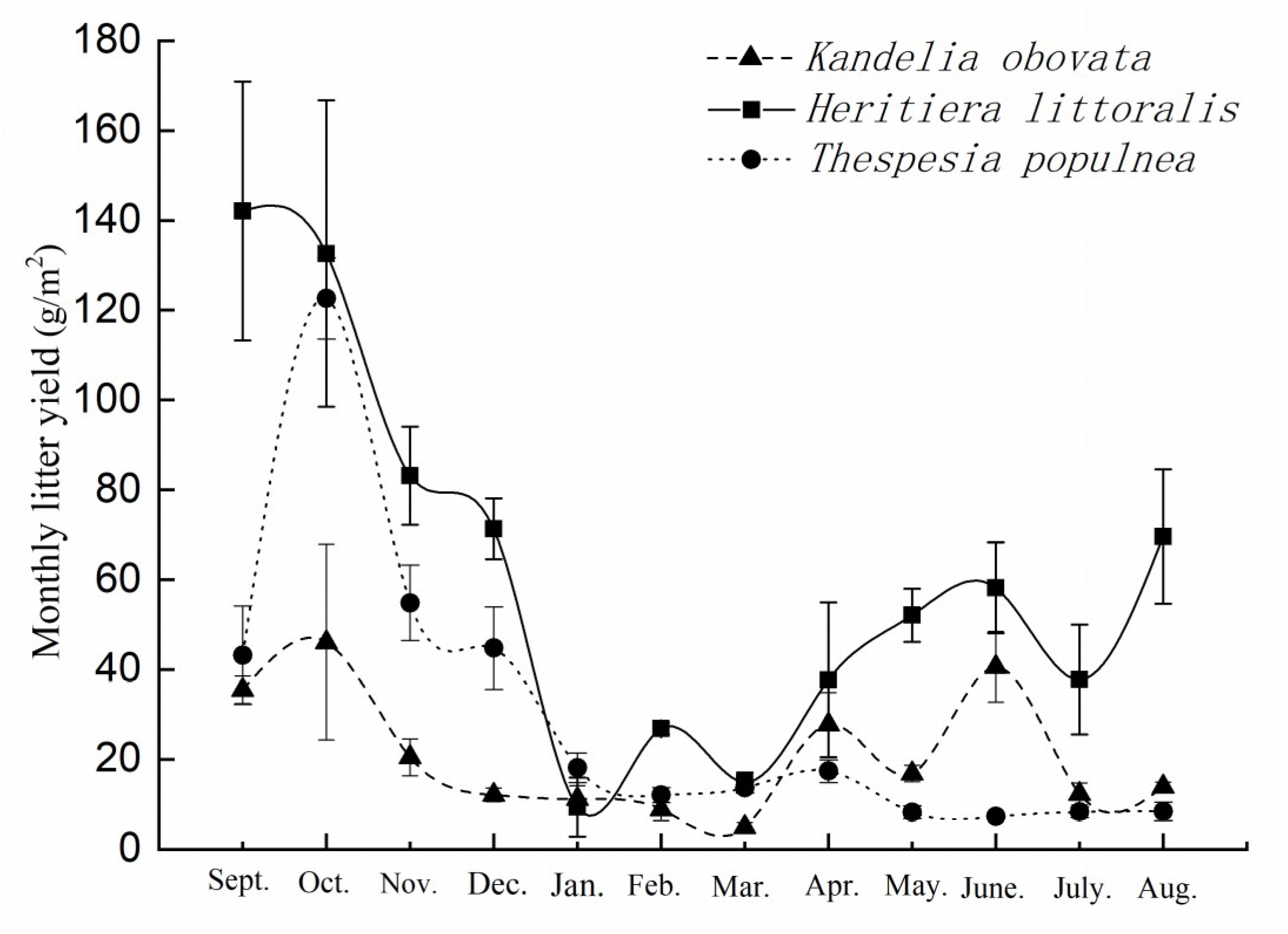
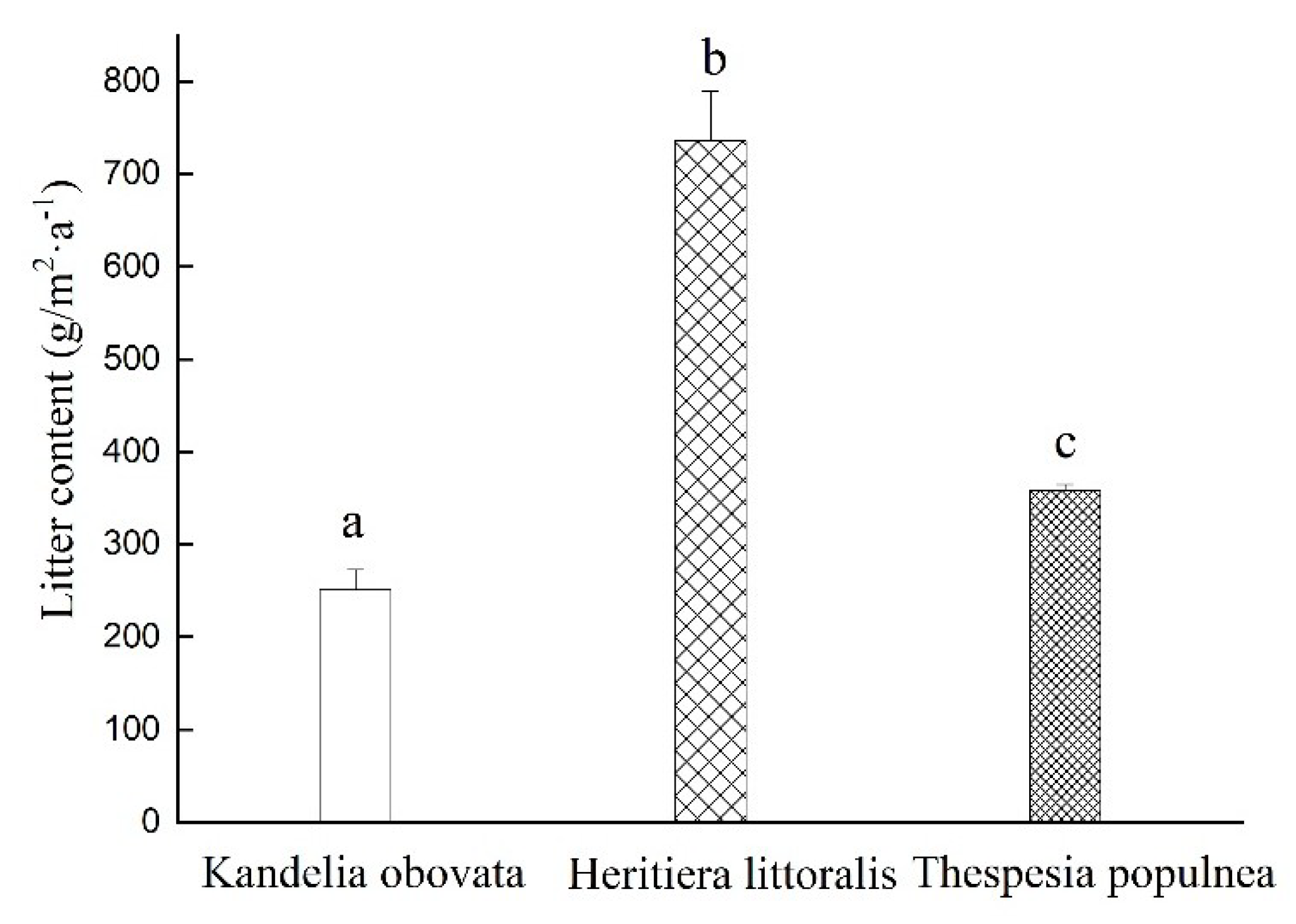
3.1. Litterfall Variation of the Three Selected Mangrove Species during the Monitoring Period
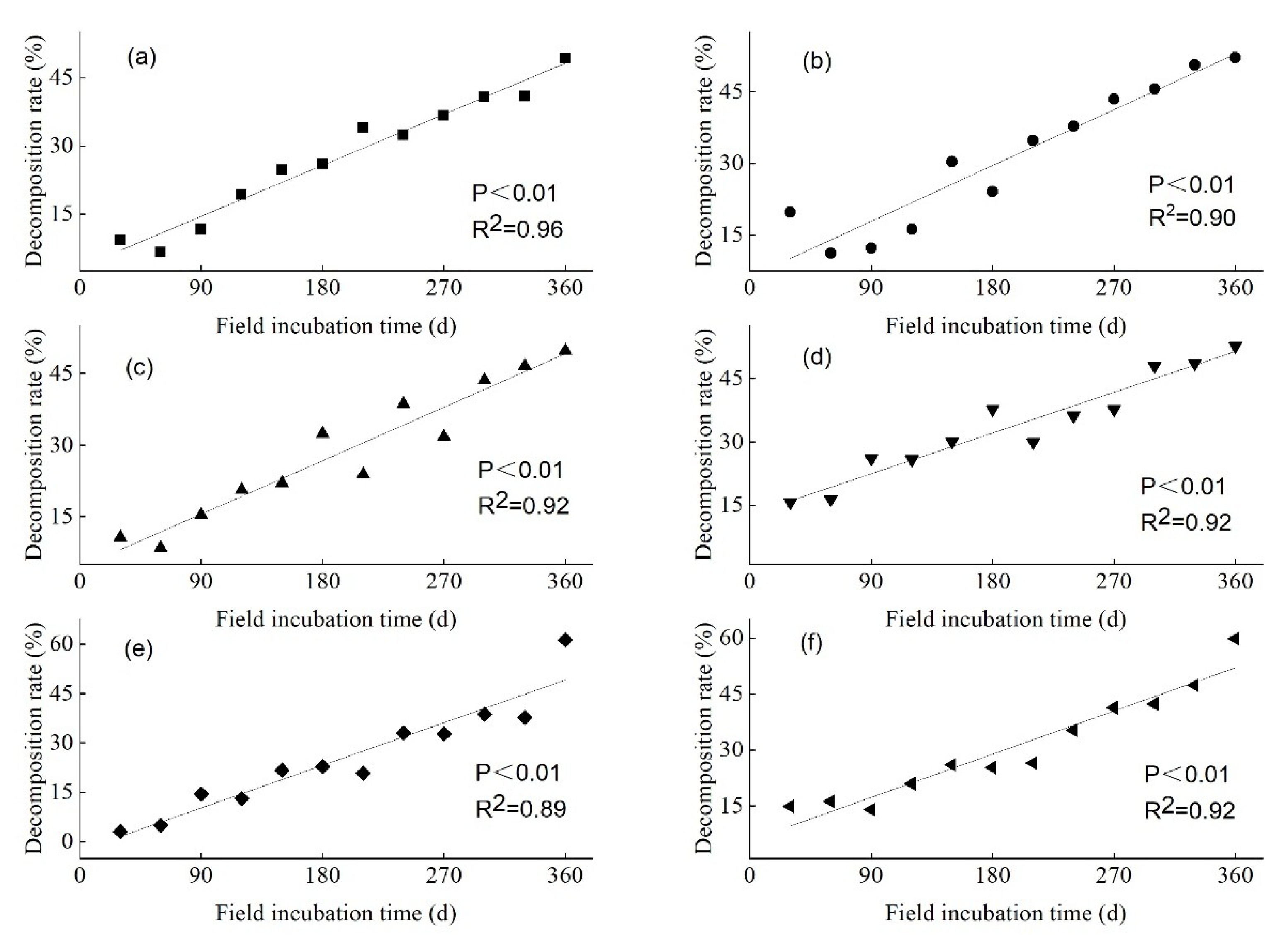
3.2. Litter WLR of the Three Mangrove Species over Varying Lengths of Field Incubation
3.3. Organic Carbon in the Soils Underneath the Added Litter Materials
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Barbier, E.B.; Hacker, S.D.; Kennedy, C.; Koch, E.W.; Stier, A.C.; Silliman, B.R. The value of estuarine and coastal ecosystem services. Ecol. Monogr. 2011, 81, 169–193. [Google Scholar] [CrossRef]
- Bao, H.; Wu, Y.; Unger, D.; Du, J.; Herbeck, L.S.; Zhang, J. Impact of the conversion of mangroves into aquaculture ponds on the sedimentary organic matter composition in a tidal flat estuary (Hainan Island, China). Cont. Shelf Res. 2013, 57, 82–91. [Google Scholar] [CrossRef]
- Chen, S.; Chen, B.; Sastrosuwondo, P.; Dharmawan, I.W.E.; Ou, D.; Yin, X.; Yu, W.; Chen, G. Ecosystem carbon stock of a tropical mangrove forest in North Sulawesi, Indonesia. Acta Oceanol. Sin. 2018, 37, 85–91. [Google Scholar] [CrossRef]
- Alongi, D.M. Present state and future of the world’s mangrove forests. Environ. Conserv. 2002, 29, 331–349. [Google Scholar] [CrossRef]
- Andres, K.; Savarese, M.; Bovard, B.; Parsons, M. Coastal wetland geomorphic and vegetative change: Effects of Sea-level rise and water management on brackish marshes. Estuaries Coasts 2019, 42, 1308–1327. [Google Scholar] [CrossRef]
- Hochard, J.P.; Hamilton, S.; Barbier, E.B. Mangroves shelter coastal economic activity from cyclones. Proc. Natl. Acad. Sci. USA 2019, 116, 12232–12237. [Google Scholar] [CrossRef] [Green Version]
- Gorman, D.; Turra, A. The role of mangrove revegetation as a means of restoring macrofaunal communities along degraded coasts. Sci. Total Environ. 2016, 566, 223–229. [Google Scholar] [CrossRef]
- Paula, A.L.D.; Lima, B.K.D.; Maia, R.C. The recovery of a degraded mangrove in Ceara through the production of Laguncularia racemosa (L.) CF Gaertn. (Combretaceae) and Avicennia sp stapf ex ridl (Acanthaceae) seedlings. Rev. Arvore 2016, 40, 377–385. [Google Scholar] [CrossRef]
- Zabbey, N.; Tanee, F.B.G. Assessment of asymmetric mangrove restoration trials in Ogoniland, Niger Delta, Nigeria: Lessons for Future Intervention. Ecol. Restor. 2016, 34, 245–257. [Google Scholar] [CrossRef]
- Bukoski, J.J.; Broadhead, J.S.; Donato, D.C.; Murdiyarso, D.; Gregoire, T.G. The Use of mixed effects models for obtaining low-cost ecosystem carbon stock estimates in mangroves of the Asia-Pacific. PLoS ONE 2017, 12, e0169096. [Google Scholar] [CrossRef]
- Atwood, T.B.; Connolly, R.M.; Almahasheer, H.; Carnell, P.E.; Duarte, C.M.; Lewis, C.J.E.; Irigoien, X.; Kelleway, J.J.; Lavery, P.S.; Macreadie, P.I.; et al. Lovelock. Global patterns in mangrove soil carbon stocks and losses. Nat. Clim. Chang. 2017, 7, 523–528. [Google Scholar] [CrossRef]
- Feng, J.X.; Cui, X.W.; Zhou, J.; Wang, L.M.; Zhu, X.S.; Lin, G.H. Effects of exotic and native mangrove forests plantation on soil organic carbon, nitrogen, and phosphorus contents and pools in Leizhou, China. Catena 2019, 180, 1–7. [Google Scholar] [CrossRef]
- Kamruzzaman, M.; Basak, K.; Paul, S.K.; Ahmed, S.; Osawa, A. Litterfall production, decomposition and nutrient accumulation in Sundarbans mangrove forests, Bangladesh. For. Sci. Technol. 2019, 15, 24–32. [Google Scholar] [CrossRef]
- Loria-Naranjo, M.; Sibaja-Cordero, J.A.; Cortes, J. Mangrove leaf litter decomposition in a seasonal tropical environment. J. Coast. Res. 2019, 35, 122–129. [Google Scholar] [CrossRef]
- Eid, E.M.; El-Bebany, A.F.; Alrumman, S.A. Distribution of soil organic carbon in the mangrove forests along the southern Saudi Arabian Red Sea coast. Rend. Lincei-Sci. Fis. E Nat. 2016, 27, 629–637. [Google Scholar] [CrossRef]
- Zhang, H.; Deng, Q.; Hui, D.; Wu, J.; Xiong, X.; Zhao, J.; Zhao, M.; Chu, G.; Zhou, G.; Zhang, D. Recovery in soil carbon stock but reduction in carbon stabilization after 56-year forest restoration in degraded tropical lands. For. Ecol. Manag. 2019, 441, 1–8. [Google Scholar] [CrossRef]
- Hergoualc’H, K.; Verchot, L.V. Stocks and fluxes of carbon associated with land use change in Southeast Asian tropical peatlands: A review. Glob. Biogeochem. Cycles 2011, 25, GB2001. [Google Scholar] [CrossRef]
- Alongi, D.M. Patterns of mangrove wood and litter production within a beach ridge-fringing reef embayment, northern great barrier reef coast. Estuaries Coasts 2011, 34, 32–44. [Google Scholar] [CrossRef]
- Numbere, A.O.; Camilo, G.R. Mangrove leaf litter decomposition under mangrove forest stands with different levels of pollution in the Niger River Delta, Nigeria. Afr. J. Ecol. 2016, 55, 162–167. [Google Scholar] [CrossRef]
- Contreras, L.M.; Fierro-Cabo, A.; Cintra-Buenrostro, C.E. Early drivers of black mangrove (Avicennia germinans) leaf litter decomposition in the water column. Hydrobiologia 2017, 803, 147–157. [Google Scholar] [CrossRef]
- Flores-Cárdenas, F.; Hurtado-Oliva, M.Á.; Doyle, T.W.; Nieves-Soto, M.; Díaz-Castro, S.; Manzano-Sarabia, M. Litterfall production of mangroves in Huizache-Caimanero lagoon system, México. J. Coast. Res. 2017, 331, 118–124. [Google Scholar] [CrossRef]
- Keuskamp, J.A.; Hefting, M.M.; Dingemans, B.J.; Verhoeven, J.T.; Feller, I.C. Effects of nutrient enrichment on mangrove leaf litter decomposition. Sci. Total Environ. 2015, 508, 402–410. [Google Scholar] [CrossRef]
- Li, R.; Chai, M.; Li, R.; Xu, H.; He, B.; Qiu, G.Y. Influence of introduced Sonneratia apetala on nutrients and heavy metals in intertidal sediments, South China. Environ. Sci. Pollut. Res. 2017, 24, 2914–2927. [Google Scholar] [CrossRef]
- Allison, L. Organic carbon, Walkley-Black method. Methods of Soil Analysis. Part 2. Agronomy 1965, 9, 1372–1378. [Google Scholar]
- Technical Specification Writing Group for Ecosystem Carbon Sequestration Projects. Observation and Investigation for Carbon Sequestration in Terrestrial Ecosystems; Science Press: Beijing, China, 2015; pp. 96–142. [Google Scholar]
- Eckstein, R.L.; Karlsson, P.S.; Weih, M. Research review: Leaf life span and nutrient resorption as determinants of plant nutrient conservation in temperate-arctic regions. New Phytol. 1999, 143, 177–189. [Google Scholar] [CrossRef]
- Wilson, K.B.; Baldocchi, D.D.; Hanson, P.J. Spatial and seasonal variability of photosynthetic parameters and their relationship to leaf nitrogen in a deciduous forest. Tree Physiol. 2000, 20, 565–578. [Google Scholar] [CrossRef] [Green Version]
- Saenger, P.; Snedaker, S.C. Pantropical trends in mangrove above-ground biomass and annual litterfall. Oecologia 1993, 96, 293–299. [Google Scholar] [CrossRef] [Green Version]
- Zheng, F.Z.; Lin, P.; Lu, C.Y.; Zheng, W.J. Interannual dynamic of litter fall of Kandelia obovata mangrove and energy flow through the litter in Jiulongjiang Estuary, Fujian Province, China. Acta Ecol. Sin. 1998, 2, 3–8. [Google Scholar]
- Kang, W.-X.; Zhao, Z.-H.; Tian, D.-L.; He, J.-N.; Deng, X.-W. CO2 exchanges between mangrove and shoal wetland ecosystems and atmosphere in Guangzhou. Chin. J. Appl. Ecol. 2008, 19, 2605–2610. [Google Scholar]
- Mao, Z.L.; Yang, X.M.; Zhao, Z.Y.; Lai, H.D.; Yang, D.Y.; Yang, D.Y.; Wu, C.L.; Xu, H.L. Preliminary study on mangrove ecosystem carbon cycle of Kandelia obovata in Futian Natural Reserve, Shenzhen, China. Ecol. Environ. Sci. 2012, 7, 1189–1199. [Google Scholar]
- Liu, S.Q.; Han, W.D.; Li, J.P. Study on the model for litter fall varying in mangrove forest based on a unit step function. Mar. Sci. 2007, 31, 35–39. [Google Scholar]
- Kalbitz, K.; Solinger, S.; Park, J.-H.; Michalzik, B.; Matzner, E. Controls on the dynamics of dissolved organic matter in soils: A review. Soil Sci. 2000, 165, 277–304. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The microbial efficiency-matrix stabilization (mems) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Chang. Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef]
| Plant Species | Average DBH * (cm) | Average Height (m) | Crown (m × m) | Clear Stem (cm) | Age (year) |
|---|---|---|---|---|---|
| Kandelia obovata | 10.35 ± 1.12 | 3.5 ± 0.5 | 2.1 × 2.3 | 90 | 10 |
| Heritiera littoralis | 15.37 ± 3.89 | 5.5 ± 0.5 | 2.4 × 2.1 | 70 | 9 |
| Thespesia populnea | 9.18 ± 2.95 | 3.7 ± 0.5 | 1.7 × 1.6 | 50 | 7 |
| Time (d) | Branch Heritiera | Leaf Heritiera | Branch Thespesia | Leaf Thespesia | Branch Kandelia | Leaf Kandelia |
|---|---|---|---|---|---|---|
| 30 | 9.18 ± 1.42gh | 19.76 ± 3.81f | 10.57 ± 2.55g | 15.67 ± 1.16f | 3.14 ± 1.1f | 14.9 ± 1.31g |
| 60 | 6.59 ± 2.61h | 11.18 ± 1.54g | 8.41 ± 2.7g | 16.43 ± 1.24f | 4.97 ± 1.1f | 16.27 ± 2.02g |
| 90 | 11.65 ± 1.21g | 12.24 ± 1.69g | 15.29 ± 1.52f | 26.11 ± 2.07e | 14.51 ± 2.63e | 14.02 ± 2.16g |
| 120 | 19.27 ± 1.71f | 16.24 ± 1.51f | 20.64 ± 1.41e | 25.86 ± 1.8e | 13.14 ± 2.7e | 20.98 ± 1.02f |
| 150 | 24.71 ± 1.25e | 30.35 ± 2.01e | 22.04 ± 1.41e | 30.06 ± 1.27d | 21.7 ± 1.07d | 25.98 ± 1.1e |
| 180 | 26 ± 2.48e | 24.12 ± 3.84d | 32.36 ± 2.18d | 37.71 ± 1.2c | 22.84 ± 1.06d | 25.29 ± 1.41e |
| 210 | 34 ± 2.98cd | 34.82 ± 1.83c | 23.82 ± 3.45e | 29.94 ± 3.71d | 20.78 ± 2.59d | 26.47 ± 1.19e |
| 240 | 32.35 ± 3.46d | 37.76 ± 1.55c | 38.6 ± 1.36c | 36.18 ± 1.04c | 33.04 ± 2.78c | 35.29 ± 1.04d |
| 270 | 36.65 ± 1.42c | 43.53 ± 2.07b | 31.72 ± 4.27d | 37.71 ± 1.46c | 32.75 ± 1.18c | 41.37 ± 1.03c |
| 300 | 40.71 ± 1.42b | 45.65 ± 1.76b | 43.57 ± 1.69b | 48.03 ± 1.24b | 38.73 ± 1.44b | 42.35 ± 1.15c |
| 330 | 40.94 ± 1.24b | 50.59 ± 1.92a | 46.5 ± 1.61ab | 48.54 ± 1.2b | 37.75 ± 1.05b | 47.35 ± 1.14b |
| 360 | 49.22 ± 1.57a | 52.15 ± 1.8a | 49.76 ± 1.71a | 52.67 ± 1.15a | 61.29 ± 4a | 59.82 ± 1.29a |
| Time (d) | Kandelia obovata | Heritiera littoralis | Thespesia populnea |
|---|---|---|---|
| Original | 11.65 ± 2.42a | 12.52 ± 4.1a | 11.34 ± 1.43b |
| 90 | 11.32 ± 2.13a | 11.34 ± 0.31a | 10.39 ± 1.42b |
| 180 | 13.99 ± 1.21b | 13.40 ± 2.18ab | 13.42 ± 0.51a |
| 270 | 13.82 ± 3.01b | 12.28 ± 1.34ab | 11.56 ± 0.52b |
| 360 | 16.29 ± 0.29b | 14.89 ± 1.68b | 14.56 ± 0.7a |
| Location | Tree Age (year) | Tree Height (m) | Litter Yield (g m−2) | Literature Source |
|---|---|---|---|---|
| Zhuhai, China (22°23′ N; 113°36′ E) | 9 | 5.5 | 745 | This study |
| Jiulong Estuary, Fujian, China (24°24′ N; 117°55′ E) | 30 | 6 | 863 | [29] |
| Nansha, Guangzhou, China (23°01′ N; 113°41′ E) | - | - | 460 | [30] |
| Shenzhen, China (22°31′ N; 114°00′ E) | 30 | 6.3 | 603 | [31] |
| Leizhou Peninsula, China (20°41′ N; 110°12′ E) | - | - | 848 | [32] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, A.; Zhou, T.; Yang, X.; Gao, Y.; Xu, S.; Lin, C. Evaluating Litter Yield and Decomposition for Re-Vegetated Mangroves in a Subtropical Mudflat. Appl. Sci. 2019, 9, 3340. https://doi.org/10.3390/app9163340
Niu A, Zhou T, Yang X, Gao Y, Xu S, Lin C. Evaluating Litter Yield and Decomposition for Re-Vegetated Mangroves in a Subtropical Mudflat. Applied Sciences. 2019; 9(16):3340. https://doi.org/10.3390/app9163340
Chicago/Turabian StyleNiu, Anyi, Ting Zhou, Xiu Yang, Yifei Gao, Songjun Xu, and Chuxia Lin. 2019. "Evaluating Litter Yield and Decomposition for Re-Vegetated Mangroves in a Subtropical Mudflat" Applied Sciences 9, no. 16: 3340. https://doi.org/10.3390/app9163340
APA StyleNiu, A., Zhou, T., Yang, X., Gao, Y., Xu, S., & Lin, C. (2019). Evaluating Litter Yield and Decomposition for Re-Vegetated Mangroves in a Subtropical Mudflat. Applied Sciences, 9(16), 3340. https://doi.org/10.3390/app9163340






