Experimental Study on the Removal of Real Exhaust Pollutants from a Diesel Engine by Activated Carbon
Abstract
:1. Introduction
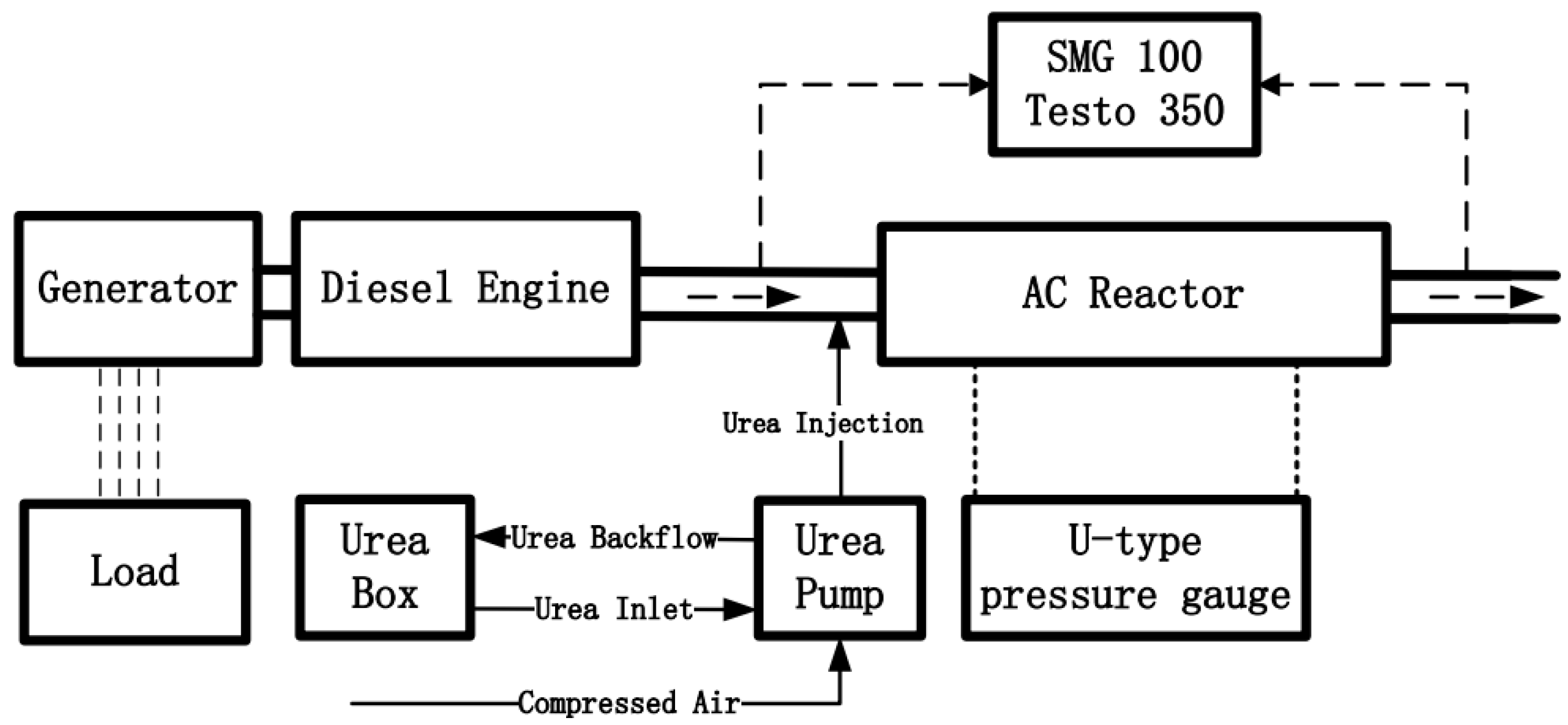
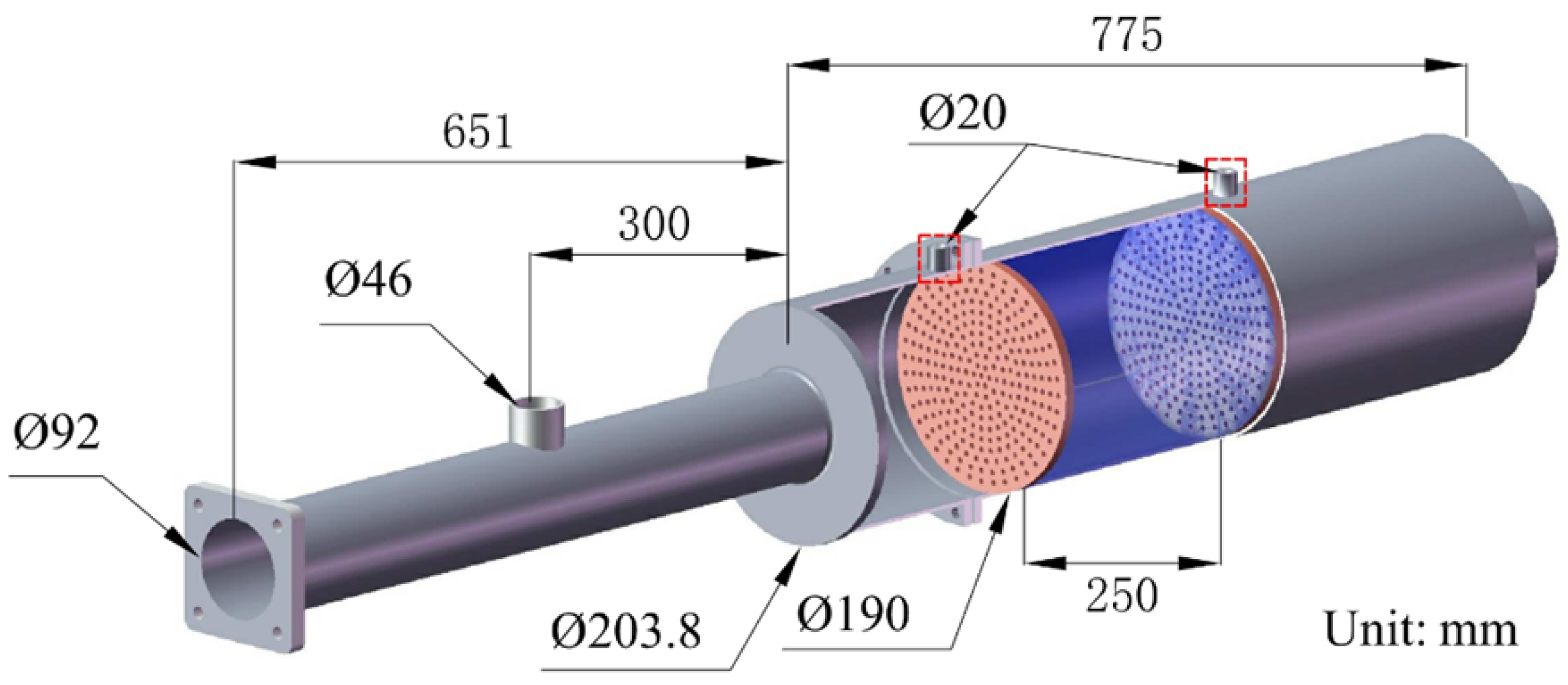
2. Experimental System and Data Processing
2.1. Experimental System and Material
2.2. Data Processing
3. Results and Discussion
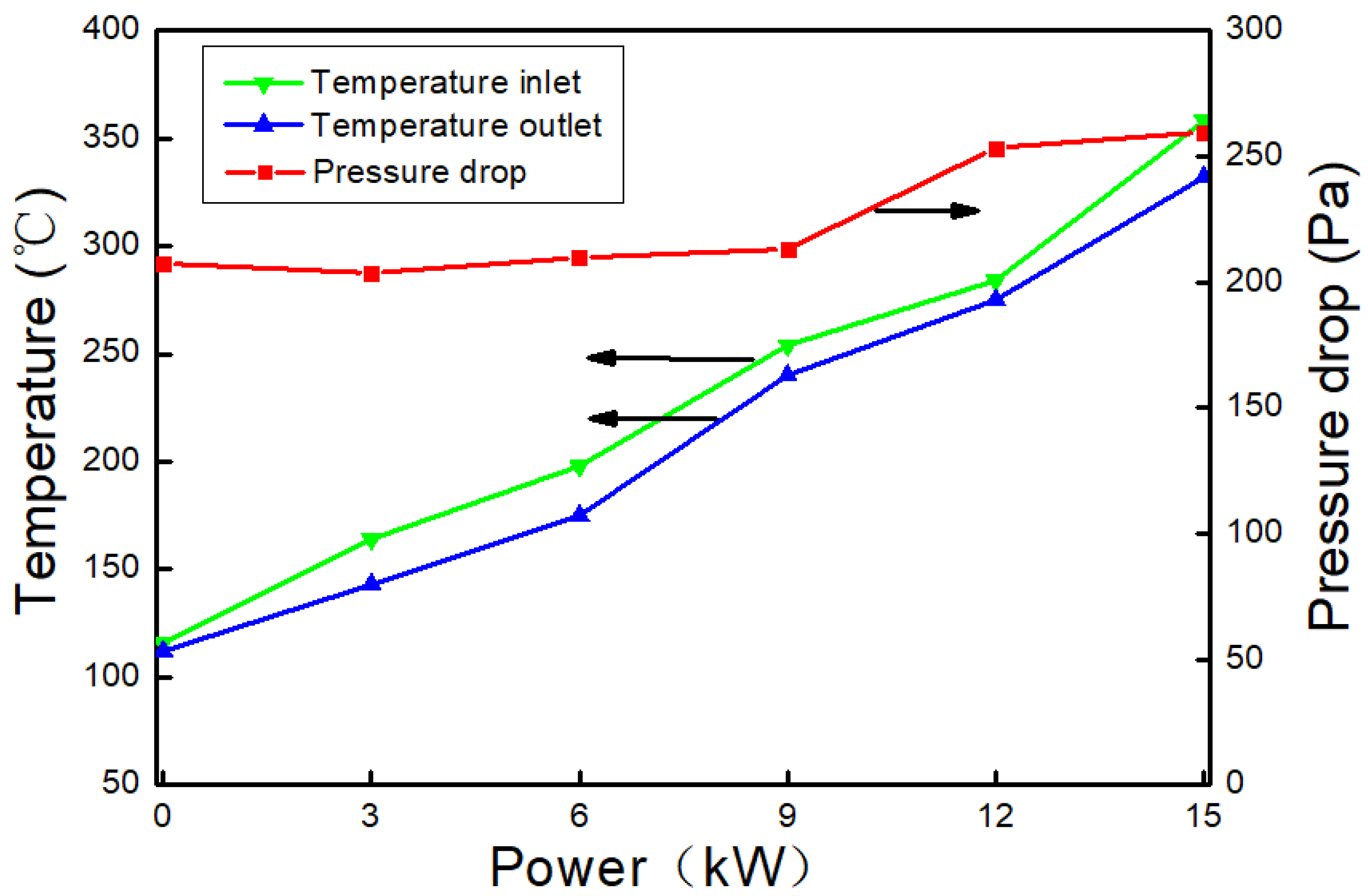
3.1. The Changes of Exhaust Gas Temperature and Pressure Drop
3.2. Effect of AC on PM
3.3. Effect of AC on CO
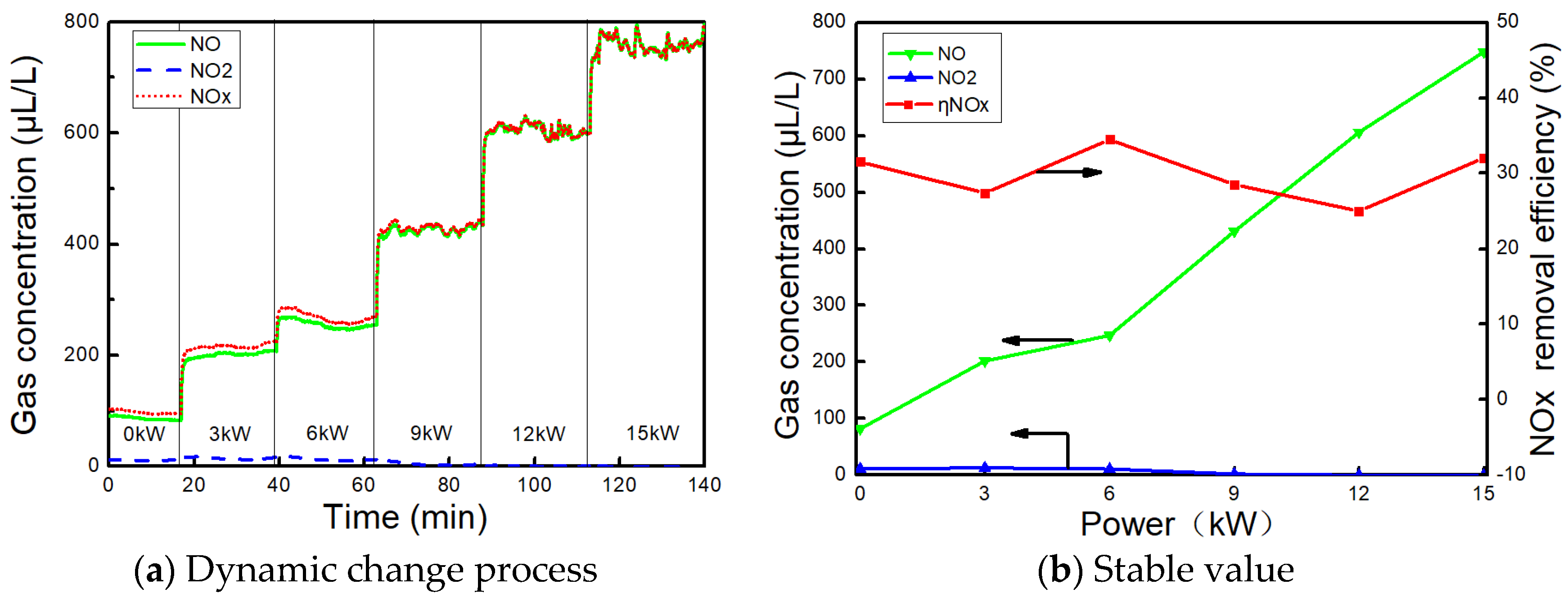
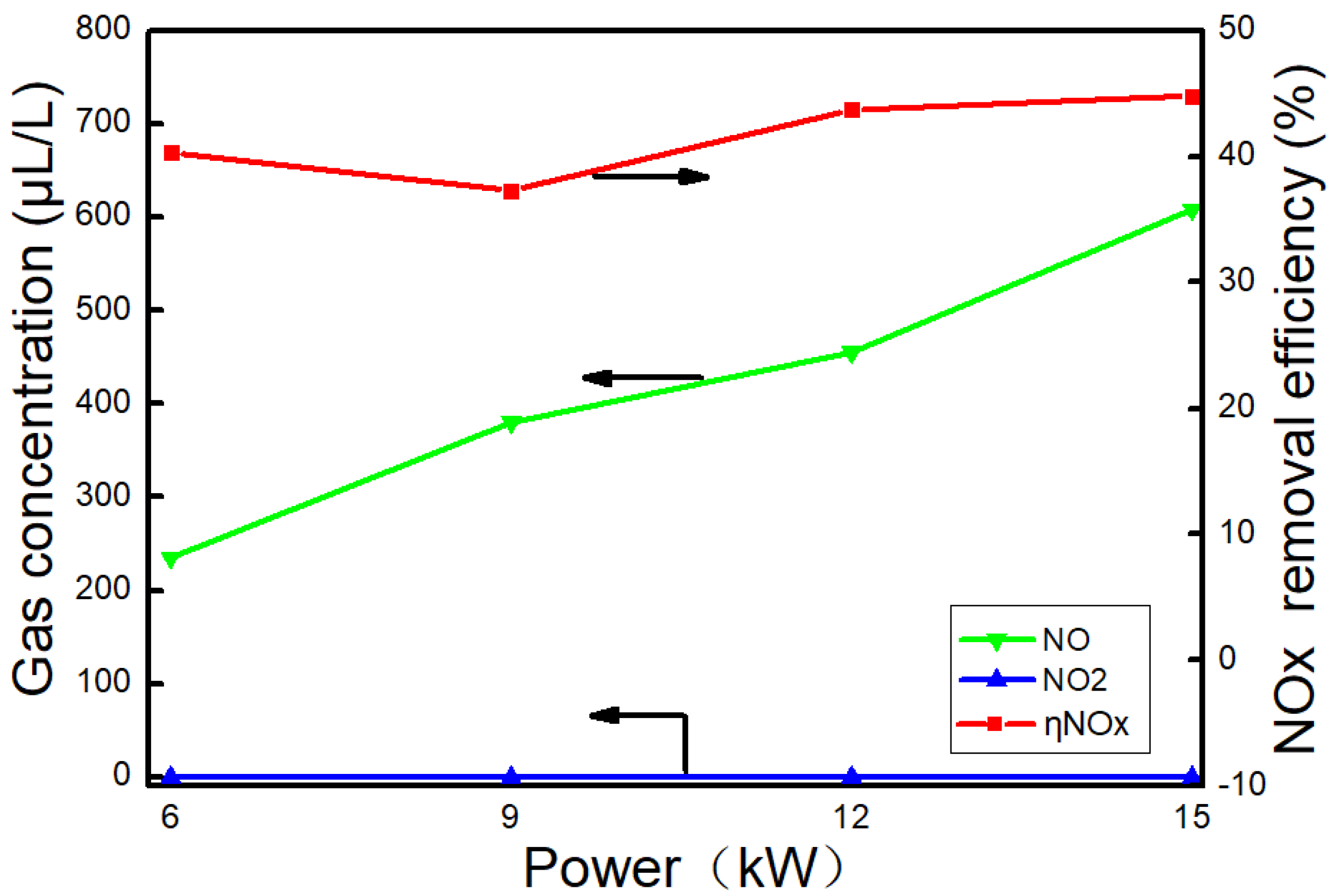
3.4. Effect of AC on NOx
4. Conclusions
- (1)
- The removal of PM was mainly by the adsorption and filtration of activated carbon. The PM removal efficiency of the AC reactor was above 60% in the entire power range, and the highest PM removal efficiency was 77% at 9 kW. However, the pressure drop of the reactor gradually increased with the running time. In the future, a fan can be installed after the reactor, or the granular AC can be made into a honeycomb structure and the regeneration method of DPF backwashing can be used to prolong the service time.
- (2)
- CO can be used as a reducing agent to participate in the denitration reaction under the catalytic action of AC. The removal of CO depends on the adsorption of AC. Since the temperature of the exhaust gas gradually increases with the power, the AC may have a certain degeneration. It is necessary to improve the thermal stability of AC by material and production process for practical purposes.
- (3)
- The NOx concentration of the exhaust gas gradually increases with the power. The denitration ability of AC was poor when urea is not sprayed, and the denitration efficiency was 34.5% at 6 kW. When urea was sprayed, NH3 was generated by urea hydrolysis. With NH3 as a reducing agent, the NO2 removal efficiency was close to 100%, and the highest denitration efficiency was 44.8% at 15 kW. Overall, the denitration efficiency of AC was still lower than other denitration catalysts, and it needs to be modified or loaded with other metal oxides to improve its denitration efficiency.
Author Contributions
Funding
Conflicts of Interest
References
- Chen, G.J.; Liu, Z.M.; Liu, T.T.; Su, S.H.; Yuan, G.J.; Cao, Y.J. Research on emission control of marine diesel engine. Proc. Adv. Mater. Res. 2012, 430, 1198–1202. [Google Scholar] [CrossRef]
- Yoo, D.-H.; Nitta, Y.; Ikame, M.; Hayashi, M.; Fujita, H.; Lim, J.-k. Exhaust characteristics of nitrous oxide from marine engine. In Proceedings of the 2012 Oceans-Yeosu, Yeosu, Korea, 21–24 May 2012; pp. 1–7. [Google Scholar]
- Zhu, Y.; Tang, X.; Li, T.; Ji, Y.; Liu, Q.; Guo, L.; Zhao, J. Shipboard trials of magnesium-based exhaust gas cleaning system. Ocean Eng. 2016, 128, 124–131. [Google Scholar] [CrossRef]
- International Maritime Organization (IMO). IMO Sets 2020 Date for Ships to Comply with Low Sulphur Fuel Oil Requirement. Available online: http://www.imo.org/en/MediaCentre/PressBriefings/Pages/MEPC-70-2020sulphur.aspxx (accessed on 2 July 2019).
- MAN Diesel & Turbo. MAN Diesel & Turbo Delivers World’s First IMO-Certified Two-Stroke Engine with Tier III NOx Control, Exhaust Gas Recirculation Systems. Available online: https://www.corporate.man.eu/en/press-and-media/presscenter/MAN-Diesel-and-Turbo-Delivers-World_s-First-IMO-Certified-Two-Stroke-Engine-with-Tier-III-NOx-Control_-Exhaust-Gas-Recirculation-Systems-241408.html (accessed on 2 July 2019).
- Brandenberger, S.; Kröcher, O.; Tissler, A.; Althoff, R. The determination of the activities of different iron species in Fe-ZSM-5 for SCR of NO by NH3. Appl. Catal. B Environ. 2010, 95, 348–357. [Google Scholar] [CrossRef]
- Hsieh, M.-F.; Wang, J. Development and experimental studies of a control-oriented SCR model for a two-catalyst urea-SCR system. Control Eng. Pract. 2011, 19, 409–422. [Google Scholar] [CrossRef]
- Colombo, M.; Nova, I.; Tronconi, E. Detailed kinetic modeling of the NH3–NO/NO2 SCR reactions over a commercial Cu-zeolite catalyst for Diesel exhausts after treatment. Catal. Today 2012, 197, 243–255. [Google Scholar] [CrossRef]
- Sjövall, H.; Olsson, L.; Fridell, E.; Blint, R.J. Selective catalytic reduction of NOx with NH3 over Cu-ZSM-5—The effect of changing the gas composition. Appl. Catal. B Environ. 2006, 64, 180–188. [Google Scholar] [CrossRef]
- Zhang, W.; Rabiei, S.; Bagreev, A.; Zhuang, M.; Rasouli, F. Study of NO adsorption on activated carbons. Appl. Catal. B Environ. 2008, 83, 63–71. [Google Scholar] [CrossRef]
- Gao, X.; Liu, S.; Zhang, Y.; Luo, Z.; Ni, M.; Cen, K. Adsorption and reduction of NO2 over activated carbon at low temperature. Fuel Process. Technol. 2011, 92, 139–146. [Google Scholar] [CrossRef]
- Ghouma, I.; Jeguirim, M.; Dorge, S.; Limousy, L.; Ghimbeu, C.M.; Ouederni, A. Activated carbon prepared by physical activation of olive stones for the removal of NO2 at ambient temperature. C. R. Chim. 2015, 18, 63–74. [Google Scholar] [CrossRef]
- Huang, Z.; Hou, Y.; Zhu, Z.; Liu, Z. Study on the NO reduction by NH3 on a SO42−/AC catalyst at low temperature. Catal. Commun. 2014, 50, 83–86. [Google Scholar] [CrossRef]
- Zhu, L.; Huang, B.; Wang, W.; Wei, Z.; Ye, D. Low-temperature SCR of NO with NH3 over CeO2 supported on modified activated carbon fibers. Catal. Commun. 2011, 12, 394–398. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Pereira, M.F.R. The role of surface chemistry in catalysis with carbons. Catal. Today 2010, 150, 2–7. [Google Scholar] [CrossRef]
- Izquierdo, M.T.; Rubio, B.; Mayoral, C.; Andrés, J.M. Low cost coal-based carbons for combined SO2 and NO removal from exhaust gas. Fuel 2003, 82, 147–151. [Google Scholar] [CrossRef]
- Ding, S.; Li, Y.; Zhu, T.; Guo, Y. Regeneration performance and carbon consumption of semi-coke and activated coke for SO2 and NO removal. J. Environ. Sci. 2015, 34, 37–43. [Google Scholar] [CrossRef]
- Ding, J.L.; Zhao, Y.Q.; Zhang, Y.F.; Fu, Y.L. Influence of Non-Pitch Based Activated Carbon on Flue Gas Desulfurization. Proc. Appl. Mech. Mater. 2013, 316–317, 1055–1058. [Google Scholar] [CrossRef]
- Mochida, I.; Korai, Y.; Shirahama, M.; Kawano, S.; Hada, T.; Seo, Y.; Yoshikawa, M.; Yasutake, A. Removal of SOx and NOx over activated carbon fibers. Carbon 2000, 38, 227–239. [Google Scholar] [CrossRef]
- Sousa, J.P.; Pereira, M.F.; Figueiredo, J.L. Modified activated carbon as catalyst for NO oxidation. Fuel Process. Technol. 2013, 106, 727–733. [Google Scholar] [CrossRef]
- Wang, H.; Wang, S.; Duan, Y.; Li, Y.-N.; Xue, Y.; Ying, Z. Activated carbon for capturing Hg in flue gas under O2/CO2 combustion conditions. Part 1: Experimental and kinetic study. Energy Fuels 2018, 32, 1900–1906. [Google Scholar] [CrossRef]
- Zhou, Q.; Duan, Y.; Chen, M.; Liu, M.; Lu, P. Studies on mercury adsorption species and equilibrium on activated carbon surface. Energy Fuels 2017, 31, 14211–14218. [Google Scholar] [CrossRef]
- Zhao, Y.; Hua, L.; Hu, J.; Tang, T.; Shuai, S.; Wang, J. Experimental study of urea solution spray characteristics in SCR system of diesel engine. Chin. Intern. Combust. Engine Eng. 2012, 33, 22–27. [Google Scholar]
- Zhuang, Q.; Kyotani, T.; Tomita, A. The change of TPD pattern of O2-gasified carbon upon air exposure. Carbon 1994, 32, 539–540. [Google Scholar] [CrossRef]
- Zielke, U.; Hüttinger, K.; Hoffman, W. Surface-oxidized carbon fibers: I. Surface structure and chemistry. Carbon 1996, 34, 983–998. [Google Scholar] [CrossRef]
- Liu, Y. Study on Removal of Particles and NOx from Diesel Engines by Application of POC and SCR Technologies; Shandong University: Jinan, China, 2015. [Google Scholar]
- Yahya, M.A.; Al-Qodah, Z.; Ngah, C.Z. Agricultural bio-waste materials as potential sustainable precursors used for activated carbon production: A review. Renew. Sustain. Energy Rev. 2015, 46, 218–235. [Google Scholar] [CrossRef]
- Wang, Z.; Kuang, H.; Zhang, J.; Chu, L.; Ji, Y. Nitrogen Oxide Removal by Coal-Based Activated Carbon for a Marine Diesel Engine. Appl. Sci. 2019, 9, 1656. [Google Scholar] [CrossRef]
- Li, Y.; Duan, Y.; Wang, H.; Zhao, S.; Chen, M.; Liu, M.; Wei, H. Effects of acidic gases on mercury adsorption by activated carbon in simulated oxy-fuel combustion flue gas. Energy Fuels 2017, 31, 9745–9751. [Google Scholar] [CrossRef]
- Ghouma, I.; Jeguirim, M.; Sager, U.; Limousy, L.; Bennici, S.; Däuber, E.; Asbach, C.; Ligotski, R.; Schmidt, F.; Ouederni, A. The potential of activated carbon made of agro-industrial residues in NOx immissions abatement. Energies 2017, 10, 1508. [Google Scholar] [CrossRef]
- Lv, H.; Yang, W.; Zhou, J.; Zhang, M.; Li, F. Investigation on thermal decomposition characteristics of urea solution under high temperature. Proc. CSEE 2010, 30, 35–40. [Google Scholar]
- Zhang, X.; Zhang, B.; Lu, X.; Xiang, X.; Xu, H. Crafts design and experimental study of urea hydrolysis to ammonia in thermal power plant. Proc. CSEE 2016, 36, 2452–2458. [Google Scholar]
- Fu, Y.; Zhang, Y.; Li, G.; Zhang, J.; Guo, Y. NO removal activity and surface characterization of activated carbon with oxidation modification. J. Energy Inst. 2017, 90, 813–823. [Google Scholar] [CrossRef]
- Samojeden, B.; Grzybek, T. The influence of the promotion of N-modified activated carbon with iron on NO removal by NH3-SCR (Selective catalytic reduction). Energy 2016, 116, 1484–1491. [Google Scholar] [CrossRef]
- Shu, Y.; Zhang, F.; Wang, F.; Wang, H. Catalytic reduction of NOx by biomass-derived activated carbon supported metals. Chin. J. Chem. Eng. 2018, 26, 2077–2083. [Google Scholar] [CrossRef]
- Wang, M.; Liu, H.; Huang, Z.-H.; Kang, F. Activated carbon fibers loaded with MnO2 for removing NO at room temperature. Chem. Eng. J. 2014, 256, 101–106. [Google Scholar] [CrossRef]



| Cylinder | Bore (mm) | Stroke (mm) | Compression Ratio | Displacement (L) | Air Inlet Method | Power (kW) | Rated Speed (r/min) |
|---|---|---|---|---|---|---|---|
| 4 | 85 | 92 | 18:1 | 2.09 | Natural | 17.5 | 1500 |
| Power (kW) | 0 | 3 | 6 | 9 | 12 | 15 |
| Exhaust flow (Nm3/h) | 119.3 | 105.5 | 98.3 | 89.9 | 88.0 | 82.8 |
| Diameter (mm) | Length (mm) | Iodine Value (mg/g) | Fire Point (°C) | Bulk Density (g/L) | SBET (m2/g) | Vtotal (cm3/g) | Vmic (cm3/g) | |
|---|---|---|---|---|---|---|---|---|
| 10 | 5.6~11.2 | 371 | 456 | 650 | 326.491 | 0.200 | 0.112 | 2.454 |
| Element | C | O | Si | Al | Ca |
|---|---|---|---|---|---|
| Mass percentage (%) | 48.07 | 20.64 | 8.83 | 5.75 | 6.58 |
| Atomic Percentage (%) | 64.26 | 20.71 | 5.05 | 3.42 | 2.64 |
| Power (kW) | 6 | 9 | 12 | 15 |
| Urea Flow (mL/h) | 146 | 206 | 269 | 345 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Kuang, H.; Zhang, J.; Chu, L.; Ji, Y. Experimental Study on the Removal of Real Exhaust Pollutants from a Diesel Engine by Activated Carbon. Appl. Sci. 2019, 9, 3175. https://doi.org/10.3390/app9153175
Wang Z, Kuang H, Zhang J, Chu L, Ji Y. Experimental Study on the Removal of Real Exhaust Pollutants from a Diesel Engine by Activated Carbon. Applied Sciences. 2019; 9(15):3175. https://doi.org/10.3390/app9153175
Chicago/Turabian StyleWang, Zongyu, Hailang Kuang, Jifeng Zhang, Lilin Chu, and Yulong Ji. 2019. "Experimental Study on the Removal of Real Exhaust Pollutants from a Diesel Engine by Activated Carbon" Applied Sciences 9, no. 15: 3175. https://doi.org/10.3390/app9153175
APA StyleWang, Z., Kuang, H., Zhang, J., Chu, L., & Ji, Y. (2019). Experimental Study on the Removal of Real Exhaust Pollutants from a Diesel Engine by Activated Carbon. Applied Sciences, 9(15), 3175. https://doi.org/10.3390/app9153175







