Application of OCT in the Gastrointestinal Tract
Abstract
:1. Introduction
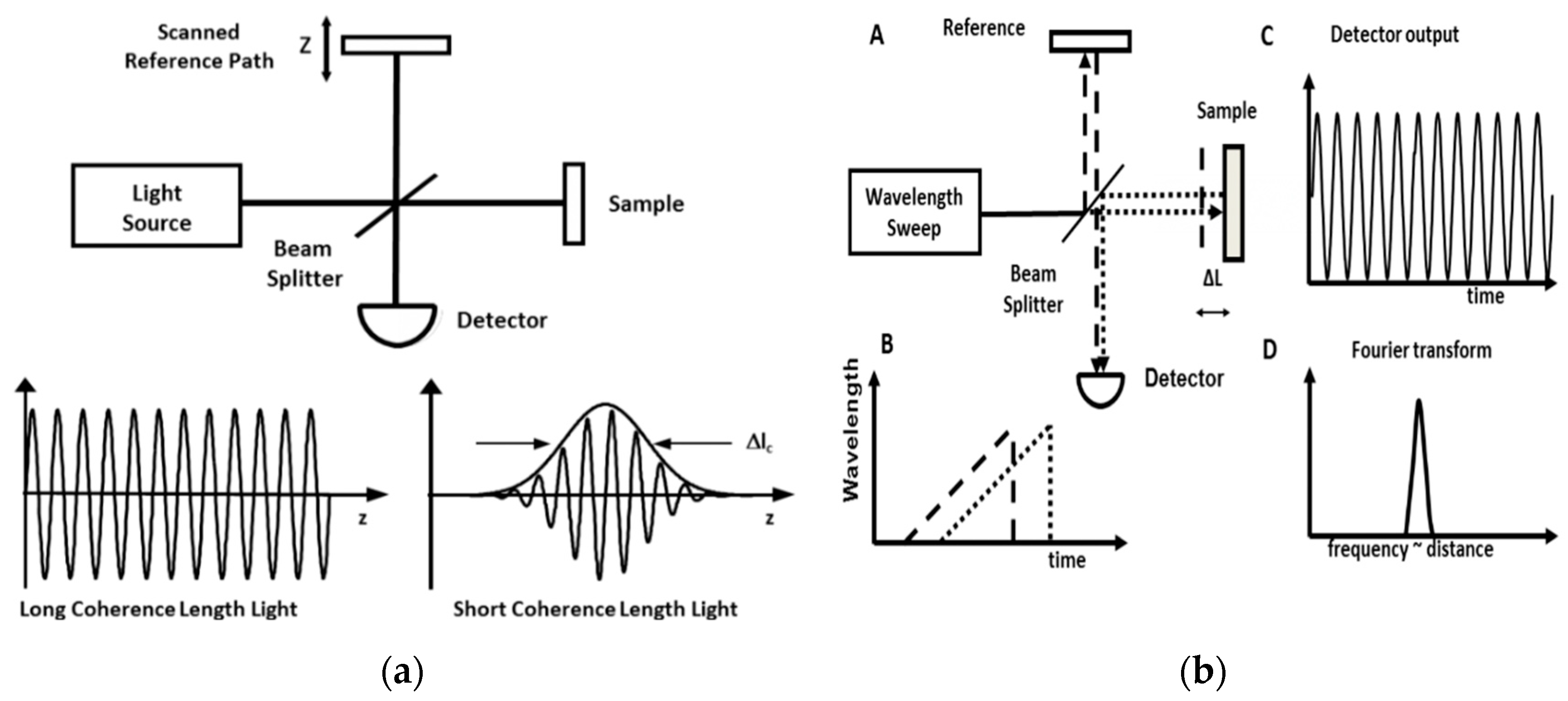
2. Evolving OCT Technology
3. OCT Applications in the GI Tract
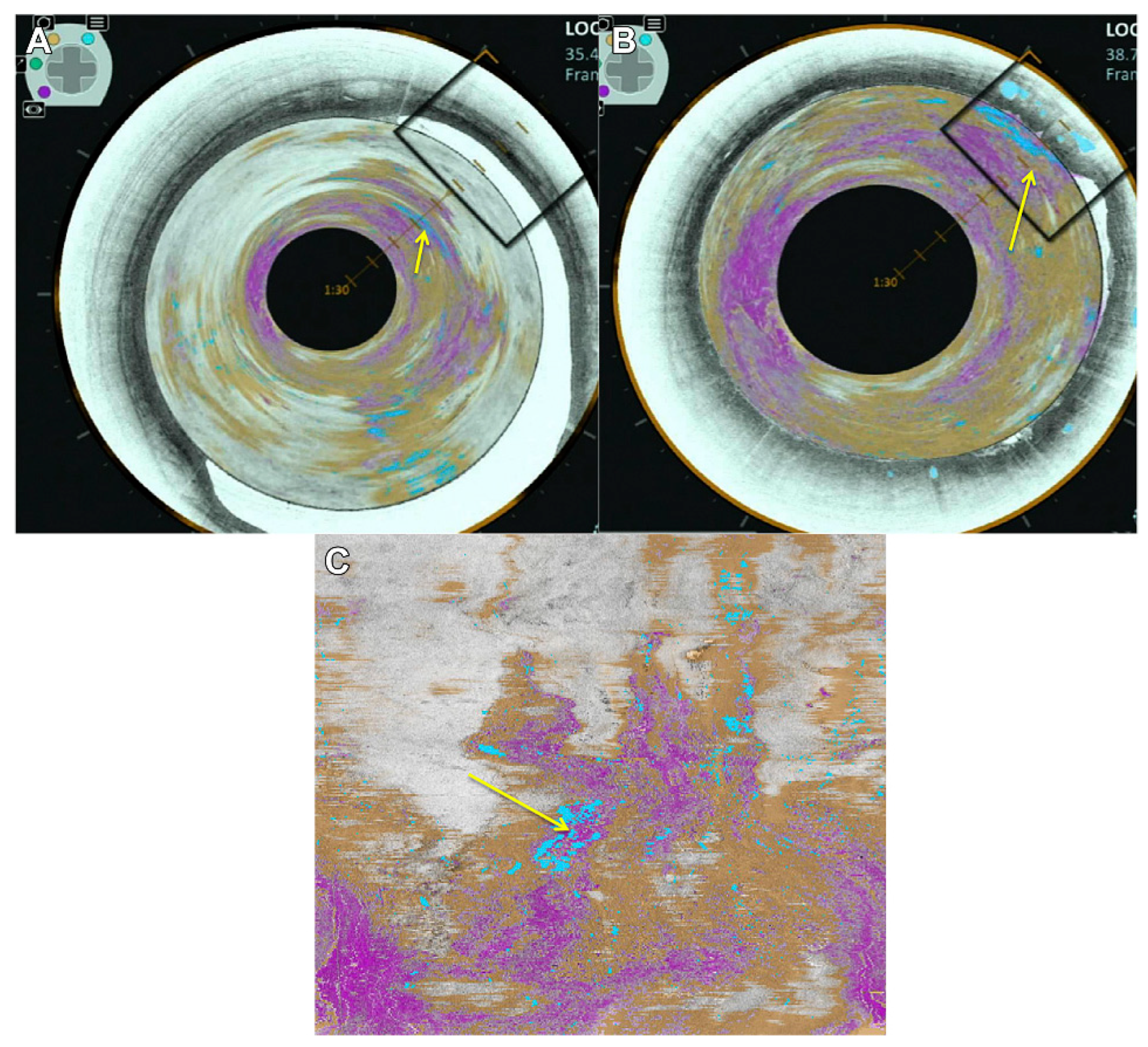
3.1. Esophagus
3.2. Colon
3.3. Hepatobiliary
3.4. Imaging of Microvasculature
3.5. Small Intestine
4. New Technologies and Future Applications
4.1. Artificial Intelligence (AI)
4.2. Capsule
5. Conclusion/Outlook for OCT
Conflicts of Interest
References
- Seward, E.; Lumley, S. Endoscopy provision: Meeting the challenges. Frontline Gastroenterol. 2017, 8, 90. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, N.; Keuchel, M. Flexible Gastro-intestinal Endoscopy—Clinical Challenges and Technical Achievements. Comput. Struct. Biotechnol. J. 2017, 15, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Bond, A.; Sarkar, S. New technologies and techniques to improve adenoma detection in colonoscopy. World J. Gastrointest. Endosc. 2015, 7, 969–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groff, R.J.; Nash, R.; Ahnen, D.J. Significance of serrated polyps of the colon. Curr. Gastroenterol. Rep. 2008, 10, 490–498. [Google Scholar] [CrossRef] [PubMed]
- A.C. Cancer Facts and Figures—2017. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2017.html. (accessed on 17 June 2019).
- Song, L.M.W.K.; Adler, D.G.; Chand, B.; Conway, J.D.; Croffie, J.M.B.; DiSario, J.A.; Mishkin, D.S.; Shah, R.J.; Somogyi, L.; Tierney, W.M. Chromoendoscopy. Gastrointest. Endosc. 2007, 66, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Manfredi, M.A.; Dayyeh, B.K.A.; Bhat, Y.M.; Chauhan, S.S.; Gottlieb, K.T.; Hwang, J.H.; Komanduri, S.; Konda, V.; Lo, S.K.; Maple, J.T. Electronic chromoendoscopy. Gastrointest. Endosc. 2015, 81, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Kiesslich, R.; Jung, M. Magnification endoscopy: Does it improve mucosal surface analysis for the diagnosis of gastrointestinal neoplasias? Endoscopy 2002, 34, 819–822. [Google Scholar] [CrossRef]
- Song, L.-M.W.K.; Banerjee, S.; Desilets, D.; Diehl, D.L.; Farraye, F.A.; Kaul, V.; Kethu, S.R.; Kwon, R.S.; Mamula, P.; Pedrosa, M.C. Autofluorescence imaging. Gastrointest. Endosc. 2011, 73, 647–650. [Google Scholar] [CrossRef]
- Zuccaro, G.; Gladkova, N.; Vargo, J.; Feldchtein, F.; Zagaynova, E.; Conwell, D.; Falk, G.; Goldblum, J.; Dumot, J.; Ponsky, J. Optical coherence tomography of the esophagus and proximal stomach in health and disease. Am. J. Gastroenterol. 2001, 96, 2633. [Google Scholar] [CrossRef]
- Chauhan, S.S.; Dayyeh, B.K.A.; Bhat, Y.M.; Gottlieb, K.T.; Hwang, J.H.; Komanduri, S.; Konda, V.; Lo, S.K.; Manfredi, M.A.; Maple, J.T. Confocal laser endomicroscopy. Gastrointest. Endosc. 2014, 80, 928–938. [Google Scholar] [CrossRef]
- Tsai, T.H.; Leggett, C.L.; Trindade, A.J.; Sethi, A.; Swager, A.F.; Joshi, V.; Bergman, J.J.; Mashimo, H.; Nishioka, N.S.; Namati, E. Optical coherence tomography in gastroenterology: A review and future outlook. J. Biomed. Opt. 2017, 22, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Takada, K.; Yokohama, I.; Chida, K.; Noda, J. New measurement system for fault location in optical waveguide devices based on an interferometric technique. Appl. Opt. 1987, 26, 1603–1606. [Google Scholar] [CrossRef] [PubMed]
- Gilgen, H.H.; Novak, R.P.; Salathe, R.P.; Hodel, W.; Beaud, P. Submillimeter optical reflectometry. J. Lightw. Technol. 1989, 7, 1225–1233. [Google Scholar] [CrossRef]
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A.; et al. Optical coherence tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef] [PubMed]
- Tearney, G.J.; Brezinski, M.E.; Bouma, B.E.; Boppart, S.A.; Pitris, C.; Southern, J.F.; Fujimoto, J.G. In vivo endoscopic optical biopsy with optical coherence tomography. Science 1997, 276, 2037–2039. [Google Scholar] [CrossRef] [PubMed]
- Swanson, E.A.; Izatt, J.A.; Hee, M.R.; Huang, D.; Lin, C.P.; Schuman, J.S.; Puliafito, C.A.; Fujimoto, J.G. In vivo retinal imaging by optical coherence tomography. Opt. Lett. 1993, 18, 1864–1866. [Google Scholar] [CrossRef] [PubMed]
- Fercher, A.F.; Hitzenberger, C.K.; Kamp, G.; El-Zaiat, S.Y. Measurement of intraocular distances by backscattering spectral interferometry. Opt. Commun. 1995, 117, 43–48. [Google Scholar] [CrossRef]
- Cense, B.; Nassif, N.A.; Chen, T.C.; Pierce, M.C.; Yun, S.-H.; Park, B.H.; Bouma, B.E.; Tearney, G.J.; de Boer, J.F. Ultrahigh-resolution high-speed retinal imaging using spectral-domain optical coherence tomography. Opt. Express 2004, 12, 2435–2447. [Google Scholar] [CrossRef]
- Wojtkowski, M.; Bajraszewski, T.; Gorczyńska, I.; Targowski, P.; Kowalczyk, A.; Wasilewski, W.; Radzewicz, C. Ophthalmic imaging by spectral optical coherence tomography. Am. J. Ophthalmol. 2004, 138, 412–419. [Google Scholar] [CrossRef]
- Popescu, D.P.; Choo-Smith, L.-P.I.; Flueraru, C.; Mao, Y.; Chang, S.; Disano, J.; Sherif, S.; Sowa, M.G. Optical coherence tomography: Fundamental principles, instrumental designs and biomedical applications. Biophys. Rev. 2011, 3, 155. [Google Scholar] [CrossRef]
- Swanson, E.A.; Huang, D.; Hee, M.R.; Fujimoto, J.G.; Lin, C.P.; Puliafito, C.A. High-speed optical coherence domain reflectometry. Opt. Lett. 1992, 17, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.-H.; Fujimoto, G.J.; Mashimo, H. Endoscopic Optical Coherence Tomography for Clinical Gastroenterology. Diagnostics 2014, 4, 57–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choma, M.; Sarunic, M.; Yang, C.; Izatt, J. Sensitivity advantage of swept source and Fourier domain optical coherence tomography. Opt. Express 2003, 11, 2183–2189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Boer, J.F.; Cense, B.; Park, B.H.; Pierce, M.C.; Tearney, G.J.; Bouma, B.E. Improved signal-to-noise ratio in spectral-domain compared with time-domain optical coherence tomography. Opt. Lett. 2003, 28, 2067–2069. [Google Scholar] [CrossRef] [PubMed]
- Leitgeb, R.; Hitzenberger, C.K.; Fercher, A.F. Performance of fourier domain vs. time domain optical coherence tomography. Opt. Express 2003, 11, 889–894. [Google Scholar] [CrossRef]
- Ko, T.H.; Adler, D.C.; Fujimoto, J.G.; Mamedov, D.; Prokhorov, V.; Shidlovski, V.; Yakubovich, S. Ultrahigh resolution optical coherence tomography imaging with a broadband superluminescent diode light source. Opt. Express 2004, 12, 2112–2119. [Google Scholar] [CrossRef] [PubMed]
- Robles, F.E.; Chowdhury, S.; Wax, A. Assessing hemoglobin concentration using spectroscopic optical coherence tomography for feasibility of tissue diagnostics. Biomed. Opt. Express 2010, 1, 310–317. [Google Scholar] [CrossRef] [Green Version]
- Tsai, T.H.; Potsaid, B.; Kraus, M.F.; Zhou, C.; Tao, Y.K.; Hornegger, J.; Fujimoto, J.G. Piezoelectric-transducer-based miniature catheter for ultrahigh-speed endoscopic optical coherence tomography. Biomed. Opt. Express 2011, 2, 2438–2448. [Google Scholar] [CrossRef]
- Wieser, W.; Biedermann, B.R.; Klein, T.; Eigenwillig, C.M.; Huber, R. Multi-Megahertz OCT: High quality 3D imaging at 20 million A-scans and 4.5 GVoxels per second. Opt. Express 2010, 18, 14685–14704. [Google Scholar] [CrossRef]
- Wang, T.; Pfeiffer, T.; Regar, E.; Wieser, W.; van Beusekom, H.; Lancee, C.T.; Springeling, G.; Krabbendam-Peters, I.; van der Steen, A.F.W.; Huber, R.; et al. Heartbeat OCT and Motion-Free 3D In Vivo Coronary Artery Microscopy. JACC Cardiovasc. Imaging 2016, 9, 622–623. [Google Scholar] [CrossRef]
- Vakoc, B.J.; Shishko, M.; Yun, S.H.; Oh, W.Y.; Suter, M.J.; Desjardins, A.E.; Evans, J.A.; Nishioka, N.S.; Tearney, G.J.; Bouma, B.E. Comprehensive esophageal microscopy by using optical frequency-domain imaging (with video). Gastrointest. Endosc. 2007, 65, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Adler, D.C.; Chen, Y.; Huber, R.; Schmitt, J.; Connolly, J.; Fujimoto, J.G. Three-dimensional endomicroscopy using optical coherence tomography. Nat. Photonics 2007, 1, 709. [Google Scholar] [CrossRef]
- Suter, M.J.; Vakoc, B.J.; Yachimski, P.S.; Shishkov, M.; Lauwers, G.Y.; Mino-Kenudson, M.; Bouma, B.E.; Nishioka, N.S.; Tearney, G.J. Comprehensive microscopy of the esophagus in human patients with optical frequency domain imaging. Gastrointest. Endosc. 2008, 68, 745–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gora, M.J.; Sauk, J.S.; Carruth, R.W.; Gallagher, K.A.; Suter, M.J.; Nishioka, N.S.; Kava, L.E.; Rosenberg, M.; Bouma, B.E.; Tearney, G.J. Tethered capsule endomicroscopy enables less invasive imaging of gastrointestinal tract microstructure. Nat. Med. 2013, 19, 238–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Supplementary Material. Available online: https://www.gastrojournal.org/article/S0016-5085(14)01073-7/fulltext#appsec1 (accessed on 19 July 2019).
- Zhang, N.; Tsai, T.-H.; Ahsen, O.O.; Liang, K.; Lee, H.-C.; Xue, P.; Li, X.; Fujimoto, J.G. Compact piezoelectric transducer fiber scanning probe for optical coherence tomography. Opt. Lett. 2014, 39, 186–188. [Google Scholar] [CrossRef] [PubMed]
- Gora, M.J.; Suter, M.J.; Tearney, G.J.; Li, X. Endoscopic optical coherence tomography: Technologies and clinical applications [Invited]. Biomed. Opt. Express 2017, 8, 2405–2444. [Google Scholar] [CrossRef] [PubMed]
- Cogliati, A.; Canavesi, C.; Hayes, A.; Tankam, P.; Duma, V.-F.; Santhanam, A.; Thompson, K.P.; Rolland, J.P. MEMS-based handheld scanning probe with pre-shaped input signals for distortion-free images in Gabor-domain optical coherence microscopy. Opt. Express 2016, 24, 13365–13374. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Zhou, G.; Yu, H.; Du, Y.; Feng, H.; Tsai, J.M.; Chau, F.S. Compact MEMS-driven pyramidal polygon reflector for circumferential scanned endoscopic imaging probe. Opt. Express 2012, 20, 6325–6339. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Conry, M.; Gu, C.; Wang, F.; Yaqoob, Z.; Yang, C. Paired-angle-rotation scanning optical coherence tomography forward-imaging probe. Opt. Lett. 2006, 31, 1265–1267. [Google Scholar] [CrossRef]
- Ahsen, O.O.; Liang, K.; Lee, H.C.; Giacomelli, M.G.; Wang, Z.; Potsaid, B.; Figueiredo, M.; Huang, Q.; Jayaraman, V.; Fujimoto, J.G.; et al. Assessment of Barrett’s esophagus and dysplasia with ultrahigh-speed volumetric en face and cross-sectional optical coherence tomography. Endoscopy 2019, 51, 355–359. [Google Scholar] [CrossRef]
- Pan, Y.; Xie, H.; Fedder, G.K. Endoscopic optical coherence tomography based on a microelectromechanical mirror. Opt. Lett. 2001, 26, 1966–1968. [Google Scholar] [CrossRef] [PubMed]
- Seibel, E.J.; Smithwick, Q.Y. Unique features of optical scanning, single fiber endoscopy. Lasers Surg. Med. 2002, 30, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.; Lee, S.-W.; Rubinstein, M.; Wong, B.J.F.; Chen, Z. Semi-resonant operation of a fiber-cantilever piezotube scanner for stable optical coherence tomography endoscope imaging. Opt. Express 2010, 18, 21183–21197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryu, S.Y.; Choi, H.Y.; Na, J.; Choi, E.S.; Lee, B.H. Combined system of optical coherence tomography and fluorescence spectroscopy based on double-cladding fiber. Opt. Lett. 2008, 33, 2347–2349. [Google Scholar] [CrossRef] [PubMed]
- Tumlinson, A.R.; Hariri, L.P.; Utzinger, U.; Barton, J.K. Miniature endoscope for simultaneous optical coherence tomography and laser-induced fluorescence measurement. Appl. Opt. 2004, 43, 113–121. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Jing, J.; Yu, J.; Zhang, B.; Huo, T.; Yang, Q.; Chen, Z. Multimodality endoscopic optical coherence tomography and fluorescence imaging technology for visualization of layered architecture and subsurface microvasculature. Opt. Lett. 2018, 43, 2074–2077. [Google Scholar] [CrossRef]
- Li, X.; Yin, J.; Hu, C.; Zhou, Q.; Shung, K.K.; Chen, Z. High-resolution coregistered intravascular imaging with integrated ultrasound and optical coherence tomography probe. Appl. Phys. Lett. 2010, 97, 133702. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Li, X.; Wang, T.; Kumavor, P.D.; Aguirre, A.; Shung, K.K.; Zhou, Q.; Sanders, M.; Brewer, M.; Zhu, Q. Integrated optical coherence tomography, ultrasound and photoacoustic imaging for ovarian tissue characterization. Biomed. Opt. Express 2011, 2, 2551–2561. [Google Scholar] [CrossRef] [Green Version]
- de Boer, J.F.; Hitzenberger, C.K.; Yasuno, Y. Polarization sensitive optical coherence tomography—A review [Invited]. Biomed. Opt. Express 2017, 8, 1838–1873. [Google Scholar] [CrossRef]
- Antonelli, M.R.; Pierangelo, A.; Novikova, T.; Validire, P.; Benali, A.; Gayet, B.; De Martino, A. Mueller matrix imaging of human colon tissue for cancer diagnostics: How Monte Carlo modeling can help in the interpretation of experimental data. Opt. Express 2010, 18, 10200–10208. [Google Scholar] [CrossRef]
- Steele, D.; Baig, K.K.K.; Peter, S. Evolving screening and surveillance techniques for Barrett’s esophagus. World J. Gastroenterol. 2019, 25, 2045–2057. [Google Scholar] [CrossRef] [PubMed]
- Maes, S.; Sharma, P.; Bisschops, R. Review: Surveillance of patients with Barrett oesophagus. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 901–912. [Google Scholar] [CrossRef] [PubMed]
- Hur, C.; Miller, M.; Kong, C.Y.; Dowling, E.C.; Nattinger, K.J.; Dunn, M.; Feuer, E.J. Trends in esophageal adenocarcinoma incidence and mortality. Cancer 2013, 119, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Lagergren, J.; Lagergren, P. Recent developments in esophageal adenocarcinoma. CA Cancer J. Clin. 2013, 63, 232–248. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.F.; Morales, C.P.; Spechler, S.J. Review article: A conceptual approach to understanding the molecular mechanisms of cancer development in Barrett’s oesophagus. Aliment. Pharmacol. Ther. 2001, 15, 1087–1100. [Google Scholar] [CrossRef]
- Weston, A.P.; Banerjee, S.K.; Sharma, P.; Tran, T.M.; Richards, R.; Cherian, R. p53 protein overexpression in low grade dysplasia (LGD) in Barrett’s esophagus: Immunohistochemical marker predictive of progression. Am. J. Gastroenterol. 2001, 96, 1355–1362. [Google Scholar] [CrossRef]
- Shaheen, N.J.; Falk, G.W.; Iyer, P.G.; Gerson, L.B. ACG Clinical Guideline: Diagnosis and Management of Barrett’s Esophagus. Am. J. Gastroenterol. 2016, 111, 30. [Google Scholar] [CrossRef]
- Zhou, C.; Tsai, T.H.; Lee, H.C.; Kirtane, T.; Figueiredo, M.; Tao, Y.K.; Ahsen, O.O.; Adler, D.C.; Schmitt, J.M.; Huang, Q.; et al. Characterization of buried glands before and after radiofrequency ablation by using 3-dimensional optical coherence tomography (with videos). Gastrointest. Endosc. 2012, 76, 32–40. [Google Scholar] [CrossRef] [Green Version]
- Pouw, R.E.; Gondrie, J.J.; Rygiel, A.M.; Sondermeijer, C.M.; ten Kate, F.J.; Odze, R.D.; Vieth, M.; Krishnadath, K.K.; Bergman, J.J. Properties of the neosquamous epithelium after radiofrequency ablation of Barrett’s esophagus containing neoplasia. Am. J. Gastroenterol. 2009, 104, 1366–1373. [Google Scholar] [CrossRef]
- Mashimo, H. Subsquamous intestinal metaplasia after ablation of Barrett’s esophagus: Frequency and importance. Curr. Opin. Gastroenterol. 2013, 29, 454–459. [Google Scholar] [CrossRef]
- Sharma, P.; Savides, T.J.; Canto, M.I.; Corley, D.A.; Falk, G.W.; Goldblum, J.R.; Wang, K.K.; Wallace, M.B.; Wolfsen, H.C. The American Society for Gastrointestinal Endoscopy PIVI (Preservation and Incorporation of Valuable Endoscopic Innovations) on imaging in Barrett’s Esophagus. Gastrointest. Endosc. 2012, 76, 252–254. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.A.; Poneros, J.M.; Bouma, B.E.; Bressner, J.; Halpern, E.F.; Shishkov, M.; Lauwers, G.Y.; Mino-Kenudson, M.; Nishioka, N.S.; Tearney, G.J. Optical coherence tomography to identify intramucosal carcinoma and high-grade dysplasia in Barrett’s esophagus. Clin. Gastroenterol. Hepatol. 2006, 4, 38–43. [Google Scholar] [CrossRef]
- Trindade, A.J.; Vamadevan, A.S.; Sejpal, D.V. Finding a needle in a haystack: Use of volumetric laser endomicroscopy in targeting focal dysplasia in long-segment Barrett’s esophagus. Gastrointest. Endosc. 2015, 82, 756–757. [Google Scholar] [CrossRef] [PubMed]
- Leggett, C.L.; Gorospe, E.C.; Chan, D.K.; Muppa, P.; Owens, V.; Smyrk, T.C.; Anderson, M.; Lutzke, L.S.; Tearney, G.; Wang, K.K. Comparative diagnostic performance of volumetric laser endomicroscopy and confocal laser endomicroscopy in the detection of dysplasia associated with Barrett’s esophagus. Gastrointest. Endosc. 2016, 83, 880–888. [Google Scholar] [CrossRef]
- Swager, A.F.; Tearney, G.J.; Leggett, C.L.; van Oijen, M.G.H.; Meijer, S.L.; Weusten, B.L.; Curvers, W.L.; Bergman, J. Identification of volumetric laser endomicroscopy features predictive for early neoplasia in Barrett’s esophagus using high-quality histological correlation. Gastrointest. Endosc. 2017, 85, 918–926. [Google Scholar] [CrossRef] [PubMed]
- Suter, M.J.; Jillella, P.A.; Vakoc, B.J.; Halpern, E.F.; Mino-Kenudson, M.; Lauwers, G.Y.; Bouma, B.E.; Nishioka, N.S.; Tearney, G.J. Image-guided biopsy in the esophagus through comprehensive optical frequency domain imaging and laser marking: A study in living swine. Gastrointest. Endosc. 2010, 71, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Ahsen, O.O.; Lee, H.C.; Liang, K.; Wang, Z.; Figueiredo, M.; Huang, Q.; Potsaid, B.; Jayaraman, V.; Fujimoto, J.G.; Mashimo, H. Ultrahigh-speed endoscopic optical coherence tomography and angiography enables delineation of lateral margins of endoscopic mucosal resection: A case report. Ther. Adv. Gastroenterol. 2017, 10, 931–936. [Google Scholar] [CrossRef]
- Trindade, A.J.; Benias, P.C.; Inamdar, S.; Fan, C.; Sethi, A.; Fukami, N.; Kahn, A.; Kahaleh, M.; Andalib, I.; Sejpal, D.V.; et al. Use of volumetric laser endomicroscopy for determining candidates for endoscopic therapy in superficial esophageal squamous cell carcinoma. United Eur. Gastroenterol. J. 2018, 6, 838–845. [Google Scholar] [CrossRef]
- Colorectal Cancer Facts and Figures 2017–2019. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/colorectal-cancer-facts-and-figures/colorectal-cancer-facts-and-figures-2017-2019.pdf (accessed on 17 June 2019).
- Wolf, A.M.D.; Fontham, E.T.H.; Church, T.R.; Flowers, C.R.; Guerra, C.E.; LaMonte, S.J.; Etzioni, R.; McKenna, M.T.; Oeffinger, K.C.; Shih, Y.-C.T.; et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J. Clin. 2018, 68, 250–281. [Google Scholar] [CrossRef]
- Kim, N.H.; Jung, Y.S.; Jeong, W.S.; Yang, H.-J.; Park, S.-K.; Choi, K.; Park, D.I. Miss rate of colorectal neoplastic polyps and risk factors for missed polyps in consecutive colonoscopies. Intest. Res. 2017, 15, 411–418. [Google Scholar] [CrossRef]
- Pfau, P.R.; Sivak, M.V., Jr.; Chak, A.; Kinnard, M.; Wong, R.C.; Isenberg, G.A.; Izatt, J.A.; Rollins, A.; Westphal, V. Criteria for the diagnosis of dysplasia by endoscopic optical coherence tomography. Gastrointest. Endosc. 2003, 58, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Zagaynova, E.; Gladkova, N.; Shakhova, N.; Gelikonov, G.; Gelikonov, V. Endoscopic OCT with forward-looking probe: Clinical studies in urology and gastroenterology. J. Biophotonics 2008, 1, 114–128. [Google Scholar] [CrossRef] [PubMed]
- Trindade, A.J.; Sultan, K.; Vamadevan, A.S.; Fan, C.; Sejpal, D.V. Successful use of volumetric laser endomicroscopy in imaging a rectal polyp. Ther. Adv. Gastroenterol. 2015, 9, 128–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, K.; Ahsen, O.O.; Wang, Z.; Lee, H.-C.; Liang, W.; Potsaid, B.M.; Tsai, T.-H.; Giacomelli, M.G.; Jayaraman, V.; Mashimo, H.; et al. Endoscopic forward-viewing optical coherence tomography and angiography with MHz swept source. Opt. Lett. 2017, 42, 3193–3196. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Zuccaro, G., Jr.; Gramlich, T.L.; Gladkova, N.; Trolli, P.; Kareta, M.; Delaney, C.P.; Connor, J.T.; Lashner, B.A.; Bevins, C.L.; et al. In vivo colonoscopic optical coherence tomography for transmural inflammation in inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 2004, 2, 1080–1087. [Google Scholar] [CrossRef]
- Adler, D.C.; Zhou, C.; Tsai, T.-H.; Schmitt, J.; Huang, Q.; Mashimo, H.; Fujimoto, J.G. Three-dimensional endomicroscopy of the human colon using optical coherence tomography. Opt. Express 2009, 17, 784–796. [Google Scholar] [CrossRef] [PubMed]
- Blechacz, B. Cholangiocarcinoma: Current Knowledge and New Developments. Gut Liver 2017, 11, 13–26. [Google Scholar] [CrossRef]
- Pugliese, V.; Pujic, N.; Saccomanno, S.; Gatteschi, B.; Pera, C.; Aste, H.; Ferrara, G.B.; Nicolò, G. Pancreatic intraductal sampling during ERCP in patients with chronic pancreatitis and pancreatic cancer: Cytologic studies and k-ras-2 codon 12 molecular analysis in 47 cases. Gastrointest. Endosc. 2001, 54, 595–599. [Google Scholar] [CrossRef]
- Rösch, T.; Hofrichter, K.; Frimberger, E.; Meining, A.; Born, P.; Weigert, N.; Allescher, H.-D.; Classen, M.; Barbur, M.; Schenck, U.; et al. ERCP or EUS for tissue diagnosis of biliary strictures? A prospective comparative study. Gastrointest. Endosc. 2004, 60, 390–396. [Google Scholar] [CrossRef]
- Selvaggi, S.M. Biliary brushing cytology. Cytopathology 2004, 15, 74–79. [Google Scholar] [CrossRef]
- de Bellis, M.; Sherman, S.; Fogel, E.L.; Cramer, H.; Chappo, J.; McHenry, L.; Watkins, J.L.; Lehman, G.A. Tissue sampling at ERCP in suspected malignant biliary strictures (Part 2). Gastrointest. Endosc. 2002, 56, 720–730. [Google Scholar] [CrossRef]
- Seitz, U.; Freund, J.; Jaeckle, S.; Feldchtein, F.; Bohnacker, S.; Thonke, F.; Gladkova, N.; Brand, B.; Schroder, S.; Soehendra, N. First in vivo optical coherence tomography in the human bile duct. Endoscopy 2001, 33, 1018–1021. [Google Scholar] [CrossRef] [PubMed]
- Poneros, J.M.; Tearney, G.J.; Shiskov, M.; Kelsey, P.B.; Lauwers, G.Y.; Nishioka, N.S.; Bouma, B.E. Optical coherence tomography of the biliary tree during ERCP. Gastrointest. Endosc. 2002, 55, 84–88. [Google Scholar] [CrossRef]
- Testoni, P.A.; Mariani, A.; Mangiavillano, B.; Arcidiacono, P.G.; Di Pietro, S.; Masci, E. Intraductal optical coherence tomography for investigating main pancreatic duct strictures. Am. J. Gastroenterol. 2007, 102, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Tyberg, A.; Xu, M.-M.; Gaidhane, M.; Sharaiha, R.Z.; Kahaleh, M. Tu2004—Second Generation Optical Coherence Tomography: Preliminary Experience in Pancreatic and Biliary Strictures. Gastroenterology 2017, 152, S1032–S1033. [Google Scholar] [CrossRef]
- Joshi, V.; Patel, S.N.; Vanderveldt, H.; Oliva, I.; Raijman, I.; Molina, C.; Carr-Locke, D.L. Mo1963 A Pilot Study of Safety and Efficacy of Directed Cannulation with a Low Profile Catheter (LP) and Imaging Characteristics of Bile Duct Wall Using Optical Coherance Tomography (OCT) for Indeterminate Biliary Strictures Initial Report on In-Vivo Evaluation During ERCP. Gastrointest. Endosc. 2017, 85, AB496–AB497. [Google Scholar] [CrossRef]
- Tsai, T.-H.; Ahsen, O.O.; Lee, H.-C.; Liang, K.; Figueiredo, M.; Tao, Y.K.; Giacomelli, M.G.; Potsaid, B.M.; Jayaraman, V.; Huang, Q. Endoscopic optical coherence angiography enables 3-dimensional visualization of subsurface microvasculature. Gastroenterology 2014, 147, 1219–1221. [Google Scholar] [CrossRef]
- Lee, M.M.; Enns, R. Narrow band imaging in gastroesophageal reflux disease and Barrett’s esophagus. Can. J. Gastroenterol. Hepatol. 2009, 23, 84–87. [Google Scholar] [CrossRef]
- Singh, R.; Anagnostopoulos, G.K.; Yao, K.; Karageorgiou, H.; Fortun, P.J.; Shonde, A.; Garsed, K.; Kaye, P.V.; Hawkey, C.J.; Ragunath, K. Narrow-band imaging with magnification in Barrett’s esophagus: Validation of a simplified grading system of mucosal morphology patterns against histology. Endoscopy 2008, 40, 457–463. [Google Scholar] [CrossRef]
- Zhou, C.; Adler, D.C.; Becker, L.; Chen, Y.; Tsai, T.-H.; Figueiredo, M.; Schmitt, J.M.; Fujimoto, J.G.; Mashimo, H. Effective treatment of chronic radiation proctitis using radiofrequency ablation. Ther. Adv. Gastroenterol. 2009, 2, 149–156. [Google Scholar] [CrossRef]
- Lenz, L.; Rohr, R.; Nakao, F.; Libera, E.; Ferrari, A. Chronic radiation proctopathy: A practical review of endoscopic treatment. World J. Gastrointest. Surg. 2016, 8, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Nojkov, B.; Cappell, M.S. Gastrointestinal bleeding from Dieulafoy’s lesion: Clinical presentation, endoscopic findings, and endoscopic therapy. World J. Gastrointest. Endosc. 2015, 7, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-C.; Ahsen, O.O.; Liang, K.; Wang, Z.; Figueiredo, M.; Potsaid, B.; Jayaraman, V.; Huang, Q.; Mashimo, H.; Fujimoto, J.G. Sa2034 Novel Ultrahigh Speed Endoscopic OCT Angiography Identifies Both Architectural and Microvascular Changes in Patients with Gastric Antral Vascular Ectasia (GAVE) Undergoing Radiofrequency Ablation (RFA) Treatment. Gastroenterology 2016, 150, S435–S436. [Google Scholar] [CrossRef]
- Masci, E.; Mangiavillano, B.; Albarello, L.; Mariani, A.; Doglioni, C.; Testoni, P.A. Pilot study on the correlation of optical coherence tomography with histology in celiac disease and normal subjects. J. Gastroenterol. Hepatol. 2007, 22, 2256–2260. [Google Scholar] [CrossRef] [PubMed]
- Masci, E.; Mangiavillano, B.; Barera, G.; Parma, B.; Albarello, L.; Mariani, A.; Doglioni, C.; Testoni, P.A. Optical coherence tomography in pediatric patients: A feasible technique for diagnosing celiac disease in children with villous atrophy. Dig. Liver Dis. 2009, 41, 639–643. [Google Scholar] [CrossRef]
- Kamboj, A.K.; Chan, D.K.; Zakko, L.; Visrodia, K.; Otaki, F.; Lutzke, L.S.; Wang, K.K.; Leggett, C.L. Mo2006 Detection of Barrett’s Esophagus Dysplasia Using a Novel Volumetric Laser Endomicroscopy Computer Algorithm. Gastrointest. Endosc. 2017, 85, AB518. [Google Scholar] [CrossRef]
- Lee, H.-C.; Ahsen, O.O.; Liang, K.; Wang, Z.; Figueiredo, M.; Giacomelli, M.G.; Potsaid, B.; Huang, Q.; Mashimo, H.; Fujimoto, J.G. Endoscopic optical coherence tomography angiography microvascular features associated with dysplasia in Barrett’s esophagus (with video). Gastrointest. Endosc. 2017, 86, 476–484. [Google Scholar] [CrossRef]
- Mohri, M.; Rostamizadeh, A.; Talwalkar, A. Foundations of Machine Learning; MIT Press: London, UK, 2018. [Google Scholar]
- Goodfellow, I.; Bengio, Y.; Courville, A. Deep Learning; MIT Press: London, UK, 2016. [Google Scholar]
- Byrne, M.F. Artificial intelligence and the future of endoscopy: Should we be quietly excited? Endoscopy 2019, 51, 511–512. [Google Scholar] [CrossRef]
- Shimodate, Y.; Mizuno, M.; Doi, A.; Nishimura, N.; Mouri, H.; Matsueda, K.; Yamamoto, H. Gastric superficial neoplasia: High miss rate but slow progression. Endosc. Int. Open 2017, 5, E722–E726. [Google Scholar] [CrossRef]
- Wu, L.; Zhou, W.; Wan, X.; Zhang, J.; Shen, L.; Hu, S.; Ding, Q.; Mu, G.; Yin, A.; Huang, X.; et al. A deep neural network improves endoscopic detection of early gastric cancer without blind spots. Endoscopy 2019, 51, 522–531. [Google Scholar] [CrossRef] [Green Version]
- Maeda, Y.; Kudo, S.-E.; Mori, Y.; Misawa, M.; Ogata, N.; Sasanuma, S.; Wakamura, K.; Oda, M.; Mori, K.; Ohtsuka, K. Fully automated diagnostic system with artificial intelligence using endocytoscopy to identify the presence of histologic inflammation associated with ulcerative colitis (with video). Gastrointest. Endosc. 2019, 89, 408–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Rijn, J.C.; Reitsma, J.B.; Stoker, J.; Bossuyt, P.M.; van Deventer, S.J.; Dekker, E. Polyp miss rate determined by tandem colonoscopy: A systematic review. Am. J. Gastroenterol. 2006, 101, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Tajbakhsh, N.; Gurudu, S.R.; Liang, J. Automatic polyp detection using global geometric constraints and local intensity variation patterns. In International Conference on Medical Image Computing and Computer-Assisted Intervention; Springer: Cham, Switzerland, 2014; Volume 17, pp. 179–187. [Google Scholar]
- Bernal, J.; Sánchez, J.; Vilarino, F. Towards automatic polyp detection with a polyp appearance model. Pattern Recognit. 2012, 45, 3166–3182. [Google Scholar] [CrossRef]
- Fernandez-Esparrach, G.; Bernal, J.; Lopez-Ceron, M.; Cordova, H.; Sanchez-Montes, C.; Rodriguez de Miguel, C.; Sanchez, F.J. Exploring the clinical potential of an automatic colonic polyp detection method based on the creation of energy maps. Endoscopy 2016, 48, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Bernal, J.; Sanchez, F.J.; Fernandez-Esparrach, G.; Gil, D.; Rodriguez, C.; Vilarino, F. WM-DOVA maps for accurate polyp highlighting in colonoscopy: Validation vs. saliency maps from physicians. Comput. Med. Imaging Graph. 2015, 43, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, V.; Rosenfeld, A.; Graham, D.G.; Banks, M.R.; Haidry, R.J.; Lovat, L.B. PTU-058 Machine Learning Creates A Simple Endoscopic Classification System for Detecting Dysplasia in Barrett’s Oesophagus with I-scan Imaging and Opens The Way To Standardised Training And Assessment Of Competence. Gut 2014, 63, A63. [Google Scholar] [CrossRef]
- Ughi, G.J.; Gora, M.J.; Swager, A.-F.; Soomro, A.; Grant, C.; Tiernan, A.; Rosenberg, M.; Sauk, J.S.; Nishioka, N.S.; Tearney, G.J. Automated segmentation and characterization of esophageal wall in vivo by tethered capsule optical coherence tomography endomicroscopy. Biomed. Opt. Express 2016, 7, 409–419. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Lee, H.-C.; Ahsen, O.O.; Liang, K.; Figueiredo, M.; Huang, Q.; Fujimoto, G.J.; Mashimo, H. Computer-Aided Analysis of Gland-Like Subsurface Hyposcattering Structures in Barrett’s Esophagus Using Optical Coherence Tomography. Appl. Sci. 2018, 8, 2420. [Google Scholar] [CrossRef]
- Kamboj, A.K.; Hoversten, P.; Kahn, A.K.; Trindade, A.J.; Iyer, P.G.; Wang, K.K.; Leggett, C.L. Interpretation of volumetric laser endomicroscopy in Barrett’s esophagus using image enhancement software. Dis. Esophagus 2019. [Google Scholar] [CrossRef]
- Lu, W.; Tong, Y.; Yu, Y.; Xing, Y.; Chen, C.; Shen, Y. Deep Learning-Based Automated Classification of Multi-Categorical Abnormalities from Optical Coherence Tomography Images. Transl. Vis. Sci. Technol. 2018, 7, 41. [Google Scholar] [CrossRef]
- Cai, S.; Zhong, Y.; Zhou, P.-H. Tu2005 development and validation of a deep learning algorithm for detection and recognition of precancerous lesion in esophagus. Gastrointest. Endosc. 2019, 89, AB654. [Google Scholar] [CrossRef]
- Shibata, J.; Ozawa, T.; Ishihara, S.; Onishi, T.; Matsuo, K.; Miura, M.; Aoyama, K.; Tada, T. Mo2050—Novel Computer-Assisted Detection System of Colorectal Carcinoid Tumors Using Convolutional Neural Networks. Gastroenterology 2019, 156, S-937. [Google Scholar] [CrossRef]
- Tokai, Y.; Yoshio, T.; Fujisaki, J.; Aoyama, K.; Tada, T. Sa1209 application of artificial intelligence using convolutional neural networks in diagnosing the invasion depth of esophageal squamous cell carcinoma. Gastrointest. Endosc. 2019, 89, AB169. [Google Scholar] [CrossRef]
- Nakagawa, K.; Ishihara, R.; Aoyama, K.; Kono, M.; Fukuda, H.; Shimamoto, Y.; Ohmori, M.; Iwagami, H.; Matsuno, K.; Inoue, S.; et al. Sa1257 specialist-level diagnostic accuracy for cancer invasion depth of superficial esophageal squamous cell carcinoma with conventional neural network. Gastrointest. Endosc. 2019, 89, AB191. [Google Scholar] [CrossRef]
- Xi, J.; Huo, L.; Wu, Y.; Cobb, M.J.; Hwang, J.H.; Li, X. High-resolution OCT balloon imaging catheter with astigmatism correction. Opt. Lett. 2009, 34, 1943–1945. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Zhang, J.; Yu, L.; Colt, H.G.; Brenner, M.; Chen, Z. Real-time swept source optical coherence tomography imaging of the human airway using a microelectromechanical system endoscope and digital signal processor. J. Biomed. Opt. 2008, 13, 030506. [Google Scholar] [CrossRef] [PubMed]
- Huo, L.; Xi, J.; Wu, Y.; Li, X. Forward-viewing resonant fiber-optic scanning endoscope of appropriate scanning speed for 3D OCT imaging. Opt. Express 2010, 18, 14375–14384. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.; Traverso, G.; Lee, H.-C.; Ahsen, O.O.; Wang, Z.; Potsaid, B.; Giacomelli, M.; Jayaraman, V.; Barman, R.; Cable, A.; et al. Ultrahigh speed en face OCT capsule for endoscopic imaging. Biomed. Opt. Express 2015, 6, 1146–1163. [Google Scholar] [CrossRef] [Green Version]
- Iddan, G.; Meron, G.; Glukhovsky, A.; Swain, P. Wireless capsule endoscopy. Nature 2000, 405, 417. [Google Scholar] [CrossRef]
- Ramirez, F.C.; Akins, R.; Shaukat, M. Screening of Barrett’s esophagus with string-capsule endoscopy: A prospective blinded study of 100 consecutive patients using histology as the criterion standard. Gastrointest. Endosc. 2008, 68, 25–31. [Google Scholar] [CrossRef]
- Liao, Z.; Gao, R.; Xu, C.; Xu, D.-F.; Li, Z.-S. Sleeve string capsule endoscopy for real-time viewing of the esophagus: A pilot study (with video). Gastrointest. Endosc. 2009, 70, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Gora, M.J.; Sauk, J.S.; Carruth, R.W.; Lu, W.; Carlton, D.T.; Soomro, A.; Rosenberg, M.; Nishioka, N.S.; Tearney, G.J. Imaging the Upper Gastrointestinal Tract in Unsedated Patients Using Tethered Capsule Endomicroscopy. Gastroenterology 2013, 145, 723–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Endo, T.; Awakawa, T.; Takahashi, H.; Arimura, Y.; Itoh, F.; Yamashita, K.; Sasaki, S.; Yamamoto, H.; Tang, X.; Imai, K. Classification of Barrett’s epithelium by magnifying endoscopy. Gastrointest. Endosc. 2002, 55, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Kudo, S.; Tamura, S.; Nakajima, T.; Yamano, H.; Kusaka, H.; Watanabe, H. Diagnosis of colorectal tumorous lesions by magnifying endoscopy. Gastrointest. Endosc. 1996, 44, 8–14. [Google Scholar] [CrossRef]
- Liang, C.P.; Dong, J.; Ford, T.; Reddy, R.; Hosseiny, H.; Farrokhi, H.; Beatty, M.; Singh, K.; Osman, H.; Vuong, B.; et al. Optical coherence tomography-guided laser marking with tethered capsule endomicroscopy in unsedated patients. Biomed. Opt. Express 2019, 10, 1207–1222. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.; Wang, Z.; Ahsen, O.O.; Lee, H.-C.; Potsaid, B.M.; Jayaraman, V.; Cable, A.; Mashimo, H.; Li, X.; Fujimoto, J.G. Cycloid scanning for wide field optical coherence tomography endomicroscopy and angiography in vivo. Optica 2018, 5, 36–43. [Google Scholar] [CrossRef] [Green Version]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samel, N.S.; Mashimo, H. Application of OCT in the Gastrointestinal Tract. Appl. Sci. 2019, 9, 2991. https://doi.org/10.3390/app9152991
Samel NS, Mashimo H. Application of OCT in the Gastrointestinal Tract. Applied Sciences. 2019; 9(15):2991. https://doi.org/10.3390/app9152991
Chicago/Turabian StyleSamel, Nicholas S., and Hiroshi Mashimo. 2019. "Application of OCT in the Gastrointestinal Tract" Applied Sciences 9, no. 15: 2991. https://doi.org/10.3390/app9152991
APA StyleSamel, N. S., & Mashimo, H. (2019). Application of OCT in the Gastrointestinal Tract. Applied Sciences, 9(15), 2991. https://doi.org/10.3390/app9152991




