Abstract
Partial discharge has become a serious threat to the stable operation of gas-insulated switchgears. SOF2 is a characteristic decomposition component of SF6 decomposition components under partial discharge, which further deteriorate the severity of partial discharge. In order to find an excellent adsorbent for SOF2, Pd/Pt-Ni(111) composite surface is raised as an adsorbent to investigate its adsorption ability of the SOF2 molecule. The results of the study show that Pd or Pt composite layer on Ni(111) atoms can significantly enhance the adsorption capacity, the adsorption ability to SOF2 is in the sequence of Pt-Ni(111) > Pd-Ni(111) > Ni(111) > Ni-Pd-Ni(111) > Ni-Pt-Ni(111). However, the adsorption of SOF2 on Pt-Ni(111) and Pd-Ni(111) surfaces is strong chemisorption, which is an irreversible adsorption process. On the contrary, Ni-Pd-Ni(111) and Ni-Pt-Ni(111) show moderate physisorption of SOF2. In addition, the density of electronic states, and electron density difference are further calculated to analyze the adsorption mechanism of SOF2. This research provides important theoretical support for developing an ideal SOF2 adsorbent.
1. Introduction
Gas-insulated switchgears (GIS) are widely used in power systems for their small volume, high stability, and long detection period. As an insulating medium filled in GISs, sulfur hexafluoride (SF6) shows excellent insulating and arc extinguishing properties, which ensures the high insulation and stable operation of GISs [1,2]. However, the application of SF6 is restricted due to its high contribution to global warming. Alternative insulating gases to SF6 are currently sought due to their sustainable and environmental properties. Currently, SF6 is the most used gas in GISs. There may be some inevitable insulation defects in GISs during the long-term operation process, which would cause partial discharges (PD) and partial overheating (POT). Under the action of PD and POT, SF6 gas decomposes to low-fluorine sulfides (SFx, x = 1–5). Due to the high chemical activity of SFx, it further reacts with traces of H2O and O2 existing in the GIS to various decomposition products, such as the main components: H2S, SO2, SOF2, and SO2F2 [3,4]. These decomposition products greatly reduce the insulation performance of GISs, and may even cause sudden malfunction of the equipment [5,6]. Therefore, it is particularly important to remove the SF6 decomposition products in a timely manner. Under the conditions of PD and POT, the proportion of SOF2 is far more than other decomposition gases. In addition, it is difficult for SOF2 to produce secondary reactions [7,8]. Therefore, it is important to remove SOF2 from SF6. At present, two types of adsorbents are widely used in gas adsorption: Activated alumina and molecular sieves [9,10,11]. However, both of them have different limitations on the adsorption of SF6 decomposition products. Therefore, it is important to explore a new adsorbent material that shows good adsorption properties of SOF2.
As a transition metal, nickel has good chemical stability and selectivity due to its unique d-electron structure, which makes it a suitable adsorbent for gas adsorption [12,13,14]. A large number of experiments with the purpose of studying various types of catalysts or sensors based on nickel have been carried out [15,16,17,18]. Moreover, other transition metal layers composited on nickel’s surface could improve the adsorption capacity of nickel [19]. Li et al. reported Pd composite layers on Ni(111) surface, verifying that the bimetallic composite surface structure of Pd-Ni(111) shows better adsorption properties than the monometallic surface of Ni(111) [20]. In our previous research, the adsorption performance of SF6 decomposition components on the surface of Ni(111) was studied. It is found that Ni(111) is more likely to adsorb SOF2, but it needs to cross a barrier of 0.54 eV [21,22].
In order to further study the adsorption capacity of Ni based materials for removing SF6 gas from SOF2, Pd and Pt layers were respectively composited to the surface of Ni(111) to improve the adsorption ability of SOF2, which was abbreviated as Pd/Pt-Ni(111). The calculations of adsorption structure, adsorption energy, charge transfer, density of states, and electron density difference of SOF2 adsorbed Ni(111) monometallic and Pd/Pt-Ni(111) bimetallic surfaces were performed based on density functional theory (DFT) calculations. This study provides a key foundation for developing a better adsorbent for SOF2 adsorption in GISs.
2. Methods and Models
All of the calculations in this paper were carried out based on DFT [23]. The electron exchange and correlation energy were calculated within the generalized gradient approximation (GGA) and the Perdew-Burke-Ernzerhof (PBE) function [24,25]. Double numerical plus polarization (DNP) basis sets were used [26]. As spin polarization has major effects on the adsorption energies for a magnetic system, all the calculations were spin-polarized [27]. The convergence criterion for the total energy and maximum force were set at 1 × 10−5 Ha and 2 × 10−3 Ha/Å, respectively. The self-consistent field (SCF) tolerance was 1 × 10−6 Ha, and direct inversion of iterative subspace (DIIS) was made to accelerate convergence of SCF [28].
The Ni(111) monometallic and Pd/Pt-Ni(111) bimetallic surfaces were modeled by periodic slabs with a supercell of a 2 × 2 unit cell. The vacuum area between the slabs is set to 20 Å in order to avoid interaction between the adjacent superlattices. The Brillouin zone was sampled by using a 5 × 5 × 1 array of k-points in the Monkhorst–Pack grid [29]. As shown in Figure 1, four surfaces were explored in this work, including two surface structures named Pd-Ni(111) and Pt-Ni(111) surfaces, and two subsurface structures named Ni-Pd-Ni(111) and Ni-Pt-Ni(111) surfaces. Furthermore, both the Ni(111) monometallic and bimetallic Pd/Pt-Ni(111) surfaces provide four typical sites for the adsorption, including the t-top site, b-bridge site, fcc-face centered cubic site, and hcp-hexagonal close packed site.
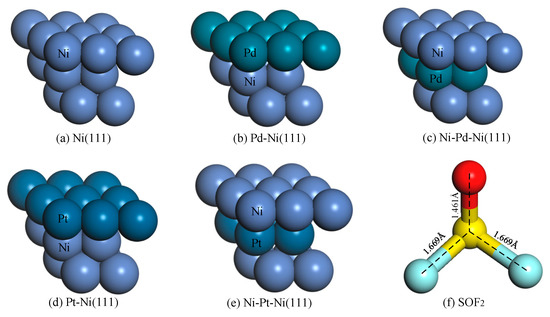
Figure 1.
(a–e) Structures of the Ni(111) monometallic and Pd/Pt-Ni(111) bimetallic surfaces, (f) Structure of SOF2.
The adsorption energy (Eads) of the molecule adsorption on Pd/Pt-Ni(111) was defined as Equation (1):
Eads = Eslab/gas − Eslab − Egas
Eslab/gas was the total energy of the adsorbed system, Eslab and Egas were the energies of the surface and the free gas molecule, respectively. Thus, negative Eads means the process of the adsorption was exothermic and that adsorption can happen spontaneously. The more energy released, the stronger the adsorption was.
The electron density distribution was calculated by Mulliken population analysis. The charge transfer (QT) in the adsorption process was defined as Equation (2):
QT = Qa − Qb
Qb and Qa were the respective total charge of the gas molecules before and after adsorption. QT > 0 means electrons transfer from the gas molecules to the surface.
3. Results and Discussion
3.1. Adsorption of SOF2 on Pd-Ni(111) and Ni-Pd-Ni(111) Surfaces
The Pd layer was used to replace the outermost layer and secondary outer layer of the Ni(111) surface to obtain stable Pd-Ni(111) and Ni-Pd-Ni(111) surfaces. Then, the SOF2 molecule was placed in different initial positions and directions to approach these two surfaces respectively. After geometric optimization, several stable structures were obtained as shown in Figure 2. The adsorption energy and relevant structural parameters are listed in Table 1.
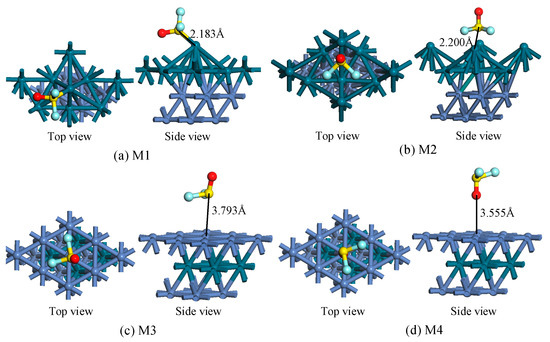
Figure 2.
The top and side views of adsorption structures. (a,b) SOF2 adsorption on Pd-Ni(111) surface for M1 and M2 structures, (c,d) SOF2 adsorption on Ni-Pd-Ni(111) surface for M3-M4 structures.

Table 1.
The adsorption parameters of SOF2 adsorbed by Ni(111), Pd-Ni(111), and Ni-Pd-Ni(111) surfaces.
Figure 2 shows the different adsorption structures of SOF2 on Pd-Ni(111) and Ni-Pd-Ni(111) surfaces. In the M1 structure, the SOF2 molecule approaches the Pd-Ni(111) surface through S atom, forming a new chemical bond between the S atom and the Pd atom. The distance of the S-Pd bond is 2.183 Å, while the bond lengths in the SOF2 molecule have almost not changed. In the M2 structure, three atoms interact with the Pd-Ni(111) surface, covering the hcp and fcc positions. Compared with the M1 structure, the deformation of M2 seems to be more serious. In the M3 and M4 structures, SOF2 adsorption on the surface had an adsorption distance of 3.793 Å and 3.555 Å, indicating that the adsorption on Ni-Pd-Ni(111) surface was much more moderate.
As listed in Table 1, the adsorption energy of the four structures in Figure 2 was negative, indicating that the adsorption process is exothermic. The Eads of M1 and M2 are −1.964 eV and −1.880 eV respectively, which are higher than the Eads of SOF2 adsorption on the Ni(111) surface (−1.55 eV). During the adsorption process, charge transferred from the SOF2 molecule to the Pd-Ni(111) surface, forming a SOF2+ cation. Compared with the results on the Ni-Pd-Ni(111) surface, Pd-Ni(111) showed greater capability of adsorbing SOF2 for its higher adsorption energy. In the M3 and M4 structures, charge transfer occurred from the Ni-Pd-Ni(111) surface to the SOF2 molecule, which is the opposite of M1 and M2.
To further study the electronic behavior of SOF2 adsorption, the total density of states (TDOS) and partial density of states (PDOS) were calculated. The structures of M1 and M3 were selected for comparison of SOF2 adsorption on the Pd-Ni(111) and Ni-Pd-Ni(111) surfaces.
As shown in Figure 3a, there are some obvious changes in TDOS after SOF2 adsorption on the Pd-Ni(111) surface. The TDOS increased from −9.5 eV to −7.5 eV and −4 eV to −1 eV, and a little decline occured from −5.5 eV to −4.5 eV. However, there are some differences with SOF2 adsorption on the Ni-Pd-Ni(111) surface in Figure 3c, the TDOS has a visible rise from −10 eV to −3 eV in the M3 structure. In viewing the PDOS in Figure 3b, the change of TDOS in M1 structure mainly consists of a 2p orbital of F atoms, a 3p orbital of S atom, a 3d of Ni atoms, and a 4d of Pd atoms. The large overlapped area between S 3p, Ni 3d, and Pd 4d signifies that their orbitals are highly hybridized during the adsorption process.
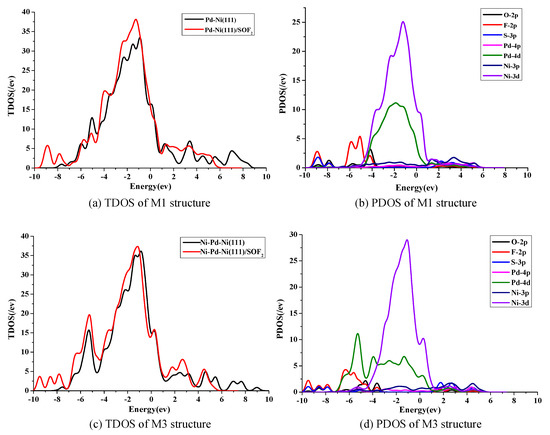
Figure 3.
(a,c) Total density of states (TDOS), (b,d) partial density of states (PDOS) for the structure of M1 and M3.
As for the M3 structure, the PDOS has a large overlapped area between F 2p and Pd 4d, indicating a strong interaction between them. Comparing the change of TDOS and PDOS, it is concluded that the interaction between the O atom, S atom and Pd atoms in M1 is stronger than that in M3. The result is consistent with the analysis of adsorption energy in Table 1.
Figure 4 shows the electron density difference of SOF2 adsorption on Pd-Ni(111) and Ni-Pd-Ni(111) surfaces. Since the electron distribution of M4 is similar to M3, M3 was selected for comparison with M1 and M2. The red region represents an increase of the electron density, while the blue region represents a decrease of the electron density.
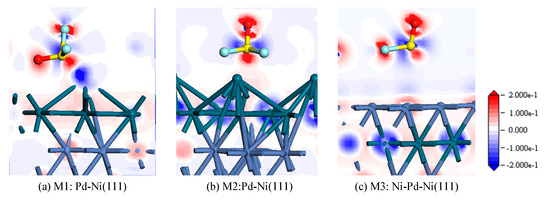
Figure 4.
Electron density difference of SOF2 adsorption on the (a) M1:Pd-Ni(111), (b) M2:Pd-Ni(111), and (c) M3:Ni-Pd-Ni(111) surfaces.
For the M1 structure in Figure 4a, it is shown that electrons transfer from the bonding Pd atom to O atom and F atom. The density of electrons between the S atom and bonding Pd atom indicates the formation of chemical bonds. As shown in Figure 4b, the electron density near the O atom and S atom increased, while the Pd atoms had varying degrees of charge transfer. In Figure 4c, a small amount of electrons transferred from the Pd atoms to the O atom and F atom, indicating a weak chemical interaction between them.
According to the results from the analysis of adsorption energy, DOS, and electron density difference, it is concluded that the Pd-Ni(111) surface shows a stronger ability of adsorbing the SOF2 molecule than the Ni-Pd-Ni(111) surface.
3.2. Adsorption of SOF2 on Pt-Ni(111) and Ni-Pt-Ni(111) Surface
Similarly, Pt atoms were used to replace the supernatant and subsurface Ni atoms to obtain stable Pt-Ni(111) and Ni-Pt-Ni(111) surfaces. After that, the SOF2 molecule was placed in different initial positions and directions to get different adsorption structures. The results are shown in Figure 5, and the adsorption energy and relevant structural parameters are listed in Table 2.
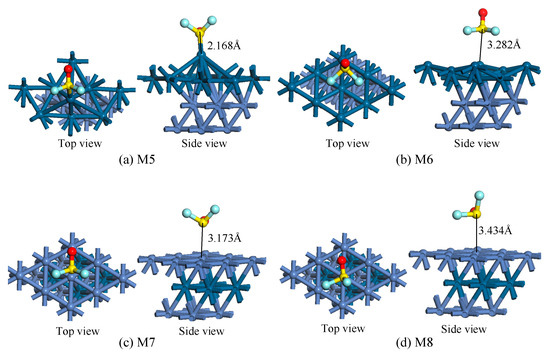
Figure 5.
The top and side views of adsorption structures. (a,b) SOF2 adsorption on Pt-Ni(111) surface for M5 and M6 structures, (c,d) SOF2 adsorption on Ni-Pt-Ni(111) surface for M7 and M8 structures.

Table 2.
The adsorption parameters of SOF2 adsorbed by Ni(111), Pt-Ni(111), and Ni-Pt-Ni(111) surfaces.
As shown in Figure 5, M5 and M6 are structures of SOF2 adsorption on the Pt-Ni(111) surface, while M7 and M8 are adsorption structures on the Ni-Pt-Ni(111) surface. In the M5 structure, the SOF2 molecule adsorbs on the top site, and forms a chemical bond between the S atom and Pt atom. After SOF2 adsorption, the position of the supernatant Pt atoms changed a lot, demonstrating a strong interaction between them. In the M6 structure, one S atom and two F atoms interacted with the Pt-Ni(111) surface, but the adsorption distance was relatively far. Therefore, it can be roughly judged that the adsorption is weaker than M1. As for the Ni-Pt-Ni(111) surface, its adsorption capacity seems to be weaker. In the M7 structure, the S-O bond occupies the bridge site, and the adsorption distance is 3.173 Å. Similarly, the M8 structure also has a long adsorption distance, indicating a weak interaction between SOF2 and Ni-Pt-Ni(111) surface.
Table 2 shows some adsorption parameters of the structures in Figure 5. The Eads of M5 and M6 are −2.123 eV and −0.745 eV respectively, which are larger than M7 (−0.344 eV) and M8 (−0.361 eV), indicating that the ability of Pt-Ni(111) to adsorb SOF2 is stronger than the Ni-Pt-Ni(111) surface. In addition, the Eads of M5 is larger than 3thf (−1.550 eV), but the adsorption distance of 3thf is shorter than M5. This is because the 3thf structure is a dissociative adsorption structure, which needs more energy to cross the energy barrier during the reaction process. As for charge transfer, M5 and M6 have a positive QT, which means electrons transfer from the SOF2 molecule to the Pt-Ni(111) surface during the adsorption process. But the circumstances on the Ni-Pt-Ni(111) surface is the opposite.
The most stable adsorption structures on the Pt-Ni(111) and Ni-Pt-Ni(111) surfaces were selected to analyze the density of states. Figure 6 shows the TDOS and PDOS of M5 and M8. In Figure 6a, it is obvious that there was an increase from −5.5 eV to −1 eV after SOF2 adsorption. According to the PDOS in Figure 6b, we found that the increase mainly consisted of F 2p, Ni 3d, and Pt 5d orbitals. The large overlapping area between Ni 3d and Pt 5d means there is a strong interaction between them. In Figure 6c, the TDOS rose in the range of −10 eV to −7 eV and −6 eV to −4 eV after SOF2 adsorption. The increased area of TDOS mainly consists of 2p orbital of F atoms and a 5d orbital of Pt atoms. Moreover, the hybridization between F 2p and Pt 5d signifies a strong interaction between the F atoms and Pt atoms.
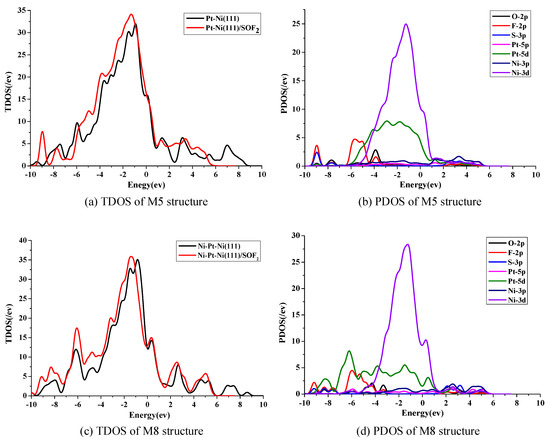
Figure 6.
TDOS and PDOS distribution of M5 and M8: (a) TDOS of M5 structure, (b) PDOS of M5 structure, (c) TDOS of M8 structure, (d) PDOS of M8 structure.
In view of the similarity between M7 and M8, only the M8 structure was selected for further analysis. Figure 7 shows the electron density difference of SOF2 adsorption on the Pt-Ni(111) and Ni-Pt-Ni(111) surface. It is shown that S atoms lose certain electrons after adsorption in M5 structure. According to the QT shown in Table 2, we can judge that the lost charge transfers to the Pt-Ni(111) surface. In Figure 7b, the electron density around the Pt atom decreases slightly, and the charge transfers to the O atom during the adsorption process. In Figure 7c, it is apparent that a small amount of electrons transfer from the Pt atoms to the S atom and F atoms, signifying a weak chemical interaction between them.
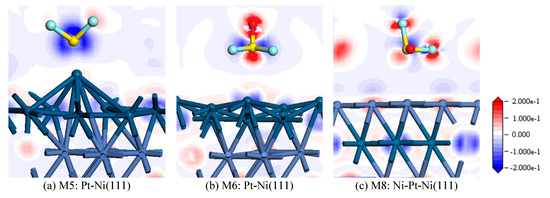
Figure 7.
Electron density difference of SOF2 adsorption on (a) M5:Pt-Ni(111), (b) M6:Pt-Ni(111), and (c) M8:Ni-Pt-Ni(111) surfaces.
As a result, we conclude that the adsorption structure on Pd-Ni(111) surface is more stable than that on Ni-Pt-Ni(111) surface, so Pt-Ni(111) surface shows a stronger ability on adsorbing SOF2 molecule than Ni-Pd-Ni(111) surface.
4. Conclusions
In this paper, DFT calculations are used to investigate the adsorption ability of Pd/Pt-Ni(111) bimetallic surfaces for SOF2 adsorption. In order to analyze the adsorption mechanism, the adsorption energy, charge transfer, density of electronic states, and electron density difference were calculated. The results show that the adsorption ability of SOF2 are in the sequence of Pt-Ni(111) > Pd-Ni(111) > Ni(111) > Ni-Pd-Ni(111) > Ni-Pt-Ni(111). It is verified that Pd and Pt composite layers on Ni(111) can significantly enhance the adsorption capacity. The adsorptions of SOF2 on Pt-Ni(111) and Pd-Ni(111) surfaces are strong chemisorption, which are irreversible adsorption processes. Although adsorption structures on Pt-Ni(111) surface are similar to those on Pd-Ni(111) surface, Pt-Ni(111) shows the most stable structure for its largest adsorption energy (−2.123 eV). The adsorption process on Ni-Pd-Ni(111) surface and Ni-Pt-Ni(111) surface belong to physical adsorption for its small adsorption energy, which is easy to desorb under external energy excitation. In summary, our research provides important theoretical support for developing an ideal SOF2 adsorbent.
Author Contributions
S.G. and X.B. proposed the project and analyzed the simulation results. Y.G. contributed to the DFT simulations. D.C. performed the analysis of the data and provided some revision of the manuscript. All authors read and approved the final manuscript.
Funding
This research was funded by Natural Science Foundation of Inner Mongolia Autonomous Region (Grant recipient: Shiming Gan).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tang, J.; Zeng, F.; Pan, J.; Zhang, X.; Yao, Q.; He, J.; Hou, X. Correlation analysis between formation process of SF6 decomposed components and partial discharge qualities. IEEE Trans. Dielectr. Electr. Insul. 2013, 20, 864–875. [Google Scholar] [CrossRef]
- Sabot, A.; Petit, A.; Taillebois, P.J. GIS insulation co-ordination: On-site tests and dielectric diagnostic techniques. A utility point of view. IEEE Trans. Power Deliv. 1996, 11, 1309–1316. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, W.; Tang, J.; Xiao, P. Study on PD detection in SF6 using multi-wall carbon nanotube films sensor. IEEE Trans. Dielectr. Electr. Insul. 2010, 17, 833–838. [Google Scholar] [CrossRef]
- Zhang, X.; Gui, Y.; Dai, Z. A simulation of Pd-doped SWCNTs used to detect SF6 decomposition components under partial discharge. Appl. Surf. Sci. 2014, 315, 196–202. [Google Scholar] [CrossRef]
- Qiu, Y.; Kuffel, E. Comparison of SF6/N2 and SF6/CO2 gas mixtures as alternatives to SF6 gas. IEEE Trans. Dielectr. Electr. Insul. 1999, 6, 892–895. [Google Scholar] [CrossRef]
- Zeng, F.; Tang, J.; Xie, Y.; Zhou, Q.; Zhang, C. Experimence study of trace water and oxygen impact on SF6 decomposition characteristics under partial discharge. J. Electr. Eng. Technol. 2015, 10, 1787–1796. [Google Scholar] [CrossRef]
- Tang, J.; Liu, F.; Zhang, X.; Meng, Q.; Zhou, J. Partial discharge recognition through an analysis of SF6 decomposition products part 1: Decomposition characteristics of SF6 under four different partial discharges. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 29–36. [Google Scholar] [CrossRef]
- Ju, T.; Fan, L.; Meng, Q.; Zhang, X.; Tao, J. Partial discharge recognition through an analysis of SF6 decomposition products part 2: Feature extraction and decision tree-based pattern recognition. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 37–44. [Google Scholar]
- Pinto, F.E.; Silva, C.F.P.M.; Tose, L.V.; Figueiredo, M.A.G.; Souza, W.C.; Vaz, B.G.; Romão, W. Evaluation of adsorbent materials for the removal of nitrogen compounds in vacuum gas oil by positive and negative electrospray ionization fourier transform ion cyclotron resonance mass spectrometry. Energy Fuels 2017, 31, 3454–3464. [Google Scholar] [CrossRef]
- Li, L.; Sun, T.H.; Shu, C.H.; Zhang, H.B. Low temperature H2S removal with 3-D structural mesoporous molecular sieves supported ZnO from gas stream. J. Hazard. Mater. 2016, 311, 142–150. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Matis, K.A. Nanoadsorbents for pollutants removal: A review. J. Mol. Liq. 2015, 203, 159–168. [Google Scholar] [CrossRef]
- Liu, M.W. Flytzanistephanopoulos, Total oxidation of carbon monoxide and methane over transition metal fluorite oxide composite catalysts: I. Catalyst composition and activity. J. Catal. 1995, 153, 304–316. [Google Scholar] [CrossRef]
- Zassinovich, G.; Mestroni, G.; Gladiali, S. Asymmetric hydrogen transfer reactions promoted by homogeneous transition metal catalysts. Chem. Rev. 1992, 23, 1051–1069. [Google Scholar] [CrossRef]
- Ren, J.; Guo, H.; Yang, J.; Qin, Z.; Lin, J.; Zhong, L. Insights into the mechanisms of CO2 methanation on Ni(111) surfaces by density functional theory. Appl. Surf. Sci. 2015, 351, 504–516. [Google Scholar] [CrossRef]
- Ma, D.; Ju, W.; Li, T.; Zhang, X.; He, C.; Ma, B.; Lu, Z.; Yang, Z. The adsorption of CO and NO on the MoS2 monolayer doped with Au, Pt, Pd, or Ni: A first-principles study. Appl. Surf. Sci. 2016, 383, 98–105. [Google Scholar] [CrossRef]
- Li, W.; Lu, X.M.; Li, G.Q.; Ma, J.J.; Zeng, P.Y.; Chen, J.F.; Pan, Z.L.; He, Q.Y. First-principle study of SO2 molecule adsorption on Ni-doped vacancy-defected single-walled (8,0) carbon nanotubes. Appl. Surf. Sci. 2016, 364, 560–566. [Google Scholar] [CrossRef]
- Yagi, Y.; Briere, T.M.; Sluiter, M.H.F.; Kumar, V.; Farajian, A.A.; Kawazoe, Y. Stable geometries and magnetic properties of single-walled carbon nanotubes doped with 3d transition metals: A first-principles study. Phys. Rev. B 2004, 69, 428–433. [Google Scholar] [CrossRef]
- Chu, F.Y. SF6 decomposition in gas-insulated equipment. IEEE Trans. Electr. Insul. 1986, 21, 693–725. [Google Scholar] [CrossRef]
- Zhong, J.Q.; Zhou, X.; Yuan, K.; Wright, C.A.; Tadich, A.; Qi, D.; Li, H.X.; Wu, K.; Xu, G.Q.; Chen, W. Probing the effect of the Pt-Ni-Pt(111) bimetallic Surface electronic structures on the ammonia decomposition reaction. Nanoscale 2016, 9, 666–672. [Google Scholar] [CrossRef]
- Yi, L.; Pan, H.; Tao, D.; Wu, J.; Mei, Q.; Xin, H.; Ding, K.; Chen, W.; Su, W.; Zhang, Y. Adsorption and dissociation of H2S on monometallic and monolayer bimetallic Ni/Pd(111) surfaces: A first-principles study. Appl. Surf. Sci. 2016, 387, 301–307. [Google Scholar]
- Li, J.; Gui, Y.; Ji, C.; Tang, C.; Zhou, Q.; Wang, Y.; Zhang, X. Theoretical study of the adsorption of SF6 decomposition components on Ni(111) surface. Comput. Mater. Sci. 2018, 152, 248–255. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, X.; Gui, Y. Theoretical and experimental study on competitive adsorption of SF 6 decomposed components on Au-modified anatase (101) surface. Appl. Surf. Sci. 2016, 387, 437–445. [Google Scholar] [CrossRef]
- Christophorou, L.G.; Olthoff, J.K. Electron interactions with SF6. J. Phys. Chem. Ref. Data 2000, 29, 267–330. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1998, 77, 3865–3868. [Google Scholar] [CrossRef]
- Denning, A.S.; Holzer, M.; Gurney, K.R.; Heimann, M.; Law, R.M.; Rayner, P.J.; Fung, I.Y.; Fan, S.M.; Taguchi, S.; Friedlingstein, P. Three-dimensional transport and concentration of SF6. Tellus Ser. B Chem. Phys. Meteorol. 1999, 51, 266–297. [Google Scholar] [CrossRef]
- Steill, J.D.; Oomens, J.; Eyler, J.R.; Compton, R.N. Gas-phase infrared multiple photon dissociation spectroscopy of isolated SF6-and SF5-anions. J. Chem. Phys. 2008, 129, 244302. [Google Scholar] [CrossRef]
- Dunne, J.A.; Mariwala, R.; Rao, M.; Sircar, S.; Gorte, A.R.J.; Myers, A.L. Calorimetric heats of adsorption and adsorption isotherms. 1. O2, N2, Ar, CO2, CH4, C2H6, and SF6 on Silicalite. Langmuir 1996, 12, 5888–5895. [Google Scholar] [CrossRef]
- Huey, L.G.; Hanson, D.R.; Howard, C.J. Reactions of SF6-and I-with atmospheric trace gases. J. Phys. Chem. 1995, 99, 5001–5008. [Google Scholar] [CrossRef]
- Kline, L.E.; Davies, D.K.; Chen, C.L.; Chantry, P.J. Dielectric properties for SF6 and SF6 mixtures predicted from basic data. J. Appl. Phys. 1979, 50, 6789–6796. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).