Photocatalytic Hydrogen Production from Glycerol Aqueous Solution Using Cu-Doped ZnO under Visible Light Irradiation
Abstract
:1. Introduction
2. Experimental
2.1. Photocatalyst Preparation
- nCu is the number of moles of Cu(CH3COO)2 used in the synthesis; and
- nZn is the number of moles of Zn(CH3COO)2 2H2O used in the synthesis.
2.2. Photocatalytic Activity Tests
3. Results and Discussion
3.1. Photocatalytic Activity Tests
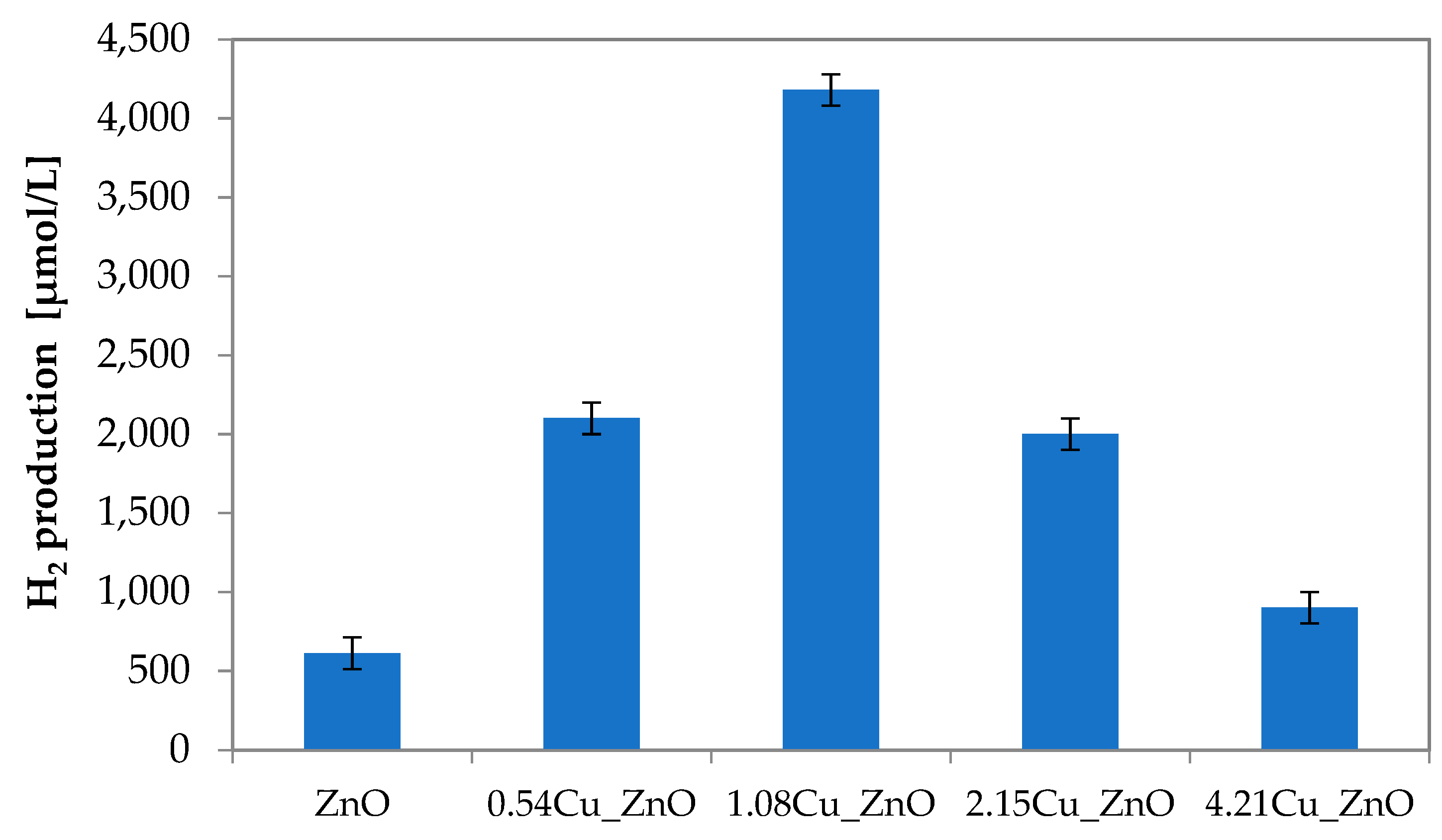
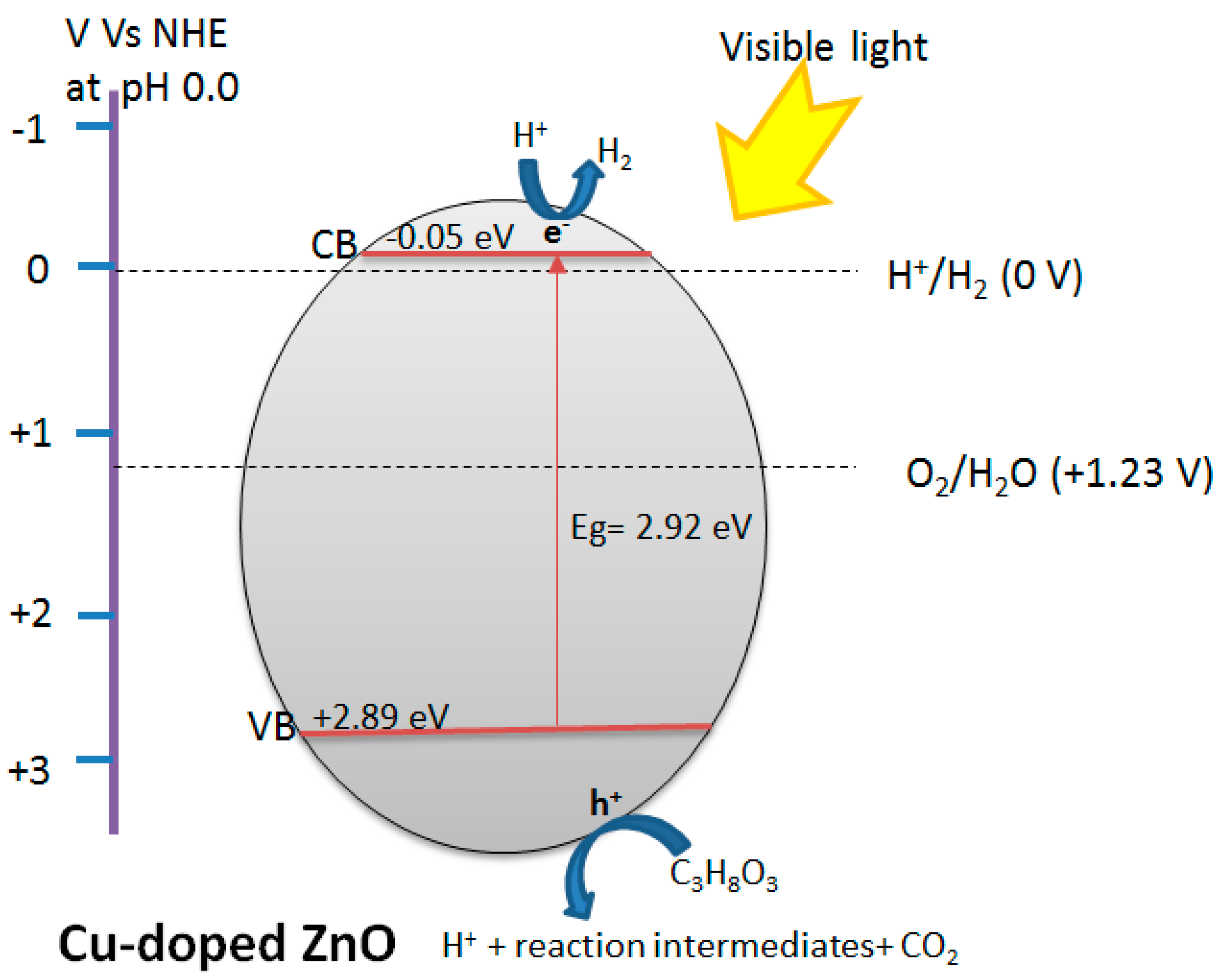
3.1.1. Influence of Cu Content on the Hydrogen Production under Visible Light
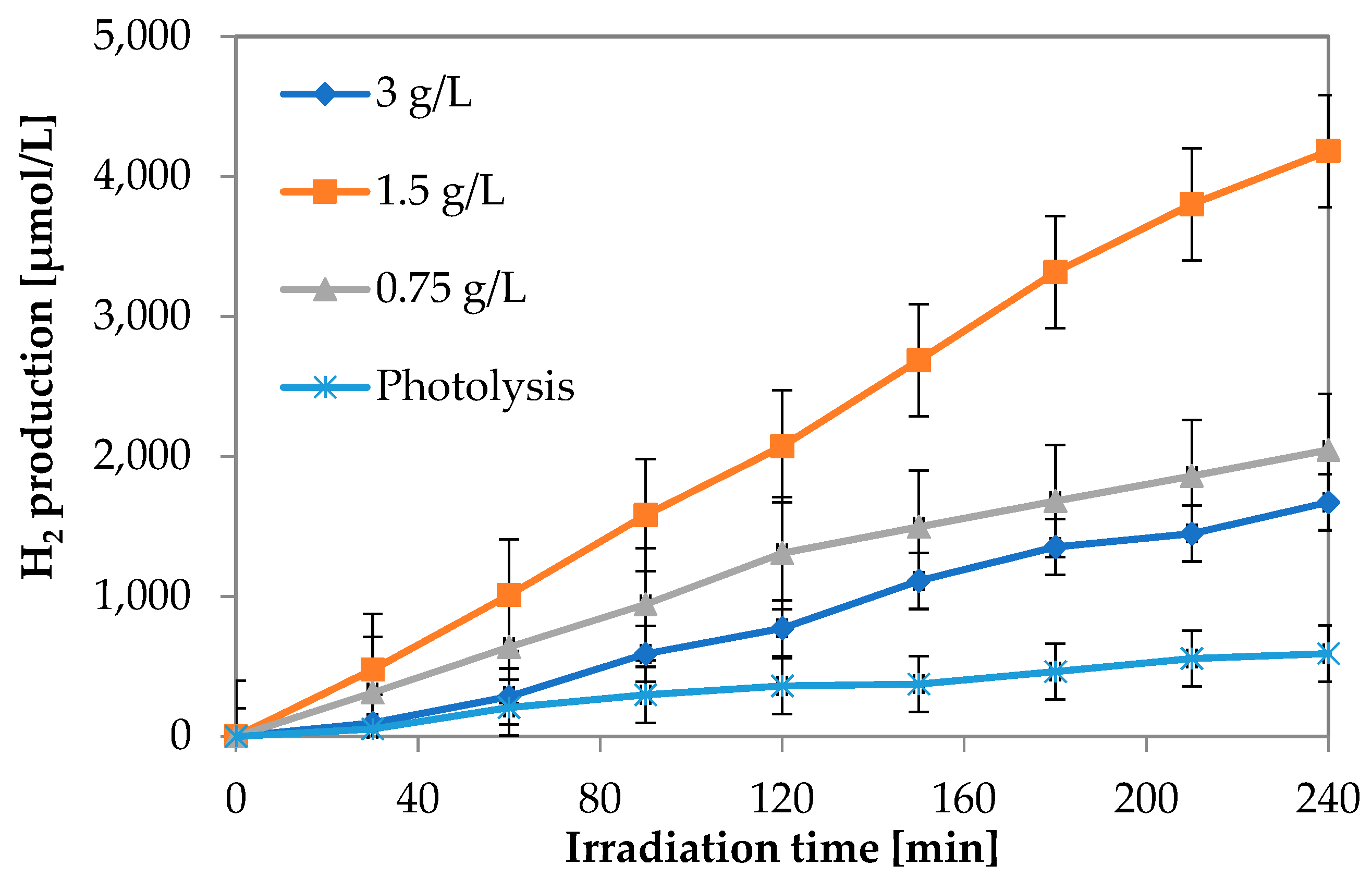
3.1.2. Influence of 1.08Cu_ZnO Catalyst Dosage in Photocatalytic Hydrogen Production
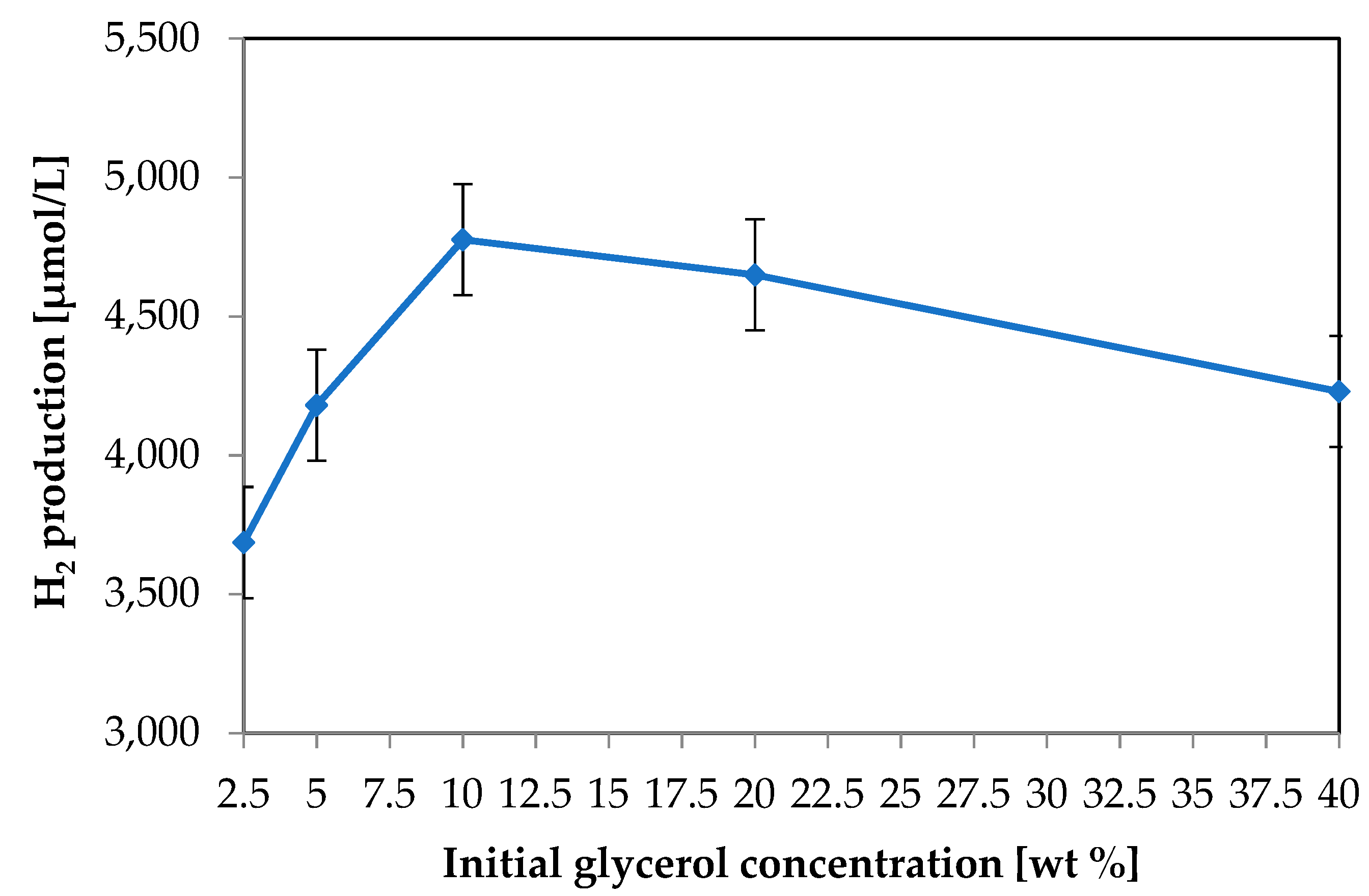
3.1.3. Effect of the Glycerol Initial Concentration on Photocatalytic Hydrogen Production
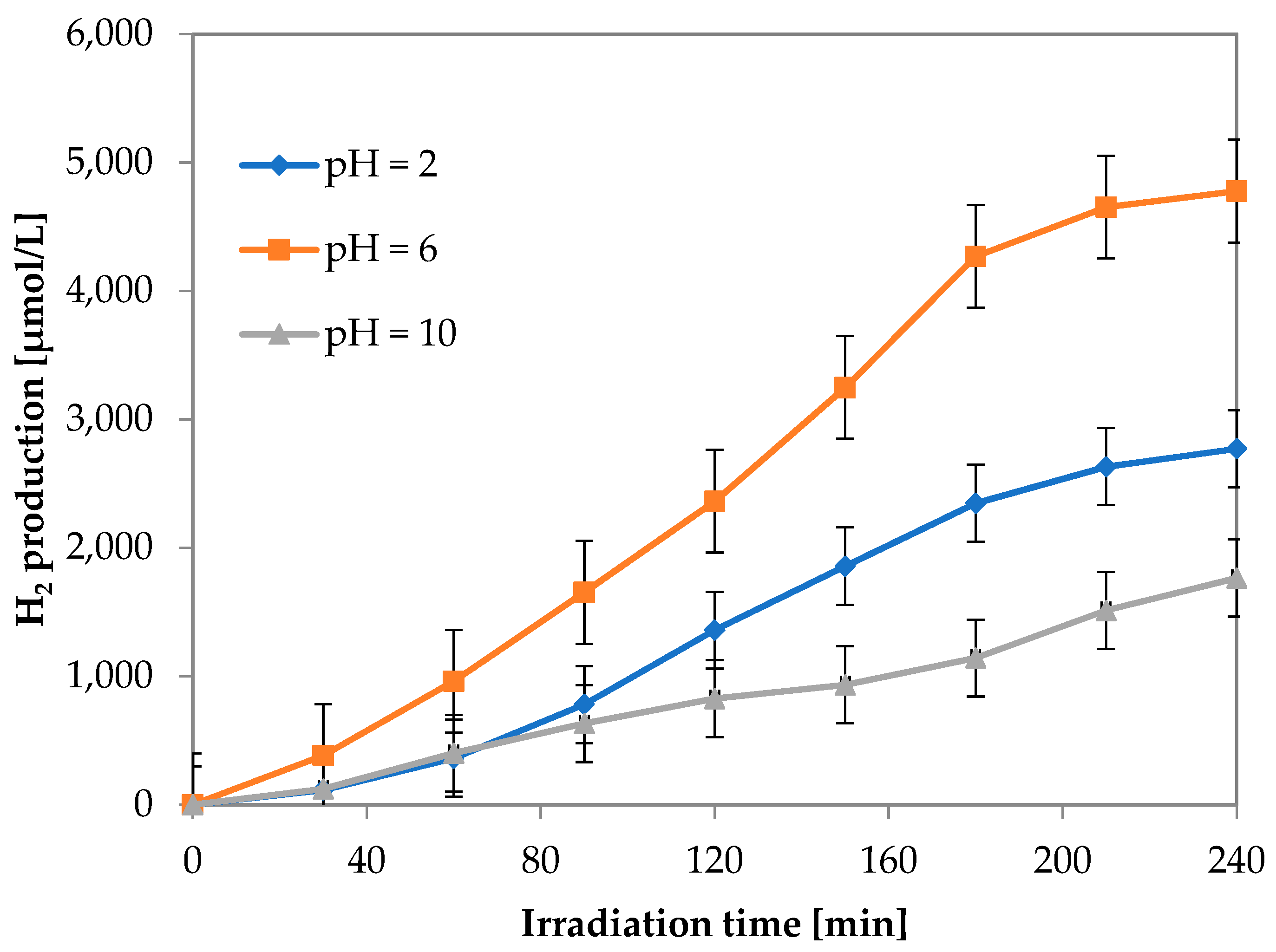
3.1.4. Effect of pH on Photocatalytic Hydrogen Production
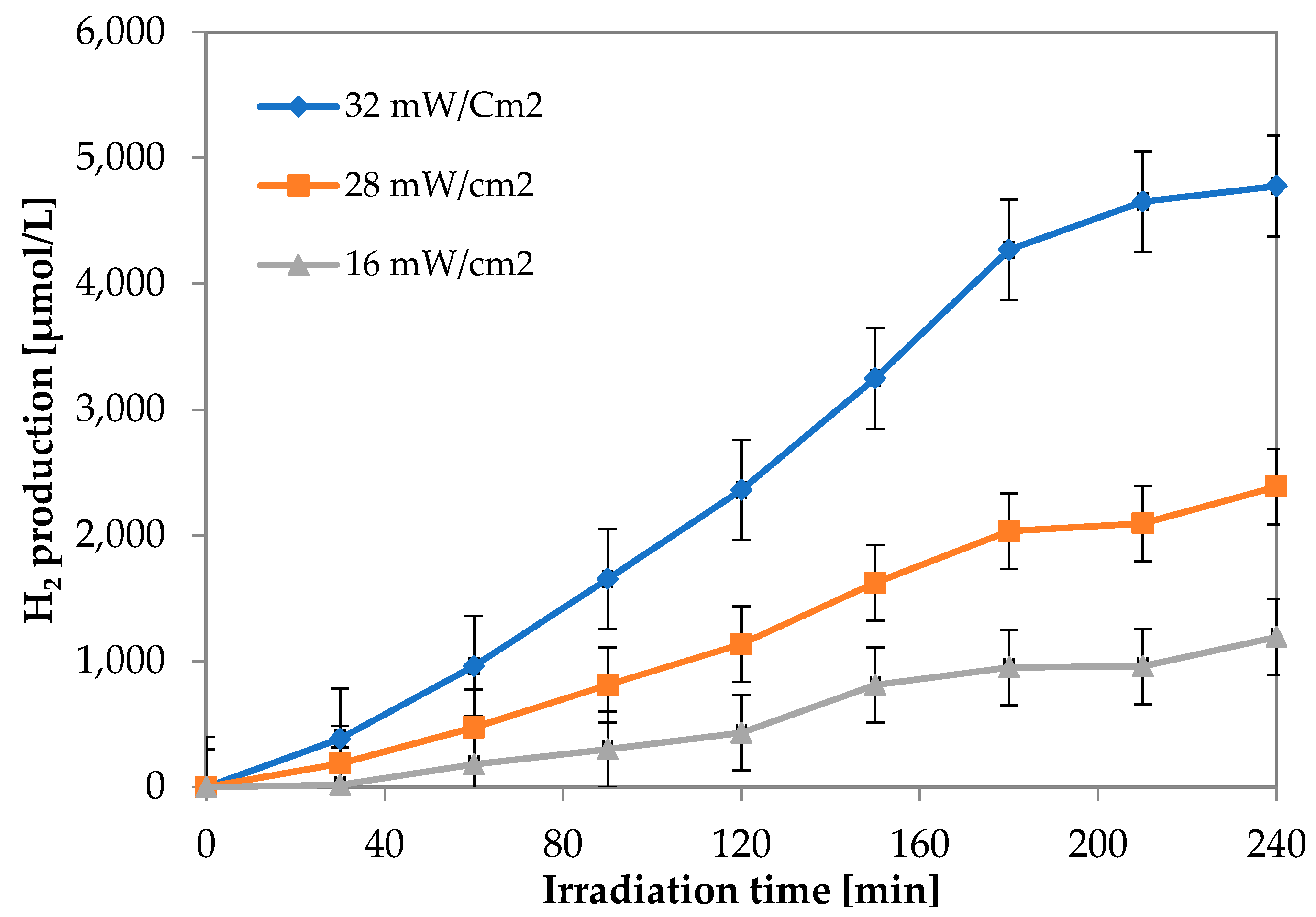
3.1.5. Influence of Visible Light Intensity on Photocatalytic Hydrogen Production
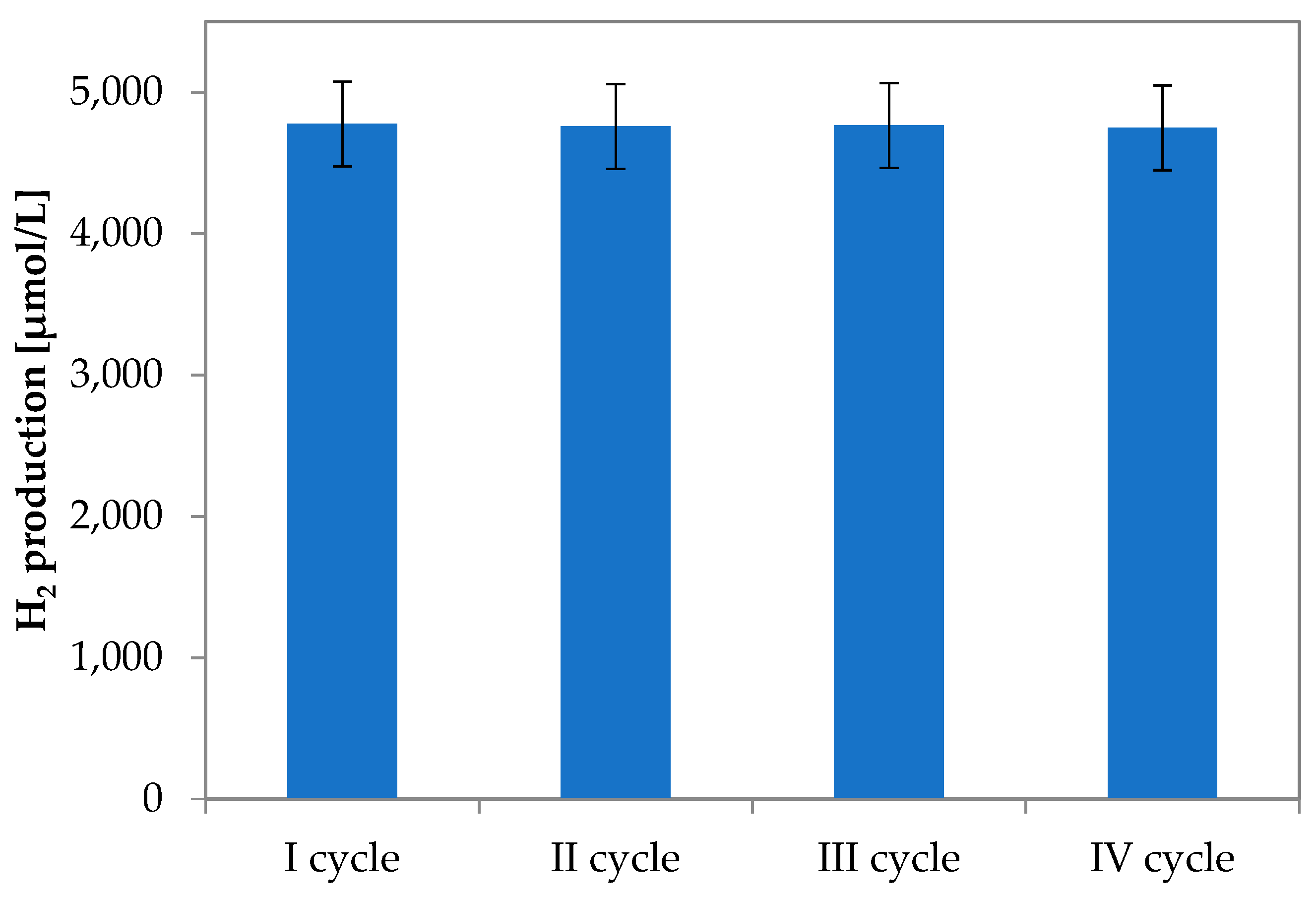
3.1.6. Recyclability Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chang, C.-J.; Lin, Y.-G.; Weng, H.-T.; Wei, Y.-H. Photocatalytic hydrogen production from glycerol solution at room temperature by zno-zns/graphene photocatalysts. Appl. Surf. Sci. 2018, 451, 198–206. [Google Scholar] [CrossRef]
- Halabi, M.; De Croon, M.; Van der Schaaf, J.; Cobden, P.; Schouten, J. Low temperature catalytic methane steam reforming over ceria–zirconia supported rhodium. Appl. Catal. A Gen. 2010, 389, 68–79. [Google Scholar] [CrossRef]
- Li, Q.; Guo, B.; Yu, J.; Ran, J.; Zhang, B.; Yan, H.; Gong, J.R. Highly efficient visible-light-driven photocatalytic hydrogen production of cds-cluster-decorated graphene nanosheets. J. Am. Chem. Soc. 2011, 133, 10878–10884. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, Y.; Park, H. Solar hydrogen production coupled with the degradation of a dye pollutant using TiO2 modified with platinum and nafion. Int. J. Photoenergy 2014, 2014, 324859. [Google Scholar] [CrossRef]
- Maeda, K.; Teramura, K.; Lu, D.; Takata, T.; Saito, N.; Inoue, Y.; Domen, K. Photocatalyst releasing hydrogen from water. Nature 2006, 440, 295. [Google Scholar] [CrossRef] [PubMed]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lou, Y.; Dayal, S.; Qiu, X.; Krolicki, R.; Burda, C.; Zhao, C.; Becker, J. Doped semiconductor nanomaterials. J. Nanosci. Nanotechnol. 2005, 5, 1408–1420. [Google Scholar] [CrossRef]
- Gallo, A.; Montini, T.; Marelli, M.; Minguzzi, A.; Gombac, V.; Psaro, R.; Fornasiero, P.; Dal Santo, V. H2 production by renewables photoreforming on Pt–Au/TiO2 catalysts activated by reduction. ChemSusChem 2012, 5, 1800–1811. [Google Scholar] [CrossRef]
- Selli, E.; Chiarello, G.L.; Quartarone, E.; Mustarelli, P.; Rossetti, I.; Forni, L. A photocatalytic water splitting device for separate hydrogen and oxygen evolution. Chem. Commun. 2007, 21, 5022–5024. [Google Scholar] [CrossRef]
- Iervolino, G.; Vaiano, V.; Sannino, D.; Rizzo, L.; Galluzzi, A.; Polichetti, M.; Pepe, G.; Campiglia, P. Hydrogen production from glucose degradation in water and wastewater treated by Ru-LaFeO3/Fe2O3 magnetic particles photocatalysis and heterogeneous photo-fenton. Int. J. Hydrogen Energy 2018, 43, 2184–2196. [Google Scholar] [CrossRef]
- Lucchetti, R.; Onotri, L.; Clarizia, L.; Di Natale, F.; Di Somma, I.; Andreozzi, R.; Marotta, R. Removal of nitrate and simultaneous hydrogen generation through photocatalytic reforming of glycerol over “in situ” prepared zero-valent nano copper/P25. Appl. Catal. B Environ. 2017, 202, 539–549. [Google Scholar] [CrossRef]
- Christoforidis, K.C.; Fornasiero, P. Photocatalytic hydrogen production: A rift into the future energy supply. ChemCatChem 2017, 9, 1523–1544. [Google Scholar] [CrossRef]
- Kawai, T.; Sakata, T. Photocatalytic hydrogen production from liquid methanol and water. J. Chem. Soc. Chem. Commun. 1980, 15, 694–695. [Google Scholar] [CrossRef]
- Sakata, T.; Kawai, T. Heterogeneous photocatalytic production of hydrogen and methane from ethanol and water. Chem. Phys. Lett. 1981, 80, 341–344. [Google Scholar] [CrossRef]
- Kondarides, D.I.; Daskalaki, V.M.; Patsoura, A.; Verykios, X.E. Hydrogen production by photo-induced reforming of biomass components and derivatives at ambient conditions. Catal. Lett. 2008, 122, 26–32. [Google Scholar] [CrossRef]
- Harada, H.; Sakata, T.; Ueda, T. Effect of semiconductor on photocatalytic decomposition of lactic acid. J. Am. Chem. Soc. 1985, 107, 1773–1774. [Google Scholar] [CrossRef]
- Li, M.; Li, Y.; Peng, S.; Lu, G.; Li, S. Photocatalytic hydrogen generation using glycerol wastewater over pt/tio 2. Front. Chem. China 2009, 4, 32–38. [Google Scholar] [CrossRef]
- Vaiano, V.; Lara, M.A.; Iervolino, G.; Matarangolo, M.; Navio, J.A.; Hidalgo, M.C. Photocatalytic H2 production from glycerol aqueous solutions over fluorinated Pt-TiO2 with high {001} facet exposure. J. Photochem. Photobiol. A Chem. 2018, 365, 52–59. [Google Scholar] [CrossRef]
- Pradima, J.; Kulkarni, M.R. Review on enzymatic synthesis of value added products of glycerol, a by-product derived from biodiesel production. Resour.-Eff. Technol. 2017, 3, 394–405. [Google Scholar] [CrossRef]
- Lalitha, K.; Sadanandam, G.; Kumari, V.D.; Subrahmanyam, M.; Sreedhar, B.; Hebalkar, N.Y. Highly stabilized and finely dispersed Cu2o/TiO2: A promising visible sensitive photocatalyst for continuous production of hydrogen from glycerol: Water mixtures. J. Phys. Chem. C 2010, 114, 22181–22189. [Google Scholar] [CrossRef]
- Montini, T.; Gombac, V.; Sordelli, L.; Delgado, J.J.; Chen, X.; Adami, G.; Fornasiero, P. Nanostructured Cu/TiO2 photocatalysts for H2 production from ethanol and glycerol aqueous solutions. ChemCatChem 2011, 3, 574–577. [Google Scholar] [CrossRef]
- Lv, R.; Wang, X.; Lv, W.; Xu, Y.; Ge, Y.; He, H.; Li, G.; Wu, X.; Li, X.; Li, Q. Facile synthesis of ZnO nanorods grown on graphene sheets and its enhanced photocatalytic efficiency. J. Chem. Technol. Biotechnol. 2015, 90, 550–558. [Google Scholar] [CrossRef]
- Jung, M.; Hart, J.N.; Boensch, D.; Scott, J.; Ng, Y.H.; Amal, R. Hydrogen evolution via glycerol photoreforming over Cu–Pt nanoalloys on TiO2. Appl. Catal. A Gen. 2016, 518, 221–230. [Google Scholar] [CrossRef]
- Patsoura, A.; Kondarides, D.I.; Verykios, X.E. Photocatalytic degradation of organic pollutants with simultaneous production of hydrogen. Catal. Today 2007, 124, 94–102. [Google Scholar] [CrossRef]
- Wang, C.; Cai, X.; Chen, Y.; Cheng, Z.; Luo, X.; Mo, S.; Jia, L.; Shu, R.; Lin, P.; Yang, Z. Efficient hydrogen production from glycerol photoreforming over Ag2OTiO2 synthesized by a sol–gel method. Int. J. Hydrogen Energy 2017, 42, 17063–17074. [Google Scholar] [CrossRef]
- Tanaka, A.; Hashimoto, K.; Kominami, H. Visible-light-induced hydrogen and oxygen formation over Pt/Au/Wo3 photocatalyst utilizing two types of photoabsorption due to surface plasmon resonance and band-gap excitation. J. Am. Chem. Soc. 2014, 136, 586–589. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Huang, B.; Qin, X.; Zhang, X.; Dai, Y.; Wei, J.; Whangbo, M.H. Ag@AgCl: A highly efficient and stable photocatalyst active under visible light. Angew. Chem. Int. Ed. 2008, 47, 7931–7933. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Yang, J.; Ma, G.; Wu, G.; Zong, X.; Lei, Z.; Shi, J.; Li, C. Visible-light-driven hydrogen production with extremely high quantum efficiency on Pt–Pds/CdS photocatalyst. J. Catal. 2009, 266, 165–168. [Google Scholar] [CrossRef]
- Anker, J.N.; Hall, W.P.; Lyandres, O.; Shah, N.C.; Zhao, J.; Van Duyne, R.P. Biosensing with plasmonic nanosensors. In Nanoscience and Technology: A Collection of Reviews from Nature Journals; World Scientific: Singapore, 2010; pp. 308–319. [Google Scholar]
- Slamet, S.; Kusrini, E.; Afrozi, A.; Ibadurrohman, M. Photocatalytic hydrogen production from glycerol-water over metal loaded and non-metal doped titanium oxide. Int. J. Technol. 2015, 6, 520–532. [Google Scholar] [CrossRef]
- Kanade, K.; Kale, B.; Baeg, J.-O.; Lee, S.M.; Lee, C.W.; Moon, S.-J.; Chang, H. Self-assembled aligned Cu doped ZnO nanoparticles for photocatalytic hydrogen production under visible light irradiation. Mater. Chem. Phys. 2007, 102, 98–104. [Google Scholar] [CrossRef]
- Tristantini, D.; Ibadurrohman, M. Photocatalytic hydrogen production from glycerol-water mixture over Pt-N-TiO2 nanotube photocatalyst. Int. J. Energy Res. 2013, 37, 1372–1381. [Google Scholar]
- Kum, J.M.; Park, Y.J.; Kim, H.J.; Cho, S.O. Plasmon-enhanced photocatalytic hydrogen production over visible-light responsive Cu/TiO2. Nanotechnology 2015, 26, 125402. [Google Scholar] [CrossRef] [PubMed]
- Clarizia, L.; Spasiano, D.; Di Somma, I.; Marotta, R.; Andreozzi, R.; Dionysiou, D.D. Copper modified-TiO2 catalysts for hydrogen generation through photoreforming of organics. A short review. Int. J. Hydrogen Energy 2014, 39, 16812–16831. [Google Scholar] [CrossRef]
- Yu, Z.; Meng, J.; Li, Y.; Li, Y. Efficient photocatalytic hydrogen production from water over a CuO and carbon fiber comodified TiO2 nanocomposite photocatalyst. Int. J. Hydrogen Energy 2013, 38, 16649–16655. [Google Scholar] [CrossRef]
- Goyal, P.; Chakraborty, S.; Misra, S.K. Multifunctional Fe3O4-ZnO nanocomposites for environmental remediation applications. Environ. Nanotechnol. Monit. Manag. 2018, 10, 28–35. [Google Scholar] [CrossRef]
- Vaiano, V.; Iervolino, G.; Rizzo, L. Cu-doped ZnO as efficient photocatalyst for the oxidation of arsenite to arsenate under visible light. Appl. Catal. B Environ. 2018, 238, 471–479. [Google Scholar] [CrossRef]
- Vaiano, V.; Iervolino, G. Facile method to immobilize ZnO particles on glass spheres for the photocatalytic treatment of tannery wastewater. J. Colloid Interface sci. 2018, 518, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef]
- Mohan, R.; Krishnamoorthy, K.; Kim, S.-J. Enhanced photocatalytic activity of Cu-doped ZnO nanorods. Solid State Commun. 2012, 152, 375–380. [Google Scholar] [CrossRef]
- Shirzad-Siboni, M.; Jonidi-Jafari, A.; Farzadkia, M.; Esrafili, A.; Gholami, M. Enhancement of photocatalytic activity of Cu-doped ZnO nanorods for the degradation of an insecticide: Kinetics and reaction pathways. J. Environ. Manag. 2017, 186, 1–11. [Google Scholar] [CrossRef]
- Ma, H.; Yue, L.; Yu, C.; Dong, X.; Zhang, X.; Xue, M.; Zhang, X.; Fu, Y. Synthesis, characterization and photocatalytic activity of Cu-doped Zn/ZnO photocatalyst with carbon modification. J. Mater. Chem. 2012, 22, 23780–23788. [Google Scholar] [CrossRef]
- Mittal, M.; Sharma, M.; Pandey, O. UV–visible light induced photocatalytic studies of Cu doped ZnO nanoparticles prepared by co-precipitation method. Sol. Energy 2014, 110, 386–397. [Google Scholar] [CrossRef]
- Wang, M.; Ren, F.; Cai, G.; Liu, Y.; Shen, S.; Guo, L. Activating ZnO nanorod photoanodes in visible light by cu ion implantation. Nano Res. 2014, 7, 353–364. [Google Scholar] [CrossRef]
- Sharma, P.K.; Kumar, M.; Pandey, A.C. Green luminescent zno: Cu2+ nanoparticles for their applications in white-light generation from uv leds. J. Nanopart. Res. 2011, 13, 1629–1637. [Google Scholar] [CrossRef]
- Martha, S.; Reddy, K.H.; Parida, K. Fabrication of In2O3 modified ZnO for enhancing stability, optical behaviour, electronic properties and photocatalytic activity for hydrogen production under visible light. J. Mater. Chem. A 2014, 2, 3621–3631. [Google Scholar] [CrossRef]
- Kadam, A.; Kim, T.G.; Shin, D.S.; Garadkar, K.; Park, J. Morphological evolution of Cu doped ZnO for enhancement of photocatalytic activity. J. Alloys Compd. 2017, 710, 102–113. [Google Scholar] [CrossRef]
- Ola, O.; Maroto-Valer, M.M. Review of material design and reactor engineering on TiO2 photocatalysis for CO2 reduction. J. Photochem. Photobiol. C Photochem. Rev. 2015, 24, 16–42. [Google Scholar] [CrossRef]
- Chen, P. A promising strategy to fabricate the Cu/BiVO4 photocatalysts and their enhanced visible-light-driven photocatalytic activities. J. Mater. Sci. Mater. Electron. 2016, 27, 2394–2403. [Google Scholar] [CrossRef]
- Yerga, R.M.N.; Alvarez-Galvan, M.C.; Vaquero, F.; Arenales, J.; Fierro, J.L.G. Hydrogen production from water splitting using photo-semiconductor catalysts. In Renewable Hydrogen Technologies; Elsevier: Amsterdam, The Netherlands, 2013; pp. 43–61. [Google Scholar]
- Nenavathu, B.P.; Kandula, S.; Verma, S. Visible-light-driven photocatalytic degradation of safranin-t dye using functionalized graphene oxide nanosheet (fgs)/Zno nanocomposites. RSC Adv. 2018, 8, 19659–19667. [Google Scholar] [CrossRef]
- Navarro Yerga, R.M.; Alvarez Galvan, M.C.; Del Valle, F.; Villoria de la Mano, J.A.; Fierro, J.L. Water splitting on semiconductor catalysts under visible-light irradiation. ChemSusChem Chem. Sustain. Energy Mater. 2009, 2, 471–485. [Google Scholar] [CrossRef]
- Vaiano, V.; Iervolino, G.; Sannino, D.; Rizzo, L.; Sarno, G.; Farina, A. Enhanced photocatalytic oxidation of arsenite to arsenate in water solutions by a new catalyst based on moox supported on TiO2. Appl. Catal. B Environ. 2014, 160, 247–253. [Google Scholar] [CrossRef]
- Li, Y.; Lu, G.; Li, S. Photocatalytic hydrogen generation and decomposition of oxalic acid over platinized TiO2. Appl. Catal. A Gen. 2001, 214, 179–185. [Google Scholar] [CrossRef]
- Wei, L.F.; Zheng, X.J.; Zhang, Z.H.; Wei, Y.J.; Xie, B.; Wei, M.B.; Sun, X.L. A systematic study of photocatalytic H2 production from propionic acid solution over Pt/TiO2 photocatalyst. Int. J. Energy Res. 2012, 36, 75–86. [Google Scholar] [CrossRef]
- Strataki, N.; Bekiari, V.; Kondarides, D.I.; Lianos, P. Hydrogen production by photocatalytic alcohol reforming employing highly efficient nanocrystalline titania films. Appl. Catal. B Environ. 2007, 77, 184–189. [Google Scholar] [CrossRef]
- Daskalaki, V.M.; Kondarides, D.I. Efficient production of hydrogen by photo-induced reforming of glycerol at ambient conditions. Catal. Today 2009, 144, 75–80. [Google Scholar] [CrossRef]
- Tambago, H.M.G.; de Leon, R.L. Intrinsic kinetic modeling of hydrogen production by photocatalytic water splitting using cadmium zinc sulfide catalyst. Int. J. Chem. Eng. Appl. 2015, 6, 220–227. [Google Scholar] [CrossRef]
- Vaiano, V.; Iervolino, G.; Sannino, D.; Rizzo, L.; Sarno, G. MoOx/TiO2 immobilized on quartz support as structured catalyst for the photocatalytic oxidation of as (iii) to as (v) in aqueous soluTiOns. Chem. Eng. Res. Des. 2016, 109, 190–199. [Google Scholar] [CrossRef]
- Sannino, D.; Vaiano, V.; Sacco, O.; Ciambelli, P. Mathematical modelling of photocatalytic degradation of methylene blue under visible light irradiation. J. Environ. Chem. Eng. 2013, 1, 56–60. [Google Scholar] [CrossRef]
- Sorathiya, K.; Mishra, B.; Kalarikkal, A.; Reddy, K.P.; Gopinath, C.S.; Khushalani, D. Enhancement in rate of photocatalysis upon catalyst recycling. Sci. Rep. 2016, 6, 35075. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, N.; Xu, Y.-J. Synthesis of graphene–zno nanorod nanocomposites with improved photoactivity and anti-photocorrosion. CrystEngComm 2013, 15, 3022–3030. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Z.; Liu, S.; Xu, Y.-J. Size effect induced activity enhancement and anti-photocorrosion of reduced graphene oxide/zno composites for degradation of organic dyes and reduction of Cr(VI) in water. Appl. Catal. B Environ. 2013, 140–141, 598–607. [Google Scholar] [CrossRef]
- Ishioka, J.; Kogure, K.; Ofuji, K.; Kawaguchi, K.; Jeem, M.; Kato, T.; Shibayama, T.; Watanabe, S. In situ direct observation of photocorrosion in zno crystals in ionic liquid using a laser-equipped high-voltage electron microscope. AIP Adv. 2017, 7, 035220. [Google Scholar] [CrossRef]
| Photocatalyst | Cu Nominal Amount (mol %) | Band Gap Energy (eV) |
|---|---|---|
| ZnO | data | 3.19 |
| 0.54Cu_ZnO | 0.54 | 3.02 |
| 1.08Cu_ZnO | 1.08 | 2.92 |
| 2.15Cu_ZnO | 2.15 | 2.94 |
| 4.21Cu_ZnO | 4.21 | 2.91 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaiano, V.; Iervolino, G. Photocatalytic Hydrogen Production from Glycerol Aqueous Solution Using Cu-Doped ZnO under Visible Light Irradiation. Appl. Sci. 2019, 9, 2741. https://doi.org/10.3390/app9132741
Vaiano V, Iervolino G. Photocatalytic Hydrogen Production from Glycerol Aqueous Solution Using Cu-Doped ZnO under Visible Light Irradiation. Applied Sciences. 2019; 9(13):2741. https://doi.org/10.3390/app9132741
Chicago/Turabian StyleVaiano, Vincenzo, and Giuseppina Iervolino. 2019. "Photocatalytic Hydrogen Production from Glycerol Aqueous Solution Using Cu-Doped ZnO under Visible Light Irradiation" Applied Sciences 9, no. 13: 2741. https://doi.org/10.3390/app9132741
APA StyleVaiano, V., & Iervolino, G. (2019). Photocatalytic Hydrogen Production from Glycerol Aqueous Solution Using Cu-Doped ZnO under Visible Light Irradiation. Applied Sciences, 9(13), 2741. https://doi.org/10.3390/app9132741






