Composite Xenohybrid Bovine Bone-Derived Scaffold as Bone Substitute for the Treatment of Tibial Plateau Fractures
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Data
2.2. Study Design, Patients’ Selection, and Endpoints
2.3. Surgical Technique and Rehabilitation
2.4. Statistical Analisis
2.5. Follow-Up
3. Results
3.1. Demographics and Type of Fracture
3.2. Clinical Assessment
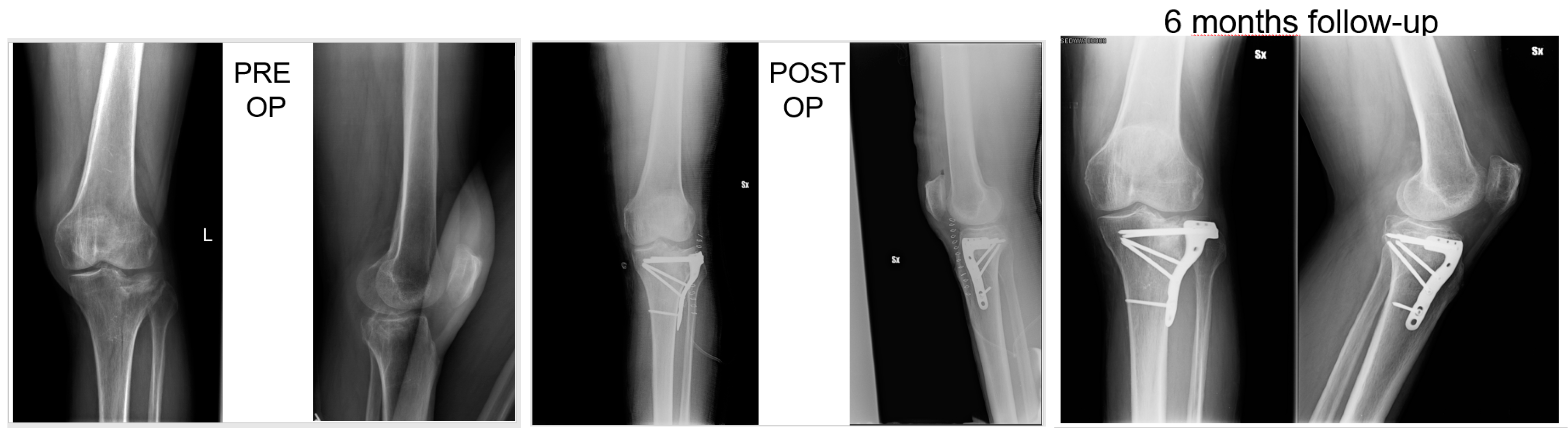
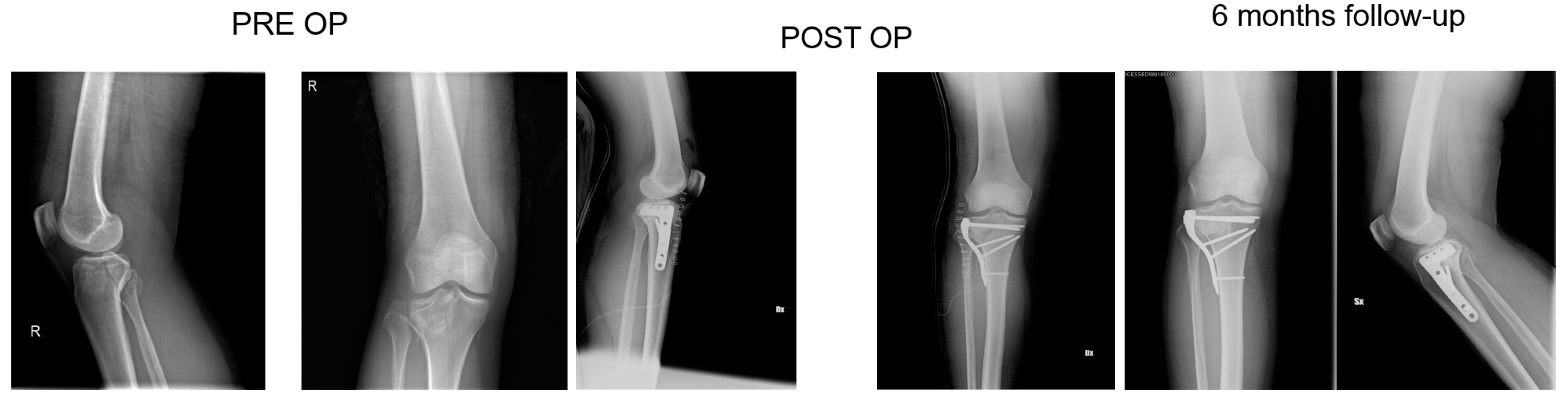
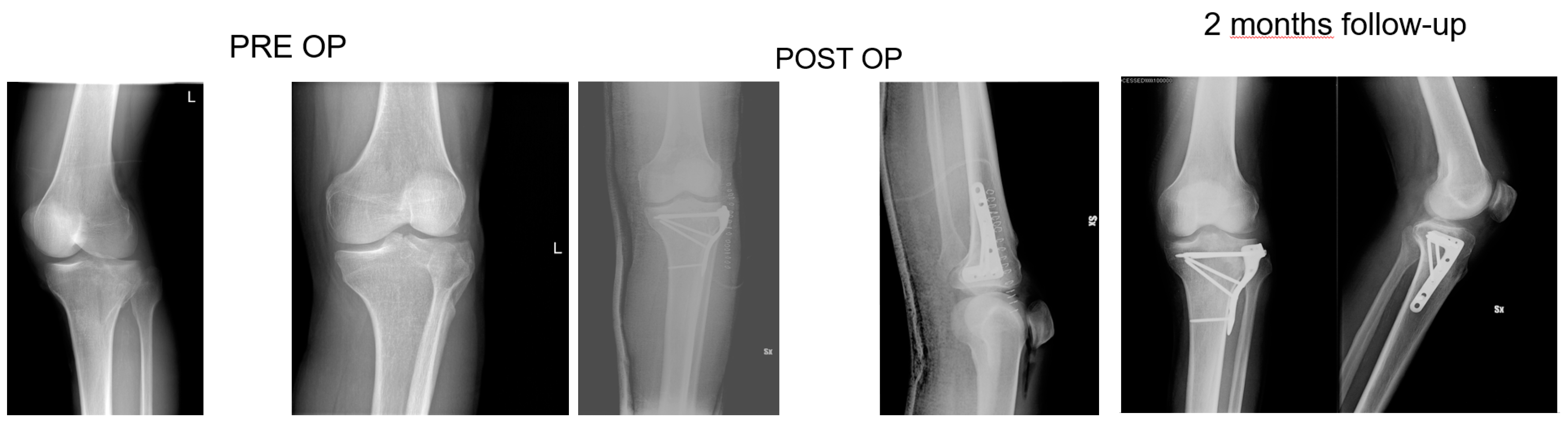
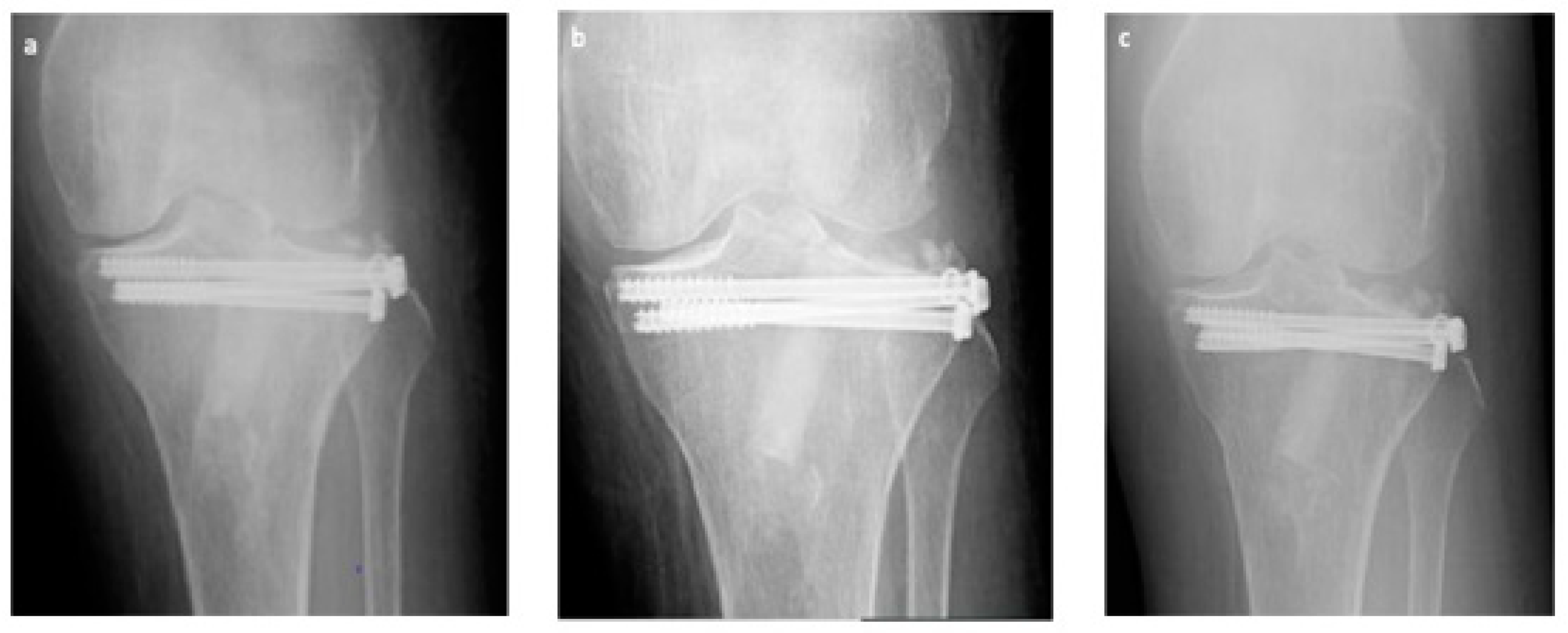
3.3. Radiological Assessment
3.4. Statistical Analysis
3.5. Long-Term Follow-Up
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Clinical Data
References
- Court-Brown, C.M.; Caesar, B. Epidemiology of Adult Fractures: A Review. Injury 2006, 37, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Koval, K.J.; Helfet, D.L. Tibial Plateau Fractures: Evaluation and Treatment. J. Am. Acad. Orthop. Surg. 1995, 3, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Schatzker, J.; McBroom, R.; Bruce, D. The Tibial Plateau Fracture. The Toronto Experience 1968–1975. Clin. Orthop. Relat. Res. 1979, 108, 94–104. [Google Scholar]
- Kandemir, U.; Maclean, J. Surgical Approaches for Tibial Plateau Fractures. J. Knee Surg. 2014, 27, 21–29. [Google Scholar] [PubMed]
- Athanasiou, V.T.; Papachristou, D.J.; Panagopoulos, A.; Saridis, A.; Scopa, C.D.; Megas, P. Histological Comparison of Autograft, Allograft-Dbm, Xenograft, and Synthetic Grafts in a Trabecular Bone Defect: An Experimental Study in Rabbits. Med. Sci. Monit. 2010, 16, BR24–BR31. [Google Scholar] [PubMed]
- Ferracini, R.; Martinez Herreros, I.; Russo, A.; Casalini, T.; Rossi, F.; Perale, G. Scaffolds as Structural Tools for Bone-Targeted Drug Delivery. Pharmaceutics 2018, 10, 122. [Google Scholar] [CrossRef]
- Roato, I.; Belisario, D.C.; Compagno, M.; Verderio, L.; Sighinolfi, A.; Mussano, F.; Genova, T.; Veneziano, F.; Pertici, G.; Perale, G.; et al. Adipose-Derived Stromal Vascular Fraction/Xenohybrid Bone Scaffold: An Alternative Source for Bone Regeneration. Stem Cells Int. 2018, 2018, 4126379. [Google Scholar] [CrossRef]
- Almaiman, M.; Al-Bargi, H.H.; Manson, P. Complication of Anterior Iliac Bone Graft Harvesting in 372 Adult Patients from May 2006 to May 2011 and a Literature Review. Craniomaxillofac. Trauma Reconstr. 2013, 6, 257–266. [Google Scholar] [CrossRef]
- Arrington, E.D.; Smith, W.J.; Chambers, H.G.; Bucknell, A.L.; Davino, N.A. Complications of Iliac Crest Bone Graft Harvesting. Clin. Orthop. Relat. Res. 1996, 300–309. [Google Scholar] [CrossRef]
- Dimitriou, R.; Mataliotakis, G.I.; Angoules, A.G.; Kanakaris, N.K.; Giannoudis, P.V. Complications Following Autologous Bone Graft Harvesting from the Iliac Crest and Using the Ria: A Systematic Review. Injury 2011, 42 (Suppl. 2), S3–S15. [Google Scholar] [CrossRef]
- Bauer, T.W.; Muschler, G.F. Bone Graft Materials. An Overview of the Basic Science. Clin. Orthop. Relat. Res. 2000, 377, 10–27. [Google Scholar] [CrossRef]
- Ali Akbari Ghavimi, S.; Ebrahimzadeh, M.H.; Solati-Hashjin, M.; Abu Osman, N.A. Polycaprolactone/Starch Composite: Fabrication, Structure, Properties, and Applications. J. Biomed. Mater. Res. A 2015, 103, 2482–2498. [Google Scholar] [CrossRef] [PubMed]
- Knaack, D.; Goad, M.E.; Aiolova, M.; Rey, C.; Tofighi, A.; Chakravarthy, P.; Lee, D.D. Resorbable Calcium Phosphate Bone Substitute. J. Biomed. Mater. Res. 1998, 43, 399–409. [Google Scholar] [CrossRef]
- Lange, T.; Schilling, A.F.; Peters, F.; Mujas, J.; Wicklein, D.; Amling, M. Size Dependent Induction of Proinflammatory Cytokines and Cytotoxicity of Particulate Beta-Tricalciumphosphate in Vitro. Biomaterials 2011, 32, 4067–4075. [Google Scholar] [CrossRef]
- Niedhart, C.; Maus, U.; Piroth, W.; Miltner, O.; Schmidt-Rohlfing, B.; Siebert, C.H. Evaluation of a Resorbable, in Situ Setting Bone Substitute in a Sheep Model. J. Biomed. Mater. Res. B Appl. Biomater. 2004, 71, 123–129. [Google Scholar] [CrossRef]
- Campana, V.; Milano, G.; Pagano, E.; Barba, M.; Cicione, C.; Salonna, G.; Lattanzi, W.; Logroscino, G. Bone Substitutes in Orthopaedic Surgery: From Basic Science to Clinical Practice. J. Mater. Sci. Mater. Med. 2014, 25, 2445–2461. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, G.; Presta, R.; Benedetti, L.; de Angelis, M.G.C.; Lupi, S.M.; Rodriguez, Y.; Baena, R. Emerging Perspectives in Scaffold for Tissue Engineering in Oral Surgery. Stem Cells Int. 2017, 2017, 4585401. [Google Scholar] [CrossRef]
- D’Alessandro, D.; Perale, G.; Milazzo, M.; Moscato, S.; Stefanini, C.; Pertici, G.; Danti, S. Bovine Bone Matrix/Poly(L-Lactic-Co-Epsilon-Caprolactone)/Gelatin Hybrid Scaffold (Smartbone((R))) for Maxillary Sinus Augmentation: A Histologic Study on Bone Regeneration. Int. J. Pharm. 2017, 523, 534–544. [Google Scholar] [CrossRef]
- Pertici, G.; Carinci, F.; Carusi, G.; Epistatus, D.; Villa, T.; Crivelli, F.; Rossi, F.; Perale, G. Composite Polymer-Coated Mineral Scaffolds for Bone Regeneration: From Material Characterization to Human Studies. J. Biol. Regul. Homeost. Agents 2015, 29 (Suppl. 1), 136–148. [Google Scholar]
- Salerno, M.; Cenni, E.; Fotia, C.; Avnet, S.; Granchi, D.; Castelli, F.; Micieli, D.; Pignatello, R.; Capulli, M.; Rucci, N.; et al. Bone-Targeted Doxorubicin-Loaded Nanoparticles as a Tool for the Treatment of Skeletal Metastases. Curr. Cancer Drug Targets 2010, 10, 649–659. [Google Scholar] [CrossRef]
- Tegner, Y.; Lysholm, J. Rating Systems in the Evaluation of Knee Ligament Injuries. Clin. Orthop. Relat. Res. 1985, 198, 43–49. [Google Scholar] [CrossRef]
- Irrgang, J.J.; Ho, H.; Harner, C.D.; Fu, F.H. Use of the International Knee Documentation Committee Guidelines to Assess Outcome Following Anterior Cruciate Ligament Reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 1998, 6, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Ware, J.E., Jr.; Sherbourne, C.D. The Mos 36-Item Short-Form Health Survey (Sf-36). I. Conceptual Framework and Item Selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Grottoli, C.F.; Ferracini, R.; Compagno, M.; Tombolesi, A.; Rampado, O.; Pilone, L.; Bistolfi, A.; Borrè, A.; Cingolani, A.; Perale, G. A Radiological Approach to Evaluate Bone Graft Integration in Reconstructive Surgeries. Appl. Sci. 2019, 9, 1469. [Google Scholar] [CrossRef]
- DeCoster, T.A.; Nepola, J.V.; El-Khoury, G.Y. Cast Brace Treatment of Proximal Tibia Fractures. A Ten-Year Follow-up Study. Clin. Orthop. Relat. Res. 1988, 231, 196–204. [Google Scholar]
- Jensen, D.B.; Rude, C.; Duus, B.; Bjerg-Nielsen, A. Tibial Plateau Fractures. A Comparison of Conservative and Surgical Treatment. J. Bone Jt. Surg. Br. 1990, 72, 49–52. [Google Scholar] [CrossRef]
- Bansal, M.R.; Bhagat, S.B.; Shukla, D.D. Bovine Cancellous Xenograft in the Treatment of Tibial Plateau Fractures in Elderly Patients. Int. Orthop. 2009, 33, 779–784. [Google Scholar] [CrossRef]
- Koval, K.J.; Sanders, R.; Borrelli, J.; Helfet, D.; DiPasquale, T.; Mast, J.W. Indirect Reduction and Percutaneous Screw Fixation of Displaced Tibial Plateau Fractures. J. Orthop. Trauma 1992, 6, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Canadian Orthopaedic Trauma Society. Open Reduction and Internal Fixation Compared with Circular Fixator Application for Bicondylar Tibial Plateau Fractures. Results of a Multicenter, Prospective, Randomized Clinical Trial. J. Bone Jt. Surg. Am. 2006, 88, 2613–2623. [Google Scholar] [CrossRef]
- Malviya, A.; Reed, M.R.; Partington, P.F. Acute Primary Total Knee Arthroplasty for Peri-Articular Knee Fractures in Patients over 65 Years of Age. Injury 2011, 42, 1368–1371. [Google Scholar] [CrossRef]
- Goff, T.; Kanakaris, N.K.; Giannoudis, P.V. Use of Bone Graft Substitutes in the Management of Tibial Plateau Fractures. Injury 2013, 44, S86–S94. [Google Scholar] [CrossRef]
- Cancedda, R.; Dozin, B.; Giannoni, P.; Quarto, R. Tissue Engineering and Cell Therapy of Cartilage and Bone. Matrix Biol. 2003, 22, 81–91. [Google Scholar] [CrossRef]
- Secondo, F.; Grottoli, C.F.; Zollino, I.; Perale, G.; Lauritano, D. Positioning of a Contextual Implant Along with a Sinus Lift Anchored with a Block of Heterologous Bone. Oral. Implantol. (Rome) 2017, 10, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Mitsou, A.; Vallianatos, P.; Piskopakis, N.; Maheras, S. Anterior Cruciate Ligament Reconstruction by over-the-Top Repair Combined with Popliteus Tendon Plasty. J. Bone Jt. Surg. Br. 1990, 72, 398–404. [Google Scholar] [CrossRef]
- Irrgang, J.J.; Anderson, A.F.; Boland, A.L.; Harner, C.D.; Kurosaka, M.; Neyret, P.; Richmond, J.C.; Shelborne, K.D. Development and Validation of the International Knee Documentation Committee Subjective Knee Form. Am. J. Sports. Med. 2001, 29, 600–613. [Google Scholar] [CrossRef] [PubMed]
- Bucholz, R.W.; Carlton, A.; Holmes, R. Interporous Hydroxyapatite as a Bone Graft Substitute in Tibial Plateau Fractures. Clin. Orthop. Relat. Res. 1989, 240, 53–62. [Google Scholar] [CrossRef]
- Keating, J.F.; Hajducka, C.L.; Harper, J. Minimal Internal Fixation and Calcium-Phosphate Cement in the Treatment of Fractures of the Tibial Plateau. A Pilot Study. J. Bone Jt. Surg. Br. 2003, 85, 68–73. [Google Scholar] [CrossRef]
- Simpson, D.; Keating, J.F. Outcome of Tibial Plateau Fractures Managed with Calcium Phosphate Cement. Injury 2004, 35, 913–918. [Google Scholar] [CrossRef]
- Lobenhoffer, P.; Gerich, T.; Witte, F.; Tscherne, H. Use of an Injectable Calcium Phosphate Bone Cement in the Treatment of Tibial Plateau Fractures: A Prospective Study of Twenty-Six Cases with Twenty-Month Mean Follow-Up. J. Orthop. Trauma. 2002, 16, 143–149. [Google Scholar] [CrossRef]
- Horstmann, W.G.; Verheyen, C.C.; Leemans, R. An Injectable Calcium Phosphate Cement as a Bone-Graft Substitute in the Treatment of Displaced Lateral Tibial Plateau Fractures. Injury 2003, 34, 141–144. [Google Scholar] [CrossRef]
- Mehta, S.; Blagg, R.; Willcockson, J.; Gociman, B.; Yamashiro, D.; Siddiqi, F. Cost-Effectiveness Analysis of Demineralized Bone Matrix and Rhbmp-2 Versus Autologous Iliac Crest Bone Grafting in Alveolar Cleft Patients. Plast. Reconstr. Surg. 2018, 142, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Russell, T.A.; Leighton, R.K.; Alpha-BSM Tibial Plateau Fracture Study Group. Comparison of Autogenous Bone Graft and Endothermic Calcium Phosphate Cement for Defect Augmentation in Tibial Plateau Fractures. A Multicenter, Prospective, Randomized Study. J. Bone Jt. Surg. Am. 2008, 90, 2057–2061. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.T.; Smith, W.R.; Ziran, B.H.; Hasenboehler, E.A.; Stahel, P.F.; Morgan, S.J. Efficacy of Composite Allograft and Demineralized Bone Matrix Graft in Treating Tibial Plateau Fractures with Bone Loss. Orthopedics 2008, 31, 649. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Chawla, A.; Yang, Y.; Li, Y.; Zhang, J.; Jang, H.L.; Khademhosseini, A. Development of Nanomaterials for Bone-Targeted Drug Delivery. Drug Discov. Today 2017, 22, 1336–1350. [Google Scholar] [CrossRef] [PubMed]
- Manidakis, N.; Dosani, A.; Dimitriou, R.; Stengel, D.; Matthews, S.; Giannoudis, P. Tibial plateau fractures: Functional outcome and incidence of osteoarthritis in 125 cases. Int. Orthop. 2010, 34, 565–570. [Google Scholar] [CrossRef] [PubMed]
| Sex | Male | 18 (53%) |
| Female | 16 (47%) | |
| Age | Means and SD | 57.5 ± 12.782 |
| Height (m) | 1.71 ± 0.10 | |
| Weight (kg) | 71.17 ± 7.78 | |
| BMI (kg/m2) | 24.23 ± 1.97 | |
| Schatzker Classification [3] | Type II | 18 (52.9%) |
| Type III | 6 (17.6%) | |
| Type VI | 10 (29.4%) |
| VAS | 2 weeks post-op | 6.33 ± 1.40 |
| 1 month post-op | 5.2 ± 0.89 | |
| 3 months post-op | 3.1 ± 0.71 | |
| 6 months post-op | 1.43 ± 0.69 | |
| 1 year post-op | 1 ± 0.79 | |
| Percentage decrease | 84.20% | |
| SF-36 [23] | Limitations in physical activities | 92.5 ± 11.65 |
| Limitations in social activities | 96.67 ± 6.23 | |
| Limitations in usual role activities because of physical health | 96.83 ± 3.34 | |
| Bodily pain | 91.8 ± 9.31 | |
| General mental health | 86.93 ± 3.78 | |
| Limitations in usual role activities because of emotional problems | 94.33 ± 12.89 | |
| Vitality | 87.5 ± 6.79 | |
| General health perception | 93.6 ± 4.90 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferracini, R.; Bistolfi, A.; Garibaldi, R.; Furfaro, V.; Battista, A.; Perale, G. Composite Xenohybrid Bovine Bone-Derived Scaffold as Bone Substitute for the Treatment of Tibial Plateau Fractures. Appl. Sci. 2019, 9, 2675. https://doi.org/10.3390/app9132675
Ferracini R, Bistolfi A, Garibaldi R, Furfaro V, Battista A, Perale G. Composite Xenohybrid Bovine Bone-Derived Scaffold as Bone Substitute for the Treatment of Tibial Plateau Fractures. Applied Sciences. 2019; 9(13):2675. https://doi.org/10.3390/app9132675
Chicago/Turabian StyleFerracini, Riccardo, Alessandro Bistolfi, Riccardo Garibaldi, Vanessa Furfaro, Agnese Battista, and Giuseppe Perale. 2019. "Composite Xenohybrid Bovine Bone-Derived Scaffold as Bone Substitute for the Treatment of Tibial Plateau Fractures" Applied Sciences 9, no. 13: 2675. https://doi.org/10.3390/app9132675
APA StyleFerracini, R., Bistolfi, A., Garibaldi, R., Furfaro, V., Battista, A., & Perale, G. (2019). Composite Xenohybrid Bovine Bone-Derived Scaffold as Bone Substitute for the Treatment of Tibial Plateau Fractures. Applied Sciences, 9(13), 2675. https://doi.org/10.3390/app9132675






