A Prototype of a Portable Gas Analyzer for Exhaled Acetone Detection
Abstract
Featured Application
Abstract
1. Introduction
2. Development of the Portable Gas Analyzer
2.1. Gas Sensor Array
2.2. Gas Chamber Design
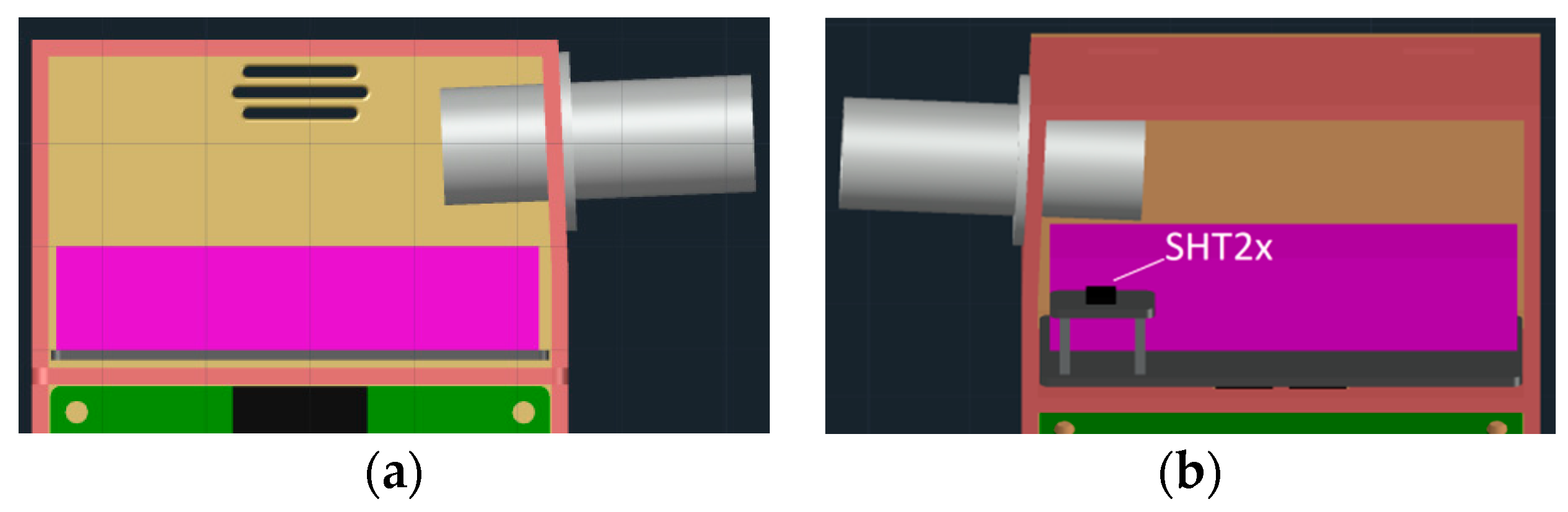
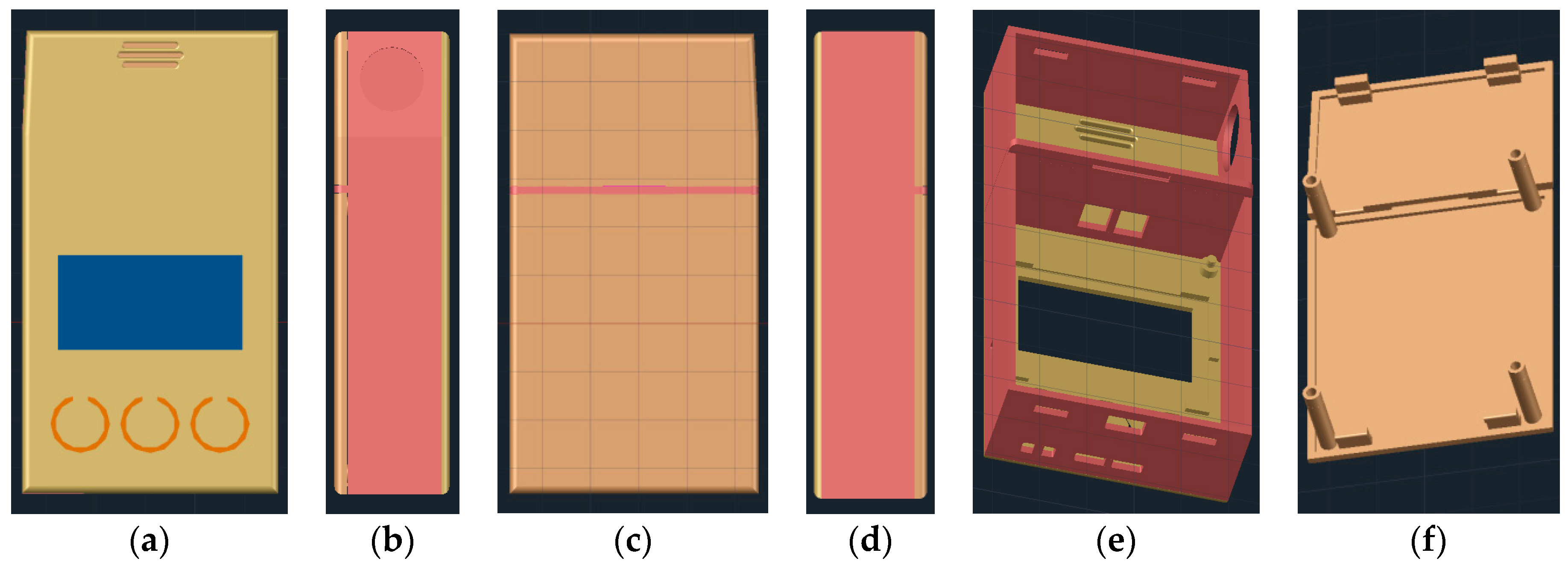
2.3. 3D Printable Housing Design
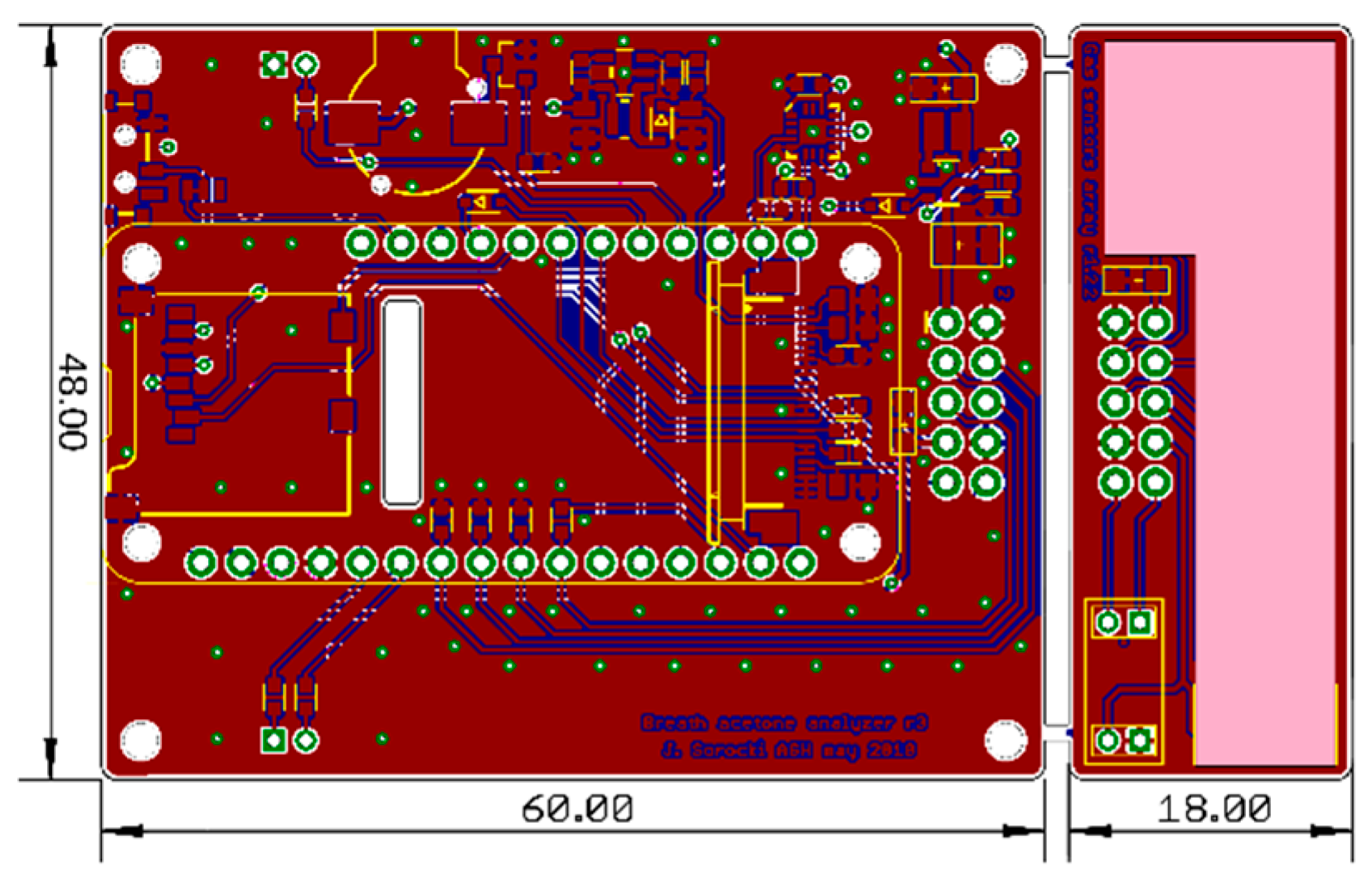
2.4. Hardware Design
2.5. Prototype Manufacturing and Assembly
3. Results and Discussion
3.1. Sensors Reference Resistance Calibration
3.2. Correction for Temperature and Humidity
3.3. Gas Concentration Calculation
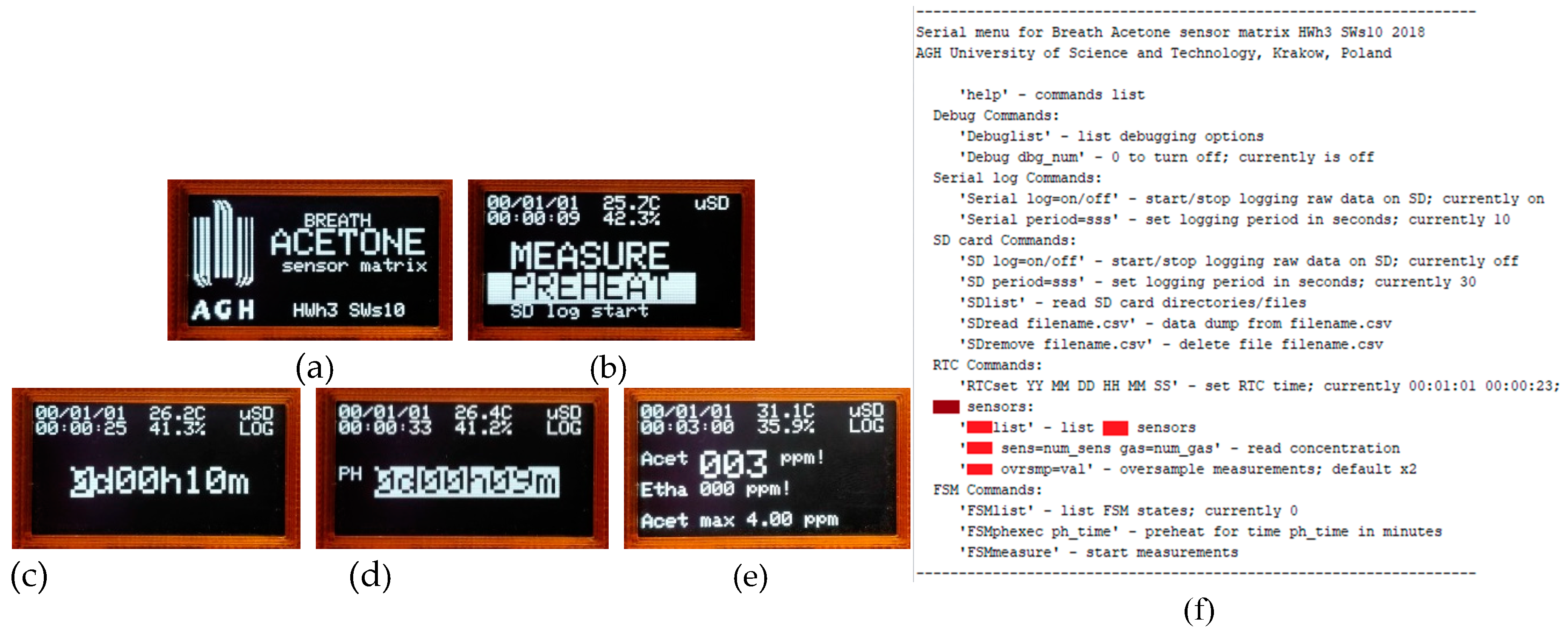
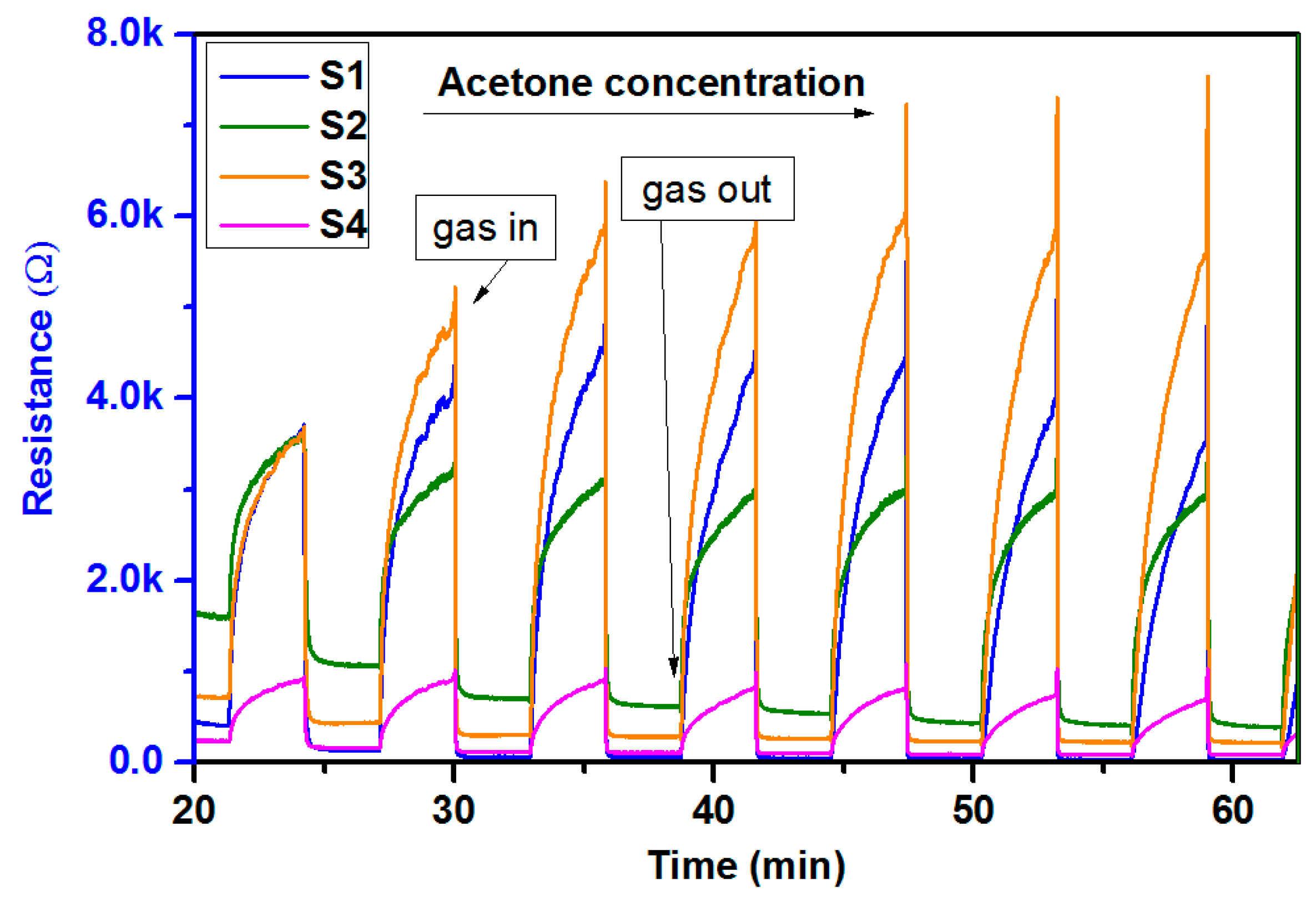
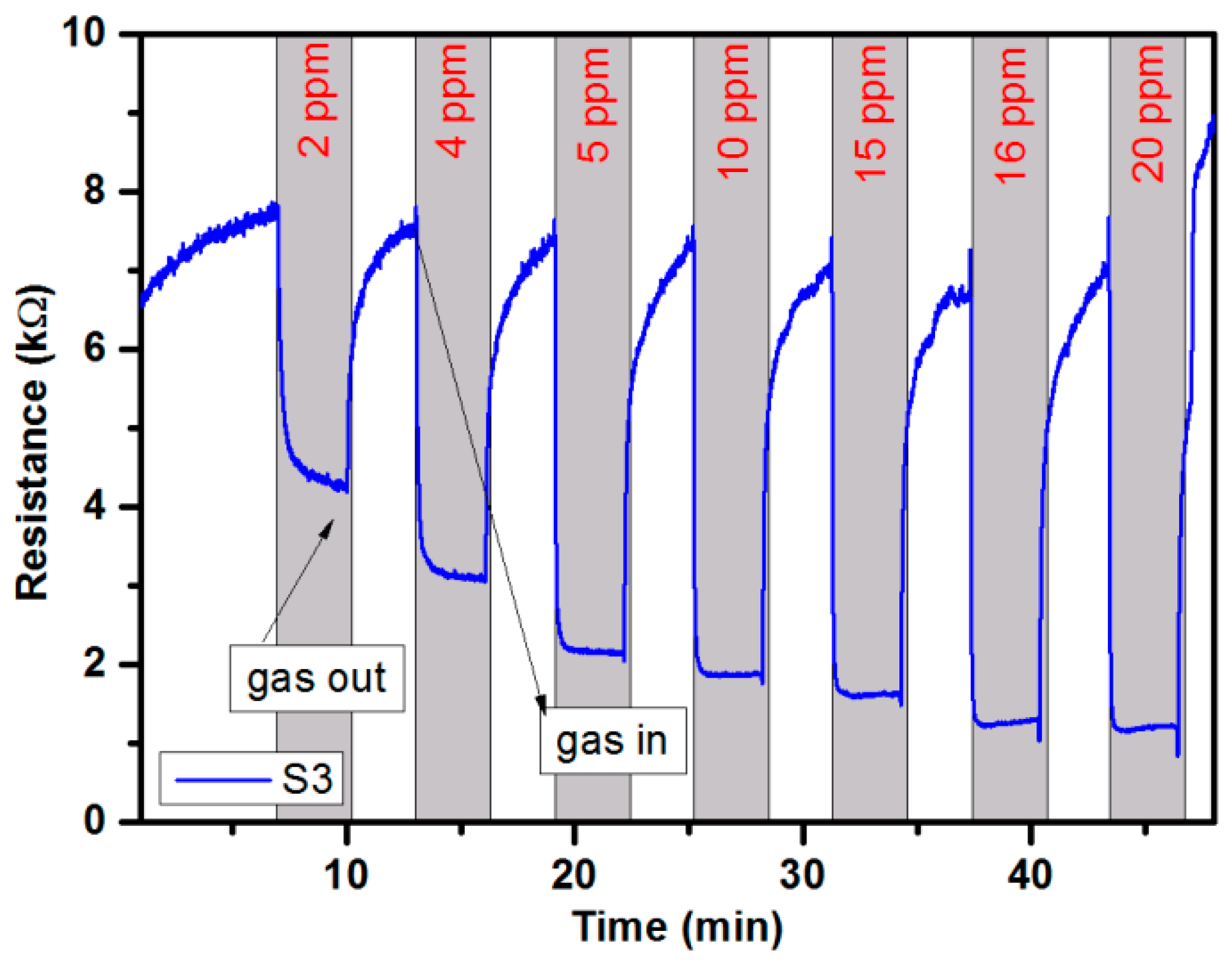
3.4. Portable Analyzer Operation and Measurements
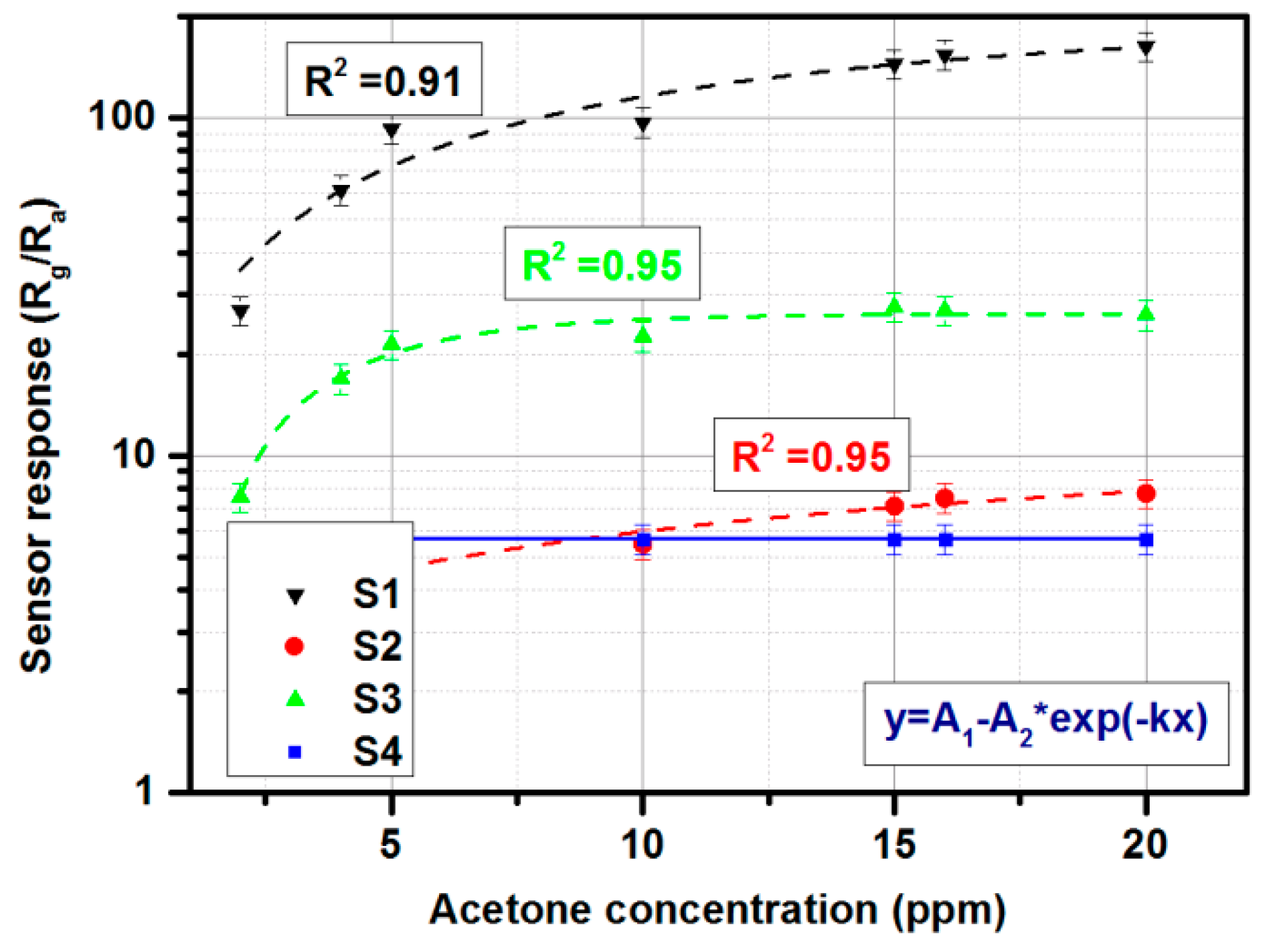
3.5. Gas-Sensing Setup Characterization
4. Conclusions and Further Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- De Lacy Costello, B.; Amann, A.; Al-Kateb, H.; Flynn, C.; Filipiak, W.; Khalid, T.; Osborne, D.; Ratcliffe, N.M. A review of the volatiles from the healthy human body. J. Breath Res. 2014, 8, 014001. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.; Byrnes, R.; Cataneo, R.N.; Chaturvedi, A.; Kaplan, P.D.; Libardoni, M.; Mehta, V.; Mundada, M.; Patel1, U.; Ramakrishna, N.; et al. Detection of volatile biomarkers of therapeutic radiation in breath. J. Breath Res. 2013, 7. [Google Scholar] [CrossRef] [PubMed]
- Al-Kateb, H.; de Lacy Costello, B.; Ratcliffe, N. An investigation of volatile organic compounds from the saliva of healthy individuals using headspace-trap/GC-MS. J. Breath Res. 2013, 7, 036004. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Zhang, J.; Yu, X.; Zhang, W.; Zhang, X. Determination of acetone in human breath by gas chromatography-mass spectrometry and solid-phase microextraction with on-fiber derivatization. J. Chromatogr. B 2004, 810, 269–275. [Google Scholar] [CrossRef]
- Lubes, G.; Goodarzi, M. GC-MS based metabolomics used for the identification of cancer volatile organic compounds as biomarkers. J. Phar. Biomed. Anal. 2018, 147, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Fedrigo, M.; Hoeschen, Ch.; Oeh, U. Multidimensional statistical analysis of PTR-MS breath samples: A test study on irradiation detection. Int. J. Mass Spectrom. 2010, 295, 13–20. [Google Scholar] [CrossRef]
- Righettoni, M.; Schmid, A.; Amann, A.; Pratsinis, S.E. Correlations between blood glucose and breath components from portable gas sensors and PTR-TOF-MS. Breath Res. 2013, 7, 037110. [Google Scholar] [CrossRef] [PubMed]
- Lapthorn, C.; Pullen, F.; Chowdhry, B.Z. Ion mobility spectrometry-mass spectrometry (IMS-MS) of small molecules: Separating and assigning structures to ions. Mass Spectrom. Rev. 2013, 32, 43–71. [Google Scholar] [CrossRef] [PubMed]
- Szymanska, E.; Tinnevelt, G.H.; Brodrick, E.; Williams, M.; Davies, A.N.; van Manen, H.-J.; Buydens, L.M.C. The increasing conclusiveness of clinical breath analysis by improved baseline correction of the multi-capillary column—Ion mobility spectrometry (MCC-IMS) data. J. Pharm. Biomed. Anal. 2016, 127, 170175. [Google Scholar] [CrossRef] [PubMed]
- Michalcikova, R.B.; Dryahina, K.; Spanel, P. SIFT-MS quantification of several breath biomarkers of inflammatory bowel disease, IBD.; A detailed study of the ion chemistry. Int. J. Mass Spect. 2016, 396, 35–41. [Google Scholar] [CrossRef]
- Smith, D.; Spanel, P. Direct, rapid quantitative analyses of BVOCs using SIFT-MS and PTR-MS obviating sample collection. TrAC 2011, 30, 945–959. [Google Scholar] [CrossRef]
- Schmidt, K.; Podmore, I. Current challenges in volatile organic compounds analysis as potential biomarkers of cancer. J. Biomark. 2015, 2015, 981458. [Google Scholar] [CrossRef] [PubMed]
- Smolinska, A.; Klaassen, E.M.M.; Dallinga, J.W.; van de Kant, K.D.G.; Jobis, Q.; Moonen, E.J.C.; van Schayck, Q.P.; Dompeling, E.; van Schooten, F.J. Profiling of volatile organic compounds in exhaled breath as a strategy to find early predictive signatures of asthma in children. PLoS ONE 2014, 9, e95668. [Google Scholar] [CrossRef] [PubMed]
- Saalberg, Y.; Wolff, M. VOC breath biomarkers in lung cancer. Clin. Chim. Acta 2016, 459, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.-E.; Lee, D.-S.; Ban, S.-W.; Oh, J.; Jung, M.Y.; Kim, S.-H.; Park, S.J.; Persaud, K.; Jheon, S. Analysis of volatile organic compounds in exhaled human breath for lung cancer diagnosis using a sensor system. Sens. Actuators B 2018, 255, 800–807. [Google Scholar] [CrossRef]
- Bos, L.D.; Sterk, P.J.; Fowler, S.J. Breathomics in the setting of asthma and chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2016, 138, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Hanania, N.A.; Pharm, M.M.; Jain, N. Measurement of fractional exhaled nitric oxide in real-world clinical practice alters asthma treatment decisions. Ann. Allergy Asthma Immunol. 2018, 120, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Anders, C.; Rømhild, D.J.; Ingrid, T.; Jørgen, V.; Jan, B. A systematic review of breath analysis and detection of volatile organic compounds in COPD. J. Breath Res. 2016, 10, 034002. [Google Scholar]
- Bregy, L.; Nussbaumer-Ochsner, Y.; Sinues, P.M.-L.; Garcia-Gomez, D.; Suter, Y.; Gaisl, T.; Stebler, N.; Gaugg, M.T.; Kohler, M.; Zenobi, R. Real-time mass spectrometric identification of metabolites characteristic of chronic obstructive pulmonary disease in exhaled breath. Clinic. Mass Spectr. 2018, 7, 29–35. [Google Scholar] [CrossRef]
- Herman-Saffar, O.; Boger, Z.; Libson, S.; Lieberman, D.; Gonen, R.; Zeiri, Y. Early non-invasive detection of breast cancer using exhaled breath and urine analysis. Comput. Biol. Med. 2018, 96, 227–232. [Google Scholar] [CrossRef]
- LI, H.; Peng, Y.; Duan, Y. Diagnosis of breast cancer based on breath analysis: An emerging method. Crit. Rev. Oncol. Hemat. 2013, 87, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Minh, T.D.Ch.; Blake, D.R.; Galassetti, P.R. The clinical potential of exhaled breath analysis for diabetes mellitus. J. Diab. Res. 2012, 97, 195–205. [Google Scholar] [CrossRef]
- Karyakin, A.A.; Nikulina, S.V.; Vokhmyanina, D.V.; Karyakina, E.E.; Anaev, E.K.H.; Chuchalin, A.G. Non-invasive monitoring of diabetes through analysis of the exhaled breath condensate (aerosol). Electrochem. Commun. 2017, 83, 81–84. [Google Scholar] [CrossRef]
- Amann, A.; Smith, D. Volatile Biomarkers: Non-Invasive Diagnosis in Physiology and Medicine; Elsevier: Edinburgh, UK, 2013. [Google Scholar]
- Piacentini, G.L.; Cattazzo, E.; Tezz, G.; Peroni, D.G. Exhaled nitric oxide in pediatrics: What is new for ptracrive purposes and clinical research in children? J. Breath Res. 2012, 6, 027103. [Google Scholar] [CrossRef] [PubMed]
- Warwick, G.; Kotluar, E.; Chow, S.; Thomas, P.S.; Yate, D.H. Exhaled breath condensate in pulmonary arterial hypertension. J. Breath Res. 2012, 6, 036006. [Google Scholar] [CrossRef]
- Diabetes—World Health Organization. Available online: http://www.who.int/diabetes/en/ (accessed on 27 October 2018).
- Rydosz, A. A negative correlation between blood glucose and acetone measured in healthy and type-1 diabetes mellitus patient breath. J. Diabetes Sci. Technol. 2015, 9, 881–884. [Google Scholar] [CrossRef]
- Rydosz, A. Micropreconcentrator in LTCC technology with mass spectrometry for the detection of acetone in healthy and type-1 diabetes mellitus patient breath. Metabolites 2014, 4, 921–931. [Google Scholar] [CrossRef]
- Rydosz, A. Sensors for enhanced detection of acetone as a potential tool for noninvasive diabetes monitoring. Sensors 2018, 18, 2298. [Google Scholar] [CrossRef]
- Saasa, V.; Malwela, T.; Beukes, M.; Mokgotho, M.; Liu, Ch.-P.; Mwakikunga, B. Sensing technologies for detection of acetone in human breath for diabetes diagnosis and monitoring. Diagnostics 2018, 8, 12. [Google Scholar] [CrossRef]
- Todd, C.; Salvetti, P.; Naylor, K.; Albatat, M. Towards non-invasive extraction and determination of blood glucose levels. Bioengineering 2017, 4, 82. [Google Scholar] [CrossRef]
- Wang, Ch.; Sahay, P. Breath analysis using laser spectroscopic techniques: Breath biomarkers, spectral fingerprints, and detection limits. Sensors 2009, 9, 8230–8262. [Google Scholar] [CrossRef] [PubMed]
- KETONIX® Breath Ketone Analyzer. Available online: https://www.ketonix.com/ (accessed on 29 May 2019).
- KetoIQ Keto Breathalyzer. Available online: https://www.amazon.com/dp/B07BKTNWF7/?tag=ketoconcern-20 (accessed on 29 May 2019).
- Ketone Breath Analyzer. Available online: https://www.amazon.com/dp/B07CKW8HZQ/?tag=ketoconcern-20 (accessed on 29 May 2019).

| J1 | |||
|---|---|---|---|
| Heaters +5 VDC supply | 1 | 2 | GND |
| Sensor 2 signal output | 3 | 4 | Sensor 1 signal output |
| Sensor 3 signal output | 5 | 6 | Sensor 3 signal output |
| SHT +3.3 VDC supp | 7 | 8 | GND |
| I2C bus SCL | 9 | 10 | I2C bus SCA |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sorocki, J.; Rydosz, A. A Prototype of a Portable Gas Analyzer for Exhaled Acetone Detection. Appl. Sci. 2019, 9, 2605. https://doi.org/10.3390/app9132605
Sorocki J, Rydosz A. A Prototype of a Portable Gas Analyzer for Exhaled Acetone Detection. Applied Sciences. 2019; 9(13):2605. https://doi.org/10.3390/app9132605
Chicago/Turabian StyleSorocki, Jakub, and Artur Rydosz. 2019. "A Prototype of a Portable Gas Analyzer for Exhaled Acetone Detection" Applied Sciences 9, no. 13: 2605. https://doi.org/10.3390/app9132605
APA StyleSorocki, J., & Rydosz, A. (2019). A Prototype of a Portable Gas Analyzer for Exhaled Acetone Detection. Applied Sciences, 9(13), 2605. https://doi.org/10.3390/app9132605






