A Comparative Study on Interactions of Antimicrobial Peptides L- and D-phenylseptin with 1,2-dimyristoyl-sn-glycero-3-phosphocholine
Abstract
1. Introduction
- L-Phenylseptin (L-Phes):
- D-Phenylseptin (D-Phes):
2. Materials and Methods
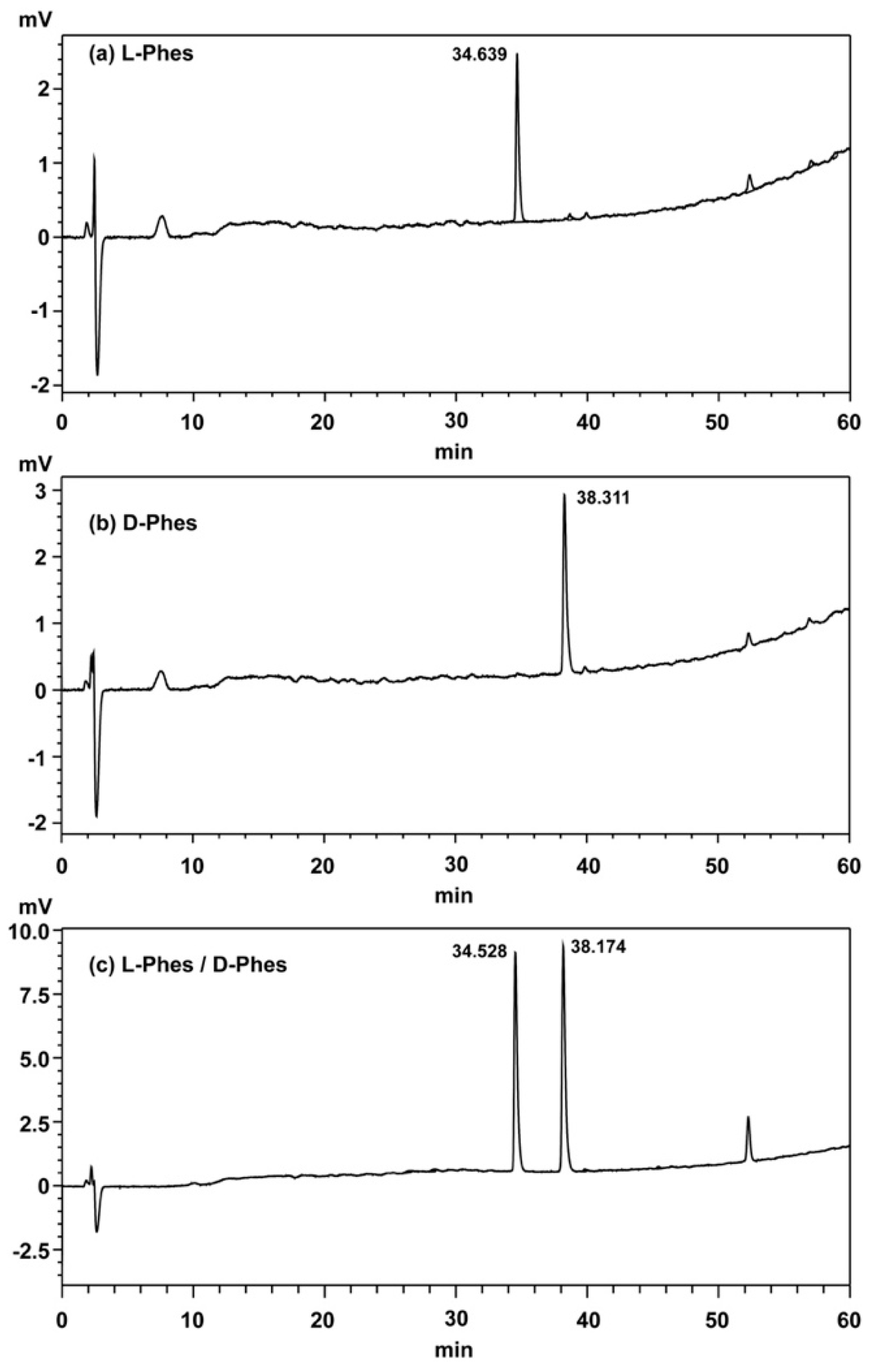
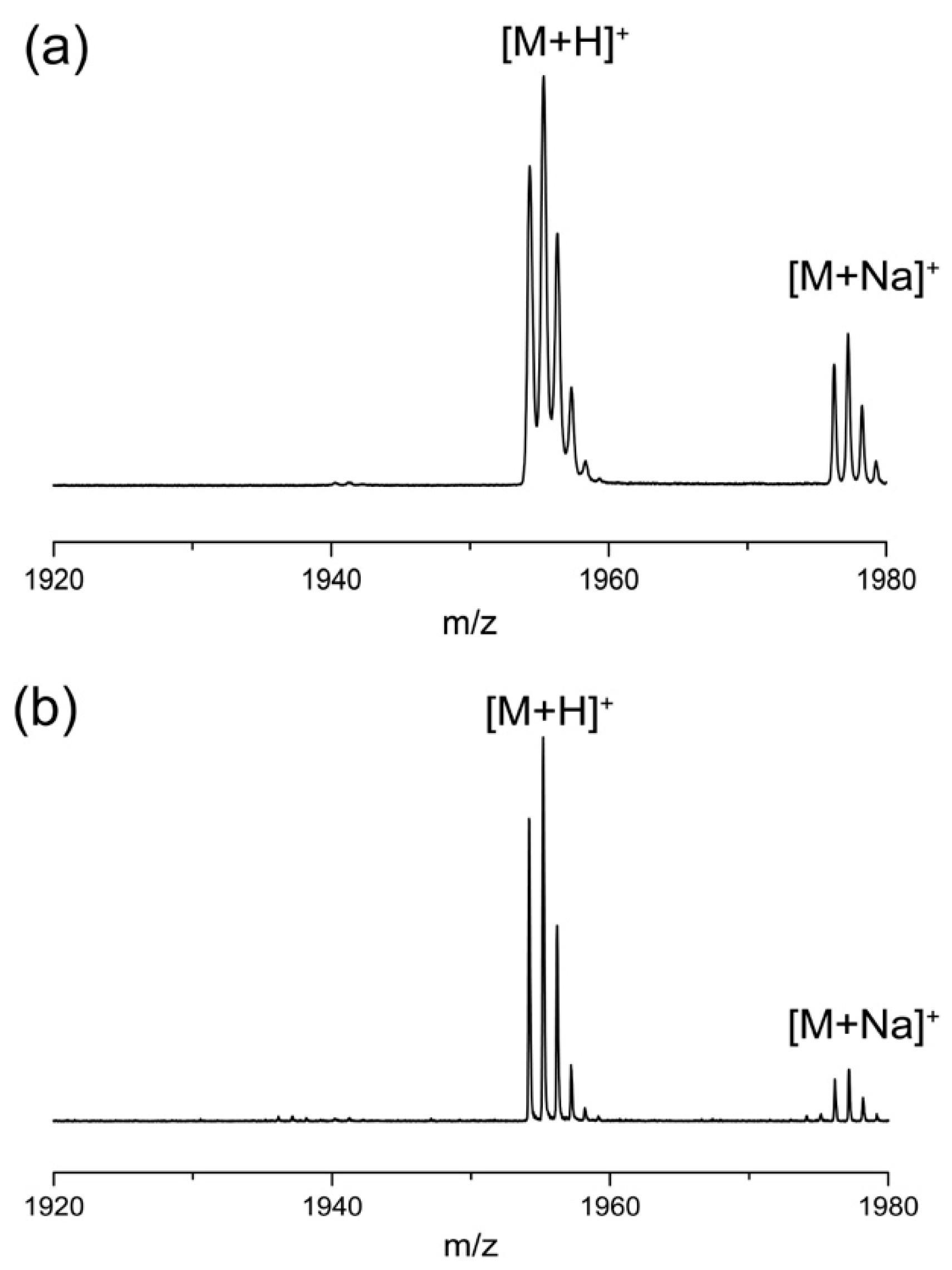
2.1. Synthesis of Peptides
2.2. Phospholipids
2.3. Quartz Crystal Microbalance
2.4. Far-Ultraviolet Circular Dichroism (Far-UV CD)
2.5. Vibrational Circular Dichroism (VCD)
2.6. P Solid-State NMR
2.7. Molecular Dynamics Simulations
3. Results and Discussions
3.1. QCM
3.2. Far-UV CD Spectroscopy
3.3. VCD and IR Spectroscopy
3.4. 31P Solid-State NMR
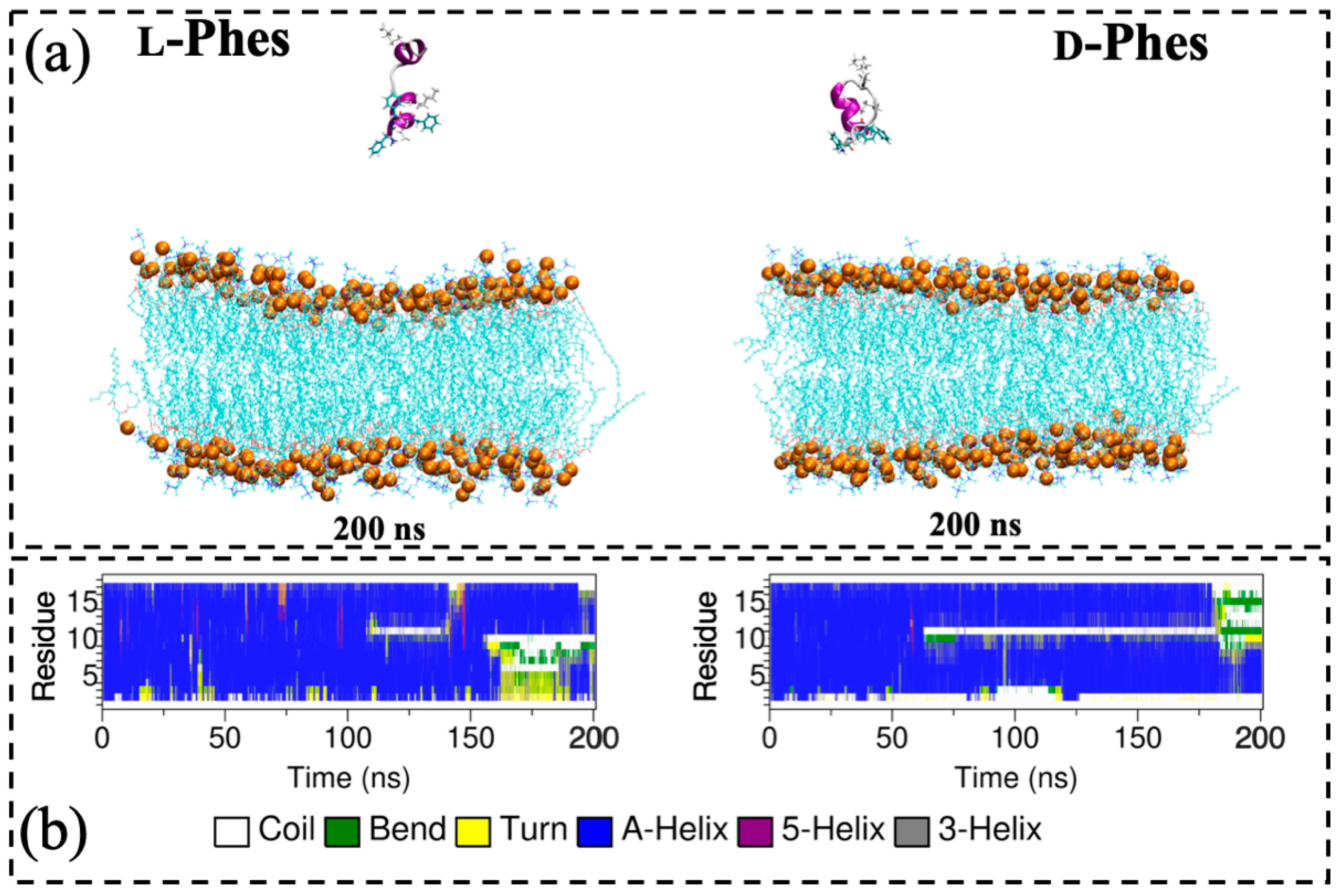
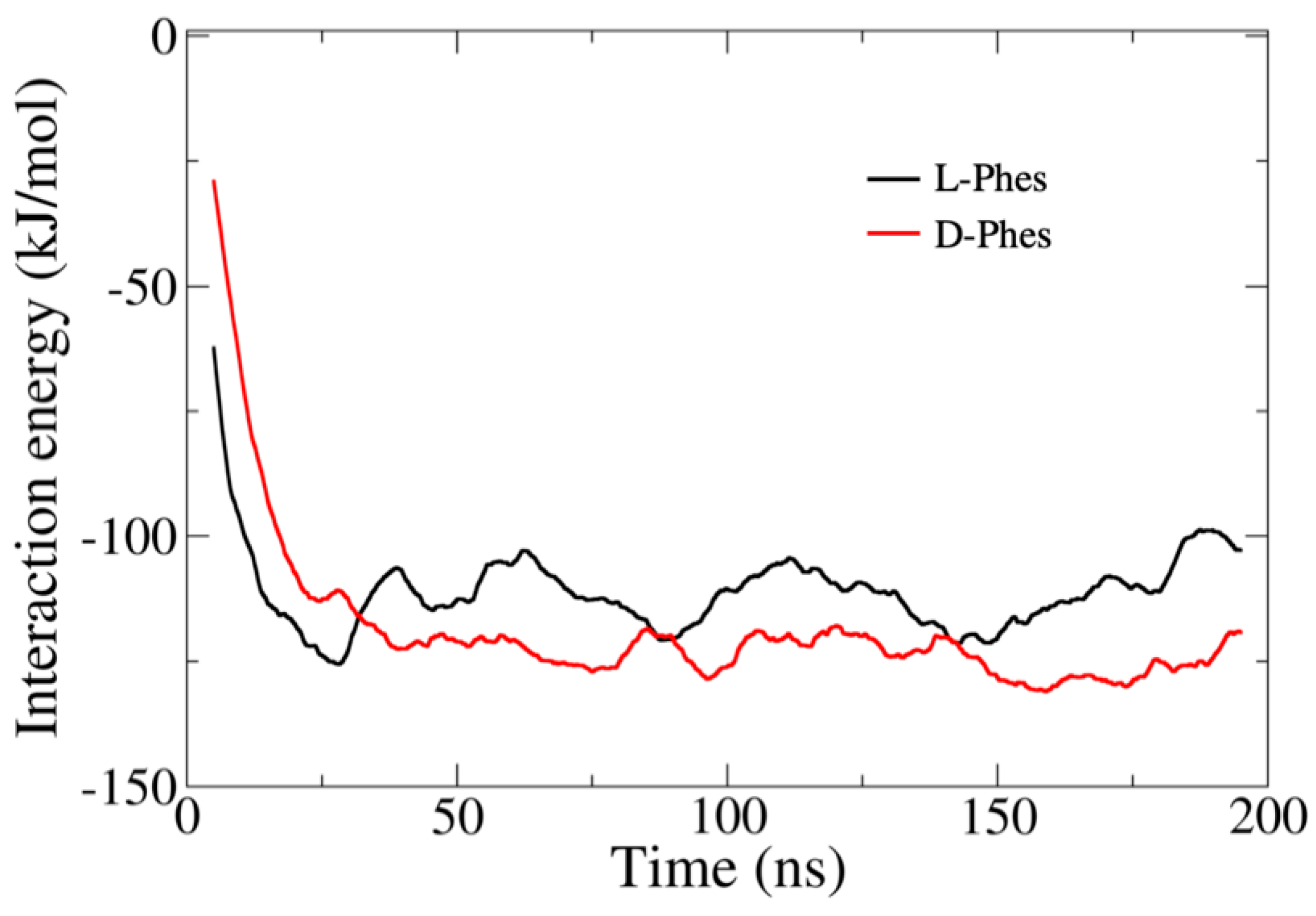
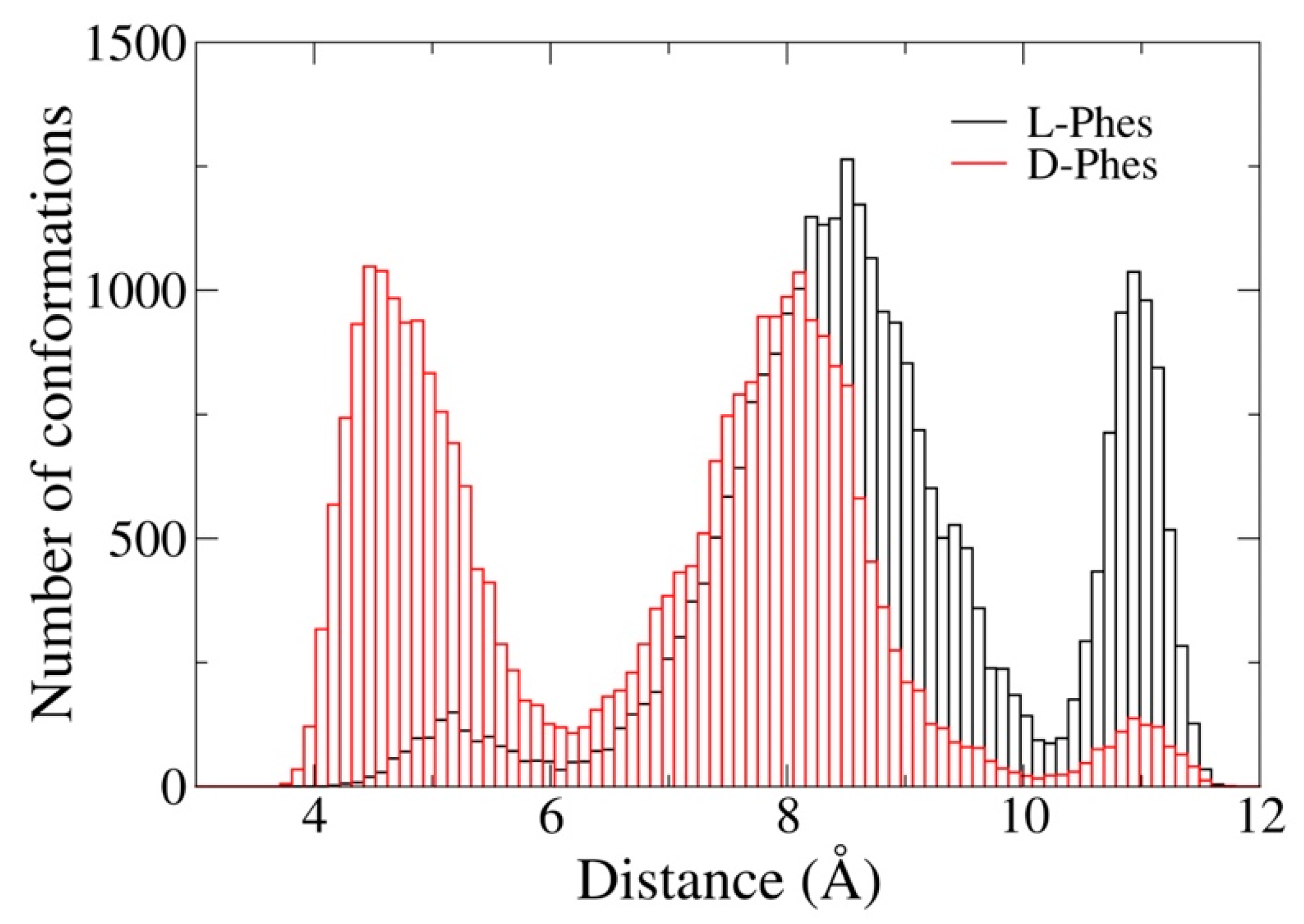
3.5. Molecular Dynamics Simulations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
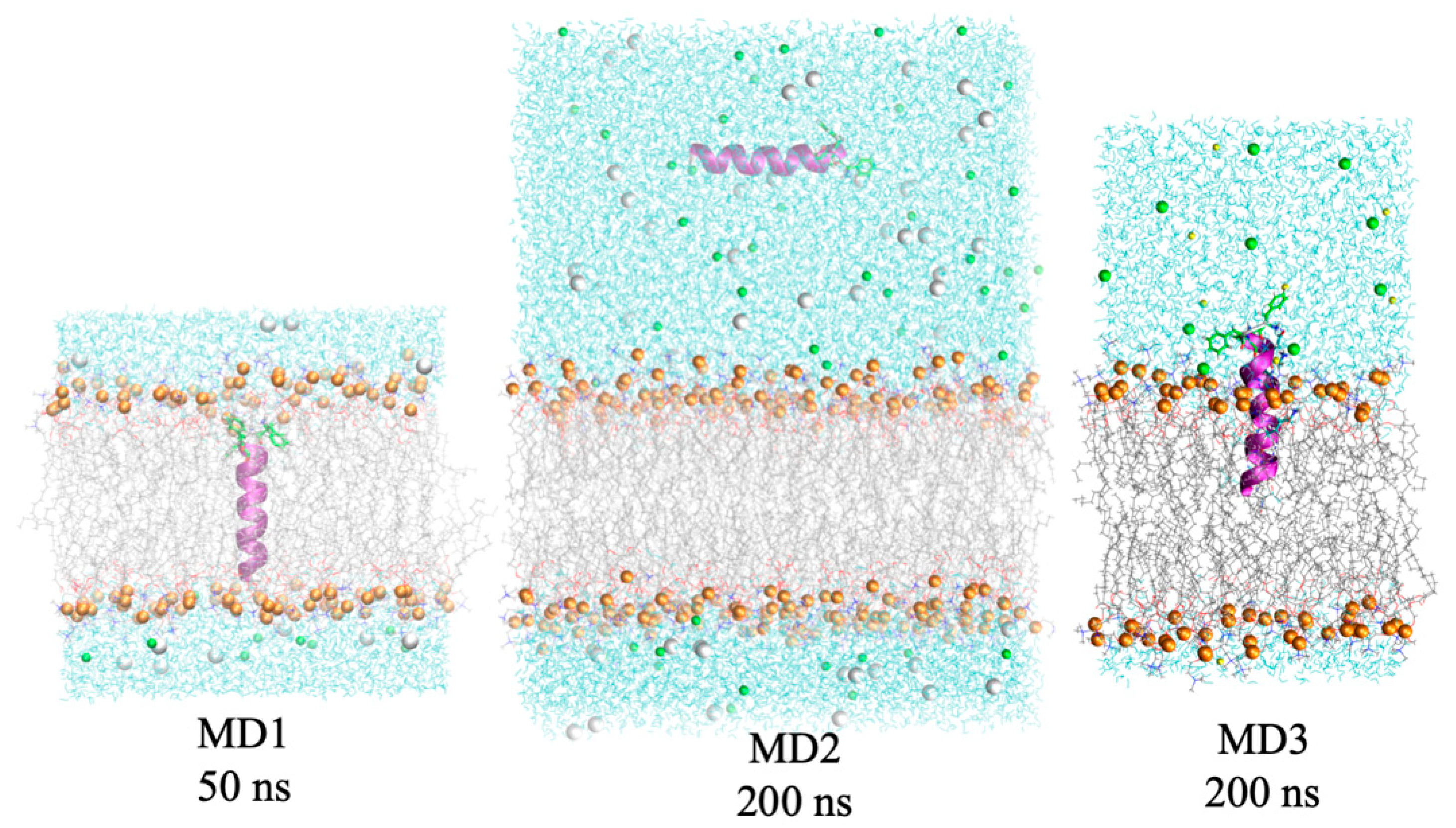

| Simulation ID | Peptide | Time, ns | Number of contents | |||
|---|---|---|---|---|---|---|
| DMPC | Water | Na+ | Cl− | |||
| MD1 | L-Phes | 50 | 128 | 3691 | 9 | 11 |
| D-Phes | 50 | 128 | 3697 | 9 | 11 | |
| MD2 | L-Phes | 200 | 240 | 17865 | 48 | 50 |
| D-Phes | 200 | 240 | 17698 | 47 | 49 | |
| MD3 | L-Phes | 200 (x3) 1 | 66 | 2959 | 7 | 9 |
| D-Phes | 200 (x3) 1 | 66 | 3119 | 8 | 10 | |
| MDs | L-Phes | D-Phes |
|---|---|---|
| Run 1 | 39.1 ± 6.0 | 38.8 ± 7.0 |
| Run 2 | 38.8 ± 7.0 | 83.1 ± 4.4 |
| Run 3 | 38.5 ± 6.2 | 83.3 ± 4.5 |
| Average | 38.8 ± 6.4 | 68.4 ± 21.6 |
References
- Rozek, T.; Wegener, K.L.; Bowie, J.H.; Olver, I.N.; Carver, J.A.; Wallace, J.C.; Tyler, M.J. The antibiotic and anticancer active aurein peptides from the Australian bell frogs Litoria aurea and Litoria raniformis. Eur. J. Biochem. 2000, 267, 5330–5341. [Google Scholar] [CrossRef] [PubMed]
- Hicks, R.P.; Russell, A.L. Application of unnatural amino acids to the de novo design of selective antibiotic peptides. In Unnatural Amino Acids: Methods and Protocols; Pollegioni, L., Servi, S., Eds.; Humana Press: Totowa, NJ, USA, 2012; pp. 135–167. [Google Scholar]
- Shai, Y. Mode of action of membrane active antimicrobial peptides. Biopolymers 2002, 66, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Marr, A.K.; McGwire, B.S.; McMaster, W.R. Modes of action of leishmanicidal antimicrobial peptides. Future Microbiol. 2012, 7, 1047–1059. [Google Scholar] [CrossRef] [PubMed]
- Bowdish, D.M.; Davidson, D.J.; Hancock, R. A re-evaluation of the role of host defence peptides in mammalian immunity. Curr. Prot. Pept. Sci. 2005, 6, 35–51. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, M.; Qiu, S.; Wang, J.; Peng, J.; Zhao, P.; Zhu, R.; Wang, H.; Li, Y.; Wang, K.; et al. Antimicrobial activity and stability of the D-amino acid substituted derivatives of antimicrobial peptide polybia-MPI. AMB Express 2016, 6, 122. [Google Scholar] [CrossRef] [PubMed]
- Ollivaux, C.; Soyez, D.; Toullec, J.-Y. Biogenesis of D-amino acid containing peptides/proteins: Where, when and how? J. Pept. Sci. 2014, 20, 595–612. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Sheeley, S.; Sweedler, J.V. Analysis of endogenous D-amino acid-containing peptides in Metazoa. Bioanal. Rev. 2009, 1, 7–24. [Google Scholar] [CrossRef]
- Glaser, T.; Hübner, K.; Castiglione, R.; de Hamprecht, B. Dermorphins, opioid peptides from amphibian skin, act on opioid receptors of mouse neuroblastoma x rat glioma hybrid cells. J. Neurochem. 1981, 37, 1613–1617. [Google Scholar] [CrossRef]
- Kreil, G. d-Amino acids in animal peptides. Annu. Rev. Biochem. 1997, 66, 337–345. [Google Scholar] [CrossRef]
- Tancredi, T.; Temussi, P.A.; Picone, D.; Amodeo, P.; Tomatis, R.; Salvadori, S.; Marastoni, M.; Santagada, V.; Balboni, G. New insights on μ/δ selectivity of opioid peptides: Conformational analysis of deltorphin analogues. Biopolymers 1991, 31, 751–760. [Google Scholar] [CrossRef]
- Barra, D.; Mignogna, G.; Simmaco, M.; Pucci, P.; Severini, C.; Falconieri-Erspamer, G.; Negri, L.; Erspamer, V. [d-Leu2] Deltorphin, A 17 amino acid opioid peptide from the skin of the Brazilian hylid frog, Phyllomedusa burmeisteri. Peptides 1994, 15, 199–202. [Google Scholar] [CrossRef]
- Mor, A.; Delfour, A.; Sagan, S.; Amiche, M.; Pradelles, P.; Rossier, J.; Nicolas, P. Isolation of dermenkephalin from amphibian skin, a high-affinity (δ-selective opioid heptapeptide containing a D-amino acid residue. FEBS Lett. 1989, 255, 269–274. [Google Scholar] [CrossRef]
- Erspamer, V.; Melchiorri, P.; Falconieri-Erspamer, G.; Negri, L.; Corsi, R.; Severini, C.; Barra, D.; Simmaco, M.; Kreil, G. Deltorphins: A family of naturally occurring peptides with high affinity and selectivity for delta opioid binding sites. Proc. Natl. Acad. Sci. USA 1989, 86, 5188–5192. [Google Scholar] [CrossRef] [PubMed]
- Mignogna, G.; Simmaco, M.; Kreil, G.; Barra, D. Antibacterial and haemolytic peptides containing D-alloisoleucine from the skin of Bombina variegata. EMBO J. 1993, 12, 4829–4832. [Google Scholar] [CrossRef] [PubMed]
- Mangoni, M.L.; Grovale, N.; Giorgi, A.; Mignogna, G.; Simmaco, M.; Barra, D. Structure-function relationships in bombinins H, antimicrobial peptides from Bombina skin secretions. Peptides 2000, 21, 1673–1679. [Google Scholar] [CrossRef]
- Mangoni, M.L.; Papo, N.; Saugar, J.M.; Barra, D.; Shai, Y.; Simmaco, M.; Rivas, L. Effect of natural L- to D-amino acid conversion on the organization, membrane binding, and biological function of the antimicrobial peptides bombinins H. Biochemistry 2006, 45, 4266–4276. [Google Scholar] [CrossRef] [PubMed]
- de Magalhães, M.T.Q.; Barbosa, E.A.; Prates, M.V.; Verly, R.M.; Munhoz, V.H.O.; de Araújo, I.E.; Bloch, C., Jr. conformational and functional effects induced by D- and L-amino acid epimerization on a single gene encoded peptide from the skin secretion of Hypsiboas punctatus. PLoS ONE 2013, 8, e59255. [Google Scholar] [CrossRef] [PubMed]
- Melchiorri, P.; Negri, L. The dermorphin peptide family. Gen. Pharm. 1996, 27, 1099–1107. [Google Scholar] [CrossRef]
- Simmaco, M.; Barra, D.; Chiarini, F.; Noviello, L.; Melchiorri, P.; Kreil, G.; Richter, K. A family of bombinin-related peptides from the skin of Bombina variegata. Eur. J. Biochem. 1991, 199, 217–222. [Google Scholar] [CrossRef]
- Mijiddorj, B.; Kaneda, S.; Sato, H.; Kitahashi, Y.; Javkhlantugs, N.; Naito, A.; Ueda, K.; Kawamura, I. The role of D-allo-isoleucine in the deposition of the anti-leishmania peptide bombinin H4 as revealed by 31P solid-state NMR, VCD spectroscopy, and MD simulation. Biochim. Biophys. Acta Proteins Proteom. 2018, 1866, 789–798. [Google Scholar] [CrossRef]
- Sekiya, Y.; Shimizu, K.; Kitahashi, Y.; Ohyama, A.; Kawamura, I.; Kawano, R. Electrophysiological analysis of membrane disruption by bombinin and its isomer using the lipid bilayer system. ACS Appl. Bio Mater. 2019, 2, 1542–1548. [Google Scholar] [CrossRef]
- Nagao, T.; Mishima, D.; Javkhlantugs, N.; Wang, J.; Ishioka, D.; Yokota, K.; Norisada, K.; Kawamura, I.; Ueda, K.; Naito, A. Structure and Orientation of antibiotic peptide alamethicin in phospholipid bilayers as revealed by chemical shift oscillation analysis of solid state nuclear magnetic resonance and molecular dynamics simulation. Biochim. Biophys. Acta Biomembr. 2015, 1848, 2789–2798. [Google Scholar] [CrossRef] [PubMed]
- Zerweck, J.; Strandberg, E.; Bürck, J.; Reichert, J.; Wadhwani, P.; Kukharenko, O.; Ulrich, A.S. Homo- and heteromeric interaction strengths of the synergistic antimicrobial peptides PGLa and Magainin 2 in membranes. Eur. Biophys. J. 2016, 45, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Sauerbrey, G. The use of quartz oscillators for weighing thin layers and for microweighing. Z. Phys. 1959, 155, 206–222. [Google Scholar] [CrossRef]
- Abraham, M.J.; van der Spoel, D.; Lindahl, E.; Hess, B. The GROMACS Development Team; GROMACS User Manual Version 5.1.2. Available online: www.gromacs.org (accessed on 27 June 2019).
- Pastor, R.W.; MacKerell, A.D., Jr. Development of the CHARMM force field for lipids. J. Phys. Chem. Lett. 2011, 2, 1526–1532. [Google Scholar] [CrossRef] [PubMed]
- Guex, N.; Peitsch, M.C. SWISS-MODEL and the Swiss-Pdb Viewer: An environment for comparative protein modeling. Electrophoresis 1997, 18, 2714–2723. [Google Scholar] [CrossRef]
- Jo, S.; Kim, T.; Iyer, V.G.; Im, W. CHARMM-GUI: A web-based graphical user interface for CHARMM. J. Comput. Chem. 2008, 29, 1859–1865. [Google Scholar] [CrossRef]
- Jo, S.; Lim, J.B.; Klauda, J.B.; Im, W. CHARMM-GUI membrane builder for mixed bilayers and its application to yeast membranes. Biophys. J. 2009, 97, 50–58. [Google Scholar] [CrossRef]
- Bussi, G.; Donadio, D.; Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 2007, 126, 014101. [Google Scholar] [CrossRef]
- Hess, B.; Bekker, H.; Berendsen, H.J.C.; Fraaije, J.G.E.M. LINCS: A linear constraint solver for molecular simulations. J. Comput. Chem. 1997, 18, 1463–1472. [Google Scholar] [CrossRef]
- Steinbach, P.J.; Brooks, B.R. New spherical-cutoff methods for long-range forces in macromolecular simulation. J. Comput. Chem. 1994, 15, 667–683. [Google Scholar] [CrossRef]
- Darden, T.; York, D.; Pedersen, L. Particle mesh Ewald: An N·log(N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef]
- Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, T.; Lee, H.; Pedersen, L.G. A smooth particle mesh Ewald method. J. Chem. Phys. 1995, 103, 8577–8593. [Google Scholar] [CrossRef]
- Nosé, S. A molecular dynamics method for simulations in the canonical ensemble. Mol. Phys. 1984, 52, 255–268. [Google Scholar] [CrossRef]
- Hoover, W.G. Canonical dynamics: Equilibrium phase-space distributions. Phys. Rev. A 1985, 31, 1695–1697. [Google Scholar] [CrossRef]
- Parrinello, M.; Rahman, A. Polymorphic transitions in single crystals: A new molecular dynamics method. J. Appl. Phys. 1981, 52, 7182–7190. [Google Scholar] [CrossRef]
- Kabsch, W.; Sander, C. Dictionary of protein secondary structure: Pattern recognition of hydrogen-bonded and geometrical features. Biopolymers 1983, 22, 2577–2637. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Tsutsumi, A.; Javkhlantugs, N.; Kira, A.; Umeyama, M.; Kawamura, I.; Nishimura, K.; Ueda, K.; Naito, A. structure and orientation of bovine lactoferrampin in the mimetic bacterial membrane as revealed by solid-state NMR and molecular dynamics simulation. Biophys. J. 2012, 103, 1735–1743. [Google Scholar] [CrossRef]
- Kira, A.; Javkhlantugs, N.; Miyamori, T.; Sasaki, Y.; Eguchi, M.; Kawamura, I.; Ueda, K.; Naito, A. Interaction of extracellular loop II of kappa-opioid receptor (196-228) with opioid peptide dynorphin in membrane environments as revealed by solid state nuclear magnetic resonance, quartz crystal microbalance and molecular dynamics simulation. J. Phys. Chem. B. 2014, 118, 9604–9612. [Google Scholar] [CrossRef]
- Hiraoki, T.; Brown, S.B.; Stevenson, K.J.; Vogel, H.J. Structural comparison between oxidized and reduced Escherichia Coli thioredoxin. Proton NMR and CD studies. Biochemistry 1988, 27, 5000–5008. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, N.J.; Fasman, G.D. Computed circular dichroism spectra for the evaluation of protein conformation. Biochemistry 1969, 8, 4108–4116. [Google Scholar] [CrossRef] [PubMed]
- Durr, H.N.U.; Gildenberg, M.; Ramamoorthy, A. The magic of bicelles lights up membrane protein structure. Chem. Rev. 2012, 112, 6054–6074. [Google Scholar] [CrossRef]
- Kurouski, D. Advances of vibrational circular dichroism (VCD) in bioanalytical chemistry. A review. Anal. Chim. Acta 2017, 990, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Nafie, L.A.; Keiderling, T.A.; Stephens, P.J. Vibrational circular dichroism. J. Am. Chem. Soc. 1976, 98, 2715–2723. [Google Scholar] [CrossRef]
- Sato, H.; Kawamura, I.; Yamagishi, A.; Sato, F. Solid-state vibrational circular dichroism spectra of isoleusine and its related compounds: Effects of interplay between two chiral centers. Chem. Lett. 2017, 46, 449–452. [Google Scholar] [CrossRef]
- Naito, A.; Nagao, T.; Norisada, K.; Mizuno, T.; Tuzi, S.; Saitô, H. Conformation and dynamics of melittin bound to magnetically oriented lipid bilayers by solid-state 31P and 13C NMR spectroscopy. Biophys. J. 2000, 78, 2405–2417. [Google Scholar] [CrossRef]
- Sinnokrot, M.O.; Valeev, E.F.; Sherrill, C.D. Estimates of the ab initio limit for π−π interactions: The benzene dimer. J. Am. Chem. Soc. 2002, 124, 10887–10893. [Google Scholar] [CrossRef]
- Ozawa, Y.; Sato, H.; Kayano, Y.; Yamaki, N.; Izato, Y.; Miyake, A.; Naito, A.; Kawamura, I. Self-assembly of tripeptides into γ-turn nanostructures. Phys. Chem. Chem. Phys. 2019, 21, 10879–10883. [Google Scholar] [CrossRef]





| Peptides | Ka/M−1 |
|---|---|
| L-Phes | 4.7 × 106 |
| D-Phes | 7.3 × 106 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mijiddorj, B.; Matsuo, Y.; Sato, H.; Ueda, K.; Kawamura, I. A Comparative Study on Interactions of Antimicrobial Peptides L- and D-phenylseptin with 1,2-dimyristoyl-sn-glycero-3-phosphocholine. Appl. Sci. 2019, 9, 2601. https://doi.org/10.3390/app9132601
Mijiddorj B, Matsuo Y, Sato H, Ueda K, Kawamura I. A Comparative Study on Interactions of Antimicrobial Peptides L- and D-phenylseptin with 1,2-dimyristoyl-sn-glycero-3-phosphocholine. Applied Sciences. 2019; 9(13):2601. https://doi.org/10.3390/app9132601
Chicago/Turabian StyleMijiddorj, Batsaikhan, Yuta Matsuo, Hisako Sato, Kazuyoshi Ueda, and Izuru Kawamura. 2019. "A Comparative Study on Interactions of Antimicrobial Peptides L- and D-phenylseptin with 1,2-dimyristoyl-sn-glycero-3-phosphocholine" Applied Sciences 9, no. 13: 2601. https://doi.org/10.3390/app9132601
APA StyleMijiddorj, B., Matsuo, Y., Sato, H., Ueda, K., & Kawamura, I. (2019). A Comparative Study on Interactions of Antimicrobial Peptides L- and D-phenylseptin with 1,2-dimyristoyl-sn-glycero-3-phosphocholine. Applied Sciences, 9(13), 2601. https://doi.org/10.3390/app9132601






