Sargassum muticum Hydrothermal Extract: Effects on Serum Parameters and Antioxidant Activity in Rats
Abstract
1. Introduction
2. Results and Discussion
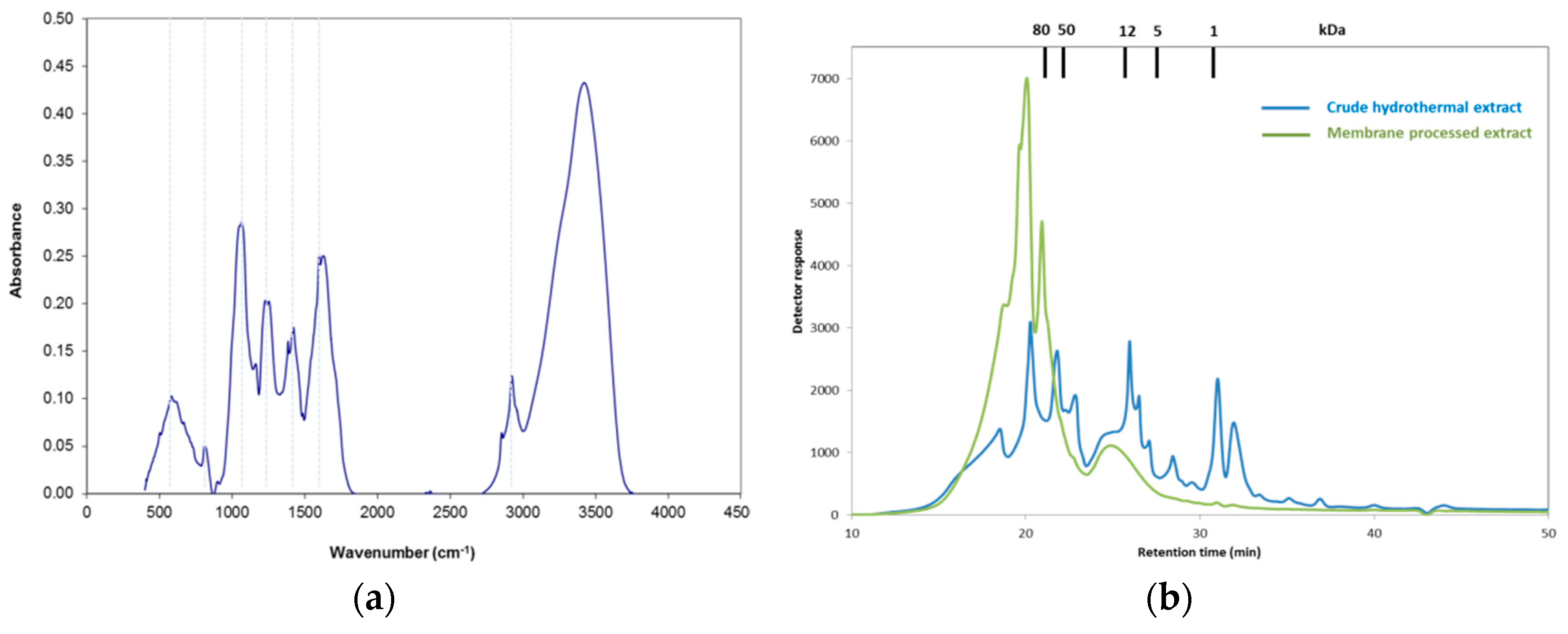
2.1. Extract Composition and Characteristics
2.2. Effect of Administration
2.2.1. Influence on Weight
2.2.2. Influence on Serum Parameters
2.2.3. Influence on Antioxidant Biomarkers
Enzymes
Other Antioxidant Compounds
3. Materials and Methods
3.1. Materials
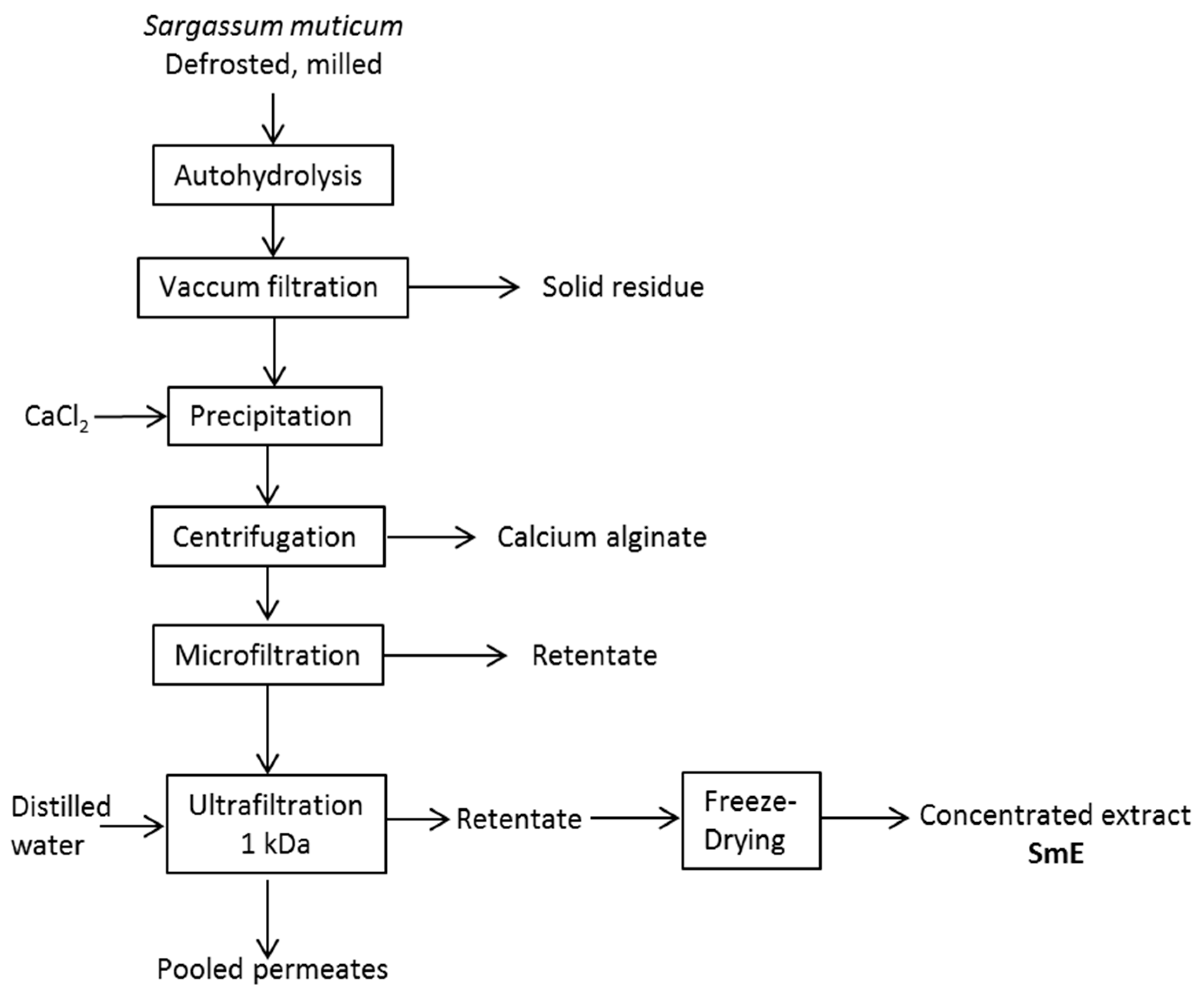
3.2. Extraction and Concentration
3.3. Experimental Animals and Diets
3.4. Serum Biochemical Parameters
3.5. Preparation of Tissues for Measurement of Antioxidant Levels
3.5.1. Preparation of Liver Samples
3.5.2. Blood Sample Preparation
3.6. Measurement of Lipid Peroxidation Levels
3.7. SOD Measurement
3.8. Measurement of GPx Activity
3.9. Measurement of Catalase Activity
4. Conclusions
5. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, Z.; Zhang, Q.; Wang, J.; Zhang, H.; Niu, X.; Li, P. Preparation of the different derivatives of the low-molecular-weight porphyran from Porphyra haitanensis and their antioxidant activities in vitro. Int. J. Macromol. 2009, 45, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Karnjanapratum, S.; You, S. Molecular characteristics of sulfated polysaccharides from Monostroma nitidum and their in vitro anticancer and immunomodulatory activities. Int. J. Biol. Macromol. 2010, 48, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Wijesekara, I.; Pangestuti, R.; Kim, S.K. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohyd. Polym. 2011, 84, 14–21. [Google Scholar] [CrossRef]
- Kang, H.K.; Deo, C.H.; Park, Y. The effects of marine carbohydrates and glycosylated compounds on human health. Int. J. Mol. Sci. 2015, 16, 6018–6056. [Google Scholar] [CrossRef] [PubMed]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Zhao, Z.; Yang, X.; Gong, Z.; Pan, M.; Han, Y.; Liu, Y. Antioxidant activities of crude phlorotannins from Sargassum hemiphyllum. J. Huazhong Univ. Sci. Technol. Med. Sci. 2016, 36, 449–45526. [Google Scholar] [CrossRef] [PubMed]
- Plaza, M.; Cifuentes, A.; Ibáñez, E. In the search of new functional food ingredients from algae. Trends Food Sci. Technol. 2008, 19, 31–39. [Google Scholar] [CrossRef]
- Sureh, V.; Anbazhagan, C.; Senthilkumar, D.; Senthilkumar, N.; Kannan, S.; Rengasamy, R.; Palani, P. Stabilization of mitochondrial and microsomal function of fucoidan from Sargassum plagiophyllum in diethylnitrosamine induced hepatocarcinogenesis. Carbohyd. Polym. 2013, 92, 1377–1385. [Google Scholar] [CrossRef]
- Mori, J.; Matsunaga, T.; Takahashi, S.; Hasegawa, C.; Saito, H. Inhibitory activity on lipid peroxidation of extracts from marine brown algae. Phytother. Res. 2003, 17, 549–551. [Google Scholar] [CrossRef]
- Murugan, K.; Iyer, V.V. Differential growth inhibition of cancer cell lines and antioxidant activity of extracts of red, brown, and green marine alge. In Vitro Cell. Dev. Biol. Anim. 2013, 49, 324–334. [Google Scholar] [CrossRef]
- Shao, P.; Chen, X.; Sun, P. In vitro antioxidant and antitumor activities of different sulfated polysaccharides isolated from three algae. Int. J. Biol. Macromol. 2013, 62, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Motshakeri, M.; Ebrahimi, M.; Goh, Y.M.; Matanjun, P.; Mohamed, S. Sargassum polycystum reduces hyperglycaemia, dyslipidaemia and oxidative stress via increasing insulin sensitivity in a rat model of type 2 diabetes. J. Sci. Food Agric. 2013, 93, 1772–1778. [Google Scholar] [CrossRef] [PubMed]
- Motshakeri, M.; Ebrahimi, M.; Goh, Y.M.; Othman, H.H.; Bejo, M.H.; Mohamed, S. Effects of brown seaweed (Sargassum polycystum) extracts on kidney, liver, and pancreas of type 2 diabetic rat model. Evid. Based Complement. Altern. Med. 2014, 379407. [Google Scholar] [CrossRef] [PubMed]
- Flórez-Fernández, N.; López-García, M.; González-Muñoz, M.J.; Vilariño, J.M.; Domínguez, H. Ultrasound-assisted extraction of fucoidan from Sargassum muticum. J. Appl. Phycol. 2017, 29, 1553–1561. [Google Scholar] [CrossRef]
- González-López, N.; Moure, A.; Domínguez, H. Hydrothermal fractionation of Sargassum muticum biomass. J. Appl. Phycol. 2012, 24, 1569–1578. [Google Scholar] [CrossRef]
- Balboa, E.M.; Rivas, S.; Moure, A.; Domínguez, H.; Parajó, J.C. Simultaneous extraction and depolymerization of fucoidan from Sargassum muticum in aqueous media. Mar. Drugs 2013, 11, 4612–4627. [Google Scholar] [CrossRef] [PubMed]
- Balboa, E.M.; Moure, A.; Domínguez, H. Valorization of Sargassum muticum biomass according to the biorefinery concept. Mar. Drugs 2015, 1, 3745–3760. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Viñas, M.; Flórez-Fernández, N.; González-Muñoz, M.J.; Domínguez, H. Influence of molecular weight on the properties of Sargassum muticum fucoidan. Algal Res. 2019, 38, 101393. [Google Scholar] [CrossRef]
- Valko, M.; Izakovic, M.; Mazur, M.; Rhodes, C.J.; Telser, J. Role of oxygen radicals in DNA damage and cancer incidence. Mol. Cell. Biochem. 2004, 266, 37–56. [Google Scholar] [CrossRef]
- Storz, P. Reactive oxygen species in tumor progression. Front. Biosci. 2005, 10, 1881–1896. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive species and antioxidants. Redox Biology is a fundamental theme of aerobic life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Porrini, M.; Riso, P.; Brusamolino, A.; Berti, C.; Guarnieri, S.; Visioli, F. Daily intake of a formulated tomato drink affects carotenoid plasma and lymphocyte concentrations and improves cellular antioxidant protection. Br. J. Nutr. 2005, 93, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Gawrieh, S.; Opara, E.C.; Koch, T.R. Oxidative stress in nonalcoholic fatty liver disease: Pathogenesis and antioxidant therapies. J. Investig. Med. 2004, 52, 506–514. [Google Scholar] [PubMed]
- Zaragozá, M.C.; López, D.; Sainz, M.P.; Poquet, M.; Perez, J.; Puig-Parrellada, P.; Mármol, F.; Simonetti, P.; Gardana, C.; Lerat, Y.; et al. Toxicity and Antioxidant activity in vitro and in vivo of two Fucus vesiculosus extracts. J. Agric. Food Chem. 2008, 56, 7773–7780. [Google Scholar] [CrossRef] [PubMed]
- Tas, S.; Celikler, S.; Ziyanok-Ayvalik, S.; Sarandol, E.; Dirican, M. Ulva rigida improves carbohydrate metabolism, hyperlipidemia and oxidative stress in streptozotocin-induced diabetic rats. Cell Biochem. Funct. 2011, 29, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Dahech, I.; Belghith, K.S.; Hamden, K.; Feki, A.; Belghith, H.; Mejdoub, H. Oral administration of levan polysaccharides reduces the alloxan-induced oxidative stress in rats. Int. J. Biol. Macromol. 2011, 49, 942–947. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, J.Y.; Choi, W.H.; Lee, S.S. Effects of seaweed supplementation on blood glucose concentration, lipid profile and antioxidant enzyme activities in patients with type 2 diabetes mellitus. Nutr. Res. Pract. 2008, 2, 62–67. [Google Scholar] [CrossRef]
- Schroder, H. Protective mechanisms of the Mediterranean diet in obesity and type 2 diabetes. J. Nutr. Biochem. 2007, 18, 149–160. [Google Scholar] [CrossRef]
- Basu, S. Carbon tetrachloride-induced lipid peroxidation: Eicosanoid formation and their regulation by antioxidant nutrients. Toxicology 2003, 189, 113–127. [Google Scholar] [CrossRef]
- Kang, K.A.; Bu, H.D.; ParK, D.S.; Go, G.M.; Jee, Y.; Shin, T.; Hyun, J.W. Antioxidant activity of ethanol extract of Callophyllis japonica. Phytother. Res. 2005, 19, 506–510. [Google Scholar] [CrossRef]
- Guerra Dore, C.M.P.; Faustino Alves, M.G.; Luiza Sheyla, E.; Pofírio Will, L.S.; Costa, T.G.; Sabry, D.A.; de Souza Rêgo, L.A.R.; Accordo, C.M.; Rocha, H.A.O.; Guimarães, A.; et al. Sulfated polysaccharide, fucans, isolated from brown algae Sargassum vulgare with anticoagulant, antithrombotic, antioxidant and anti-inflammatory effects. Carbohyd. Polym. 2013, 91, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Kellogt, J.; Lila, M.A. Chemical in vitro assessment of Alaskan Coastal vegetation antioxidant capacity. J. Agric. Food Chem. 2013, 61, 11025–11032. [Google Scholar] [CrossRef] [PubMed]
- Marudhupandi, T.; Kumar, T.T.; Senthil, S.L.; Devi, K.N. In vitro antioxidant properties of fucoidan fractions from Sargassum tenerrimum. Pak. J. Biol. Sci. 2014, 17, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Rocha de Souza, M.C.; Marques, C.T.; Guerra Dore, C.M.; Ferreira da Silva, F.R.; Oliveira Rocha, H.A.; Lisboa Leite, E. Antioxidant activities of sulfated polysaccharides from brown and red seaweeds. J. Appl. Phycol. 2007, 19, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Sabeena, F.K.N.; Jacobsen, C. Phenolic compounds and antioxidant activities of selected species of seaweeds from Danish coast. Food Chem. 2013, 138, 1670–1681. [Google Scholar] [CrossRef] [PubMed]
- Muroney, N.C.; O’Grady, M.N.; O’Doherty, J.V.; Kerry, J.P. Addition of seaweed (Laminaria digitata) extracts containing laminarin and fucoidan to porcine diets: Influence on the quality and shelf-life of fresh pork. Meat Sci. 2012, 92, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Gorham, J.; Lewey, S.A. Seasonal changes in the chemical composition of Sargassum muticum. Mar. Biol. 1984, 80, 103–107. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Far, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 267–275. [Google Scholar]
- Fairbanks, V.F.; Klee, G.G. Biochemical aspects of hematology. In Fundamentals of Clinical Chemistry, 3rd ed.; Tiez, N.W., Ed.; W.R. Saunders Co.: Philadelphia, PA, USA, 1987; pp. 197–212. [Google Scholar]
- Esterbauer, H.; Cheeseman, K.H. Determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990, 186, 407–421. [Google Scholar]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Labor. Clin. Med. 1967, 70, 158–169. [Google Scholar]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
| Trophic Balance | Control | 0.5 g kg−1 | 1.0 g kg−1 | 2.0 g kg−1 |
|---|---|---|---|---|
| Weight gain (g day−1) | 6.79 ± 0.15 | 7.02 ± 0.11 | 6.93 ± 0.11 | 6.79 ± 0.22 |
| Liver/body weight (%) | 3.30 ± 0.04 | 3.43 ± 0.07 | 3.35 ± 0.08 | 3.42 ± 0.04 |
| Kidney/body weight (%) | 0.86 ± 0.00 | 0.79 ± 0.02 * | 0.79 ± 0.02 * | 0.81 ± 0.01 * |
| Intestinal mucosa/body weight (%) | 0.85 ± 0.01 | 0.87 ± 0.03 | 0.74 ± 0.02 | 0.80 ± 0.05 |
| Heart/body weight (%) | 0.41 ± 0.00 | 0.41 ± 0.00 | 0.42 ± 0.01 | 0.39 ± 0.01 |
| Lung/body weight (%) | 0.55 ± 0.01 | 0.48 ± 0,03 | 0.59 ± 0.02 * | 0.53 ± 0.07 |
| Stomach/body weight (%) | 0.62 ± 0.03 | 0.59 ± 0.02 | 0.57 ± 0.02 | 0.64 ± 0.02 |
| Serum Parameters | Control | 0.5 g kg−1 | 1 g kg−1 | 2 g kg−1 |
|---|---|---|---|---|
| CHO total (mg dL−1) | 121.50 ± 3.96 | 118.17 ± 3.24 | 118.50 ± 5.36 | 114.83 ± 2.96 |
| HDL (mg dL−1) | 49.50 ± 1.30 | 48.80 ± 1.53 | 48.10 ± 2.09 | 45.33 ± 1.15 |
| LDL (mg dL−1) | 51.70 ± 2.94 | 50.70 ± 1.71 | 45.08 ± 2.27 | 46.43 ± 2.14 |
| Triglycerides (mg dL−1) | 105.75 ± 6.21 | 98.23 ± 6.23 | 86.50 ± 5.70 | 97.17 ± 8.19 |
| BUN (mg dL−1) | 11.25 ± 0.34 | 11.33 ± 0.76 | 13.00 ± 0.71 | 12.00 ± 0.95 |
| Total protein (g dL−1) | 5.40 ± 0.05 | 5.57 ± 0.08 | 5.50 ± 0.11 | 5.37 ± 0.09 |
| Albumin (g dL−1) | 4.15 ± 0.058 | 4.30 ± 0.05 | 4.13 ± 0.12 | 4.13 ± 0.03 |
| GOT (UI L−1) | 127.50 ± 8.14 | 157.83 ± 11.46 | 167.50 ± 24.46 | 164.67 ± 19.81 |
| GPT (UI L−1) | 23.25 ± 2.28 | 21.83 ± 4.00 | 26.00 ± 1.71 | 29.00 ± 3.97 |
| Glucose (mg dL−1) | 100.80 ± 3.28 | 95.83 ± 3.06 | 90.78 ± 2.76 | 82.40 ± 2.55 |
| Enzyme | Control | 0.5 g kg−1 | 1.0 g kg−1 | 2.0 g kg−1 |
|---|---|---|---|---|
| SOD hemolyzed (U·mg−1 Hb) | 11.33 ± 2.02 | 9.00 ± 1.81 | 14.46 ± 2.78 | 13.27 ± 3.57 |
| SOD liver (U·mg−1 prot.) | 35.09 ± 1.80 | 34.55 ± 1.24 | 32.71 ± 1.18 | 35.51 ± 0.96 |
| GPx hemolyzed (U·mg−1 Hb) | 1422.02 ± 162.87 | 1036.83 ± 183.74 | 1568.59 ± 212.84 | 1368.04 ± 283.80 |
| GPx liver (U·mg−1 prot) | 1306.18 ± 39.35 | 536.75 ± 69.13 * | 487.49 ± 35.90 * | 528.55 ± 44.6 * |
| CAT hemolyzed (µmol H2O2·mg−1 Hb) | 752.60 ± 95.72 | 630.82 ± 143.89 | 884.28 ± 167.33 | 726.53 ± 182.14 |
| CAT liver (µmol H2O2·mg−1 Hb) | 1551.92 ± 38.42 | 1450.28 ± 37.02 | 1460.20 ± 61.52 | 1458.94 ± 35.48 |
| TBARS liver (nmol MDA·mg−1 prot) | 2.43 ± 0.21 | 1.33 ± 0.09 * | 1.53 ± 0.08 * | 1.82 ± 0.12 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balboa, E.M.; Millán, R.; Domínguez, H.; Taboada, C. Sargassum muticum Hydrothermal Extract: Effects on Serum Parameters and Antioxidant Activity in Rats. Appl. Sci. 2019, 9, 2570. https://doi.org/10.3390/app9122570
Balboa EM, Millán R, Domínguez H, Taboada C. Sargassum muticum Hydrothermal Extract: Effects on Serum Parameters and Antioxidant Activity in Rats. Applied Sciences. 2019; 9(12):2570. https://doi.org/10.3390/app9122570
Chicago/Turabian StyleBalboa, Elena M., Rosendo Millán, Herminia Domínguez, and Cristina Taboada. 2019. "Sargassum muticum Hydrothermal Extract: Effects on Serum Parameters and Antioxidant Activity in Rats" Applied Sciences 9, no. 12: 2570. https://doi.org/10.3390/app9122570
APA StyleBalboa, E. M., Millán, R., Domínguez, H., & Taboada, C. (2019). Sargassum muticum Hydrothermal Extract: Effects on Serum Parameters and Antioxidant Activity in Rats. Applied Sciences, 9(12), 2570. https://doi.org/10.3390/app9122570




