Study of Green Synthesis of Ultrasmall Gold Nanoparticles Using Citrus Sinensis Peel
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. C. Sinensis Peels Extract
2.3. Synthesis of AuNPs Using Fresh C. sinensis Peel Extract
2.4. Effect of pH on AuNPs Size Distribution and Morphology
2.5. Analysis and Characterization of AuNPs by UV-vis, EDS, TEM, DLS, FT-IR
2.6. Aging and 72 h-Incubation Test
2.7. Image Processing
3. Results and Discussion
3.1. Formation of AuNPs Using Fresh C. sinensis Peel Extract at Different pHs
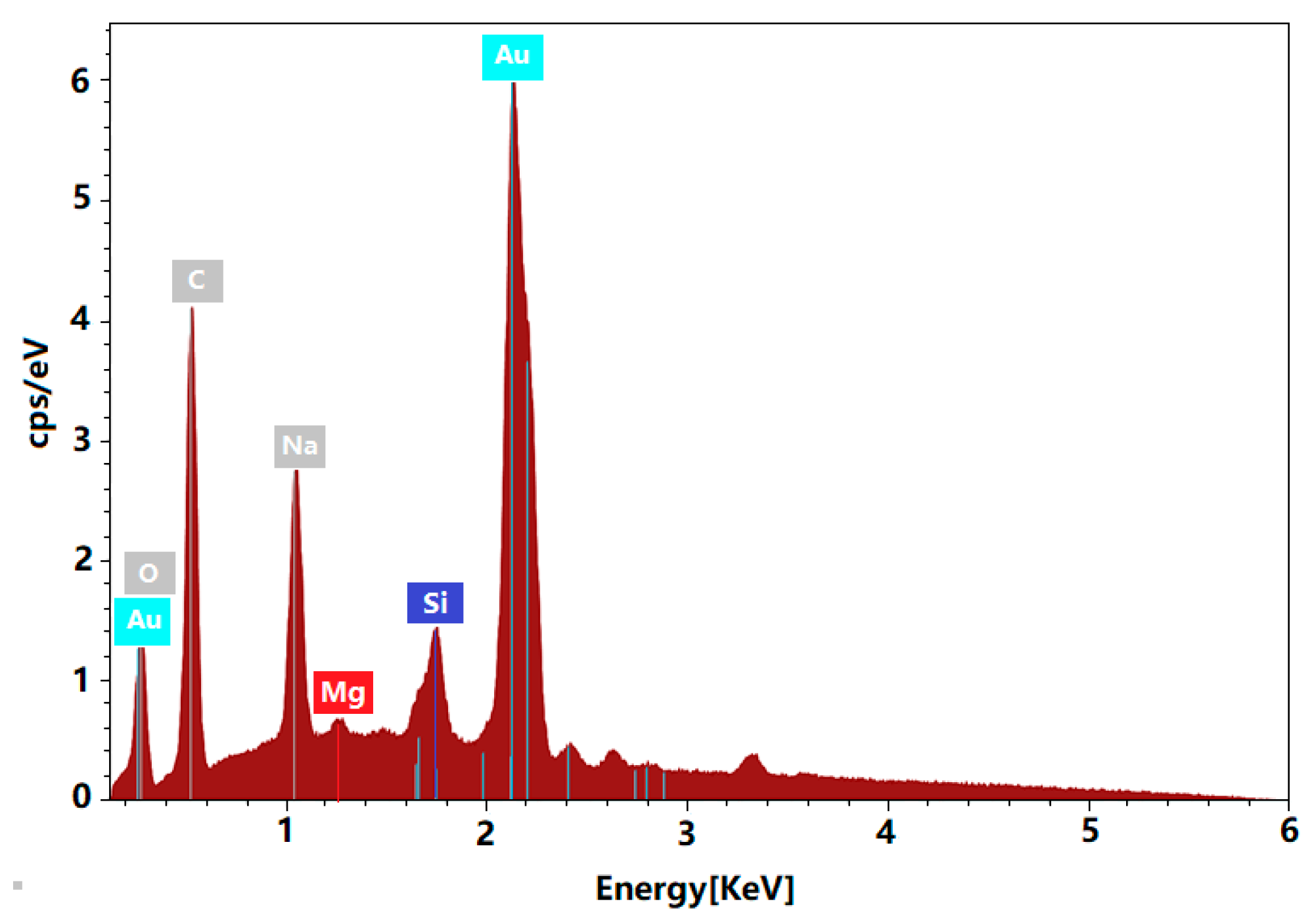
3.2. Elements Contained Analysis of AuNPs by EDS
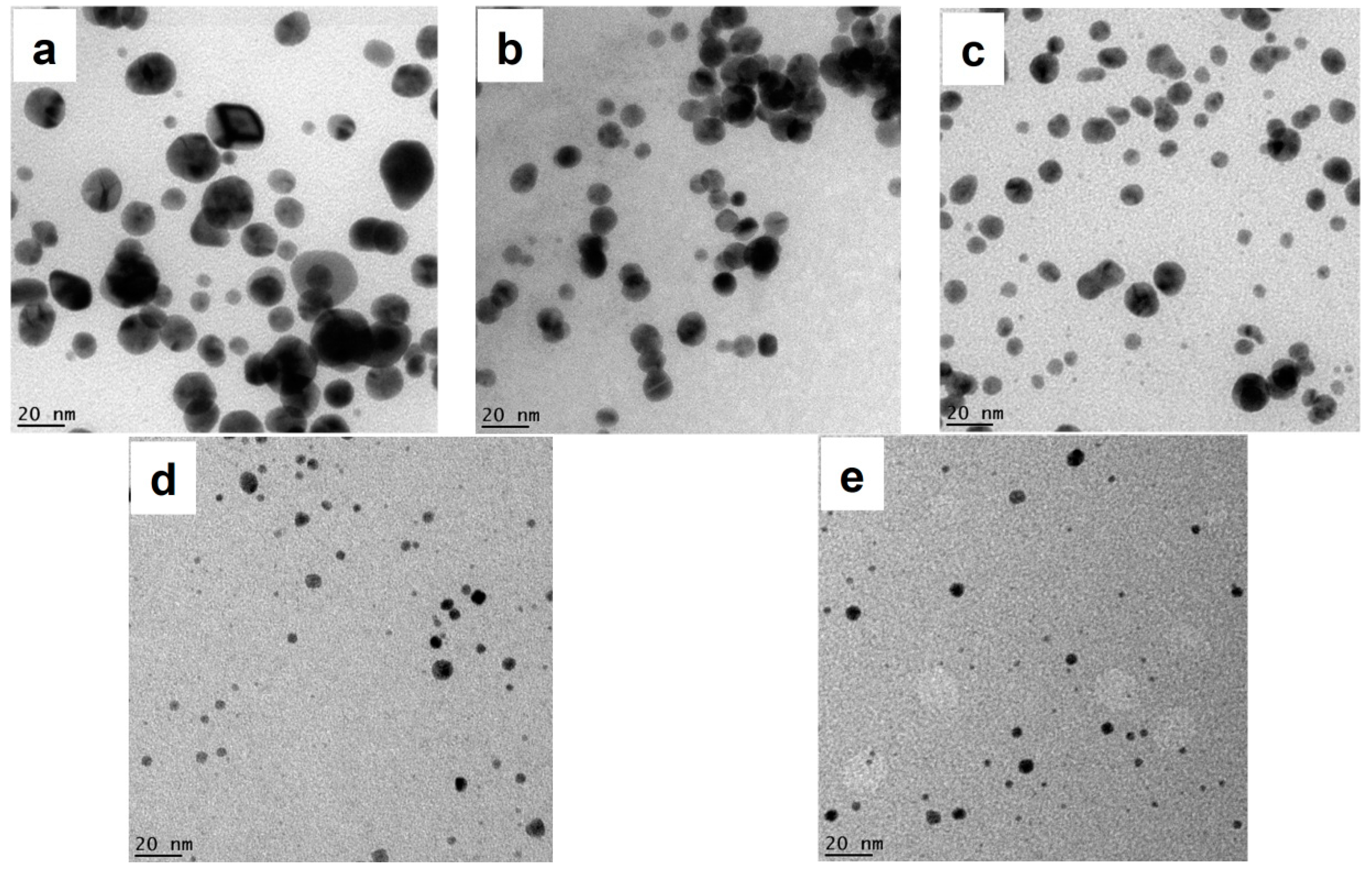
3.3. Morphology Analysis of AuNPs by TEM
3.4. Tuning AuNPs Size and Size Distribution
3.5. Hydrodynamic Diameter Analysis of AuNPs by DLS
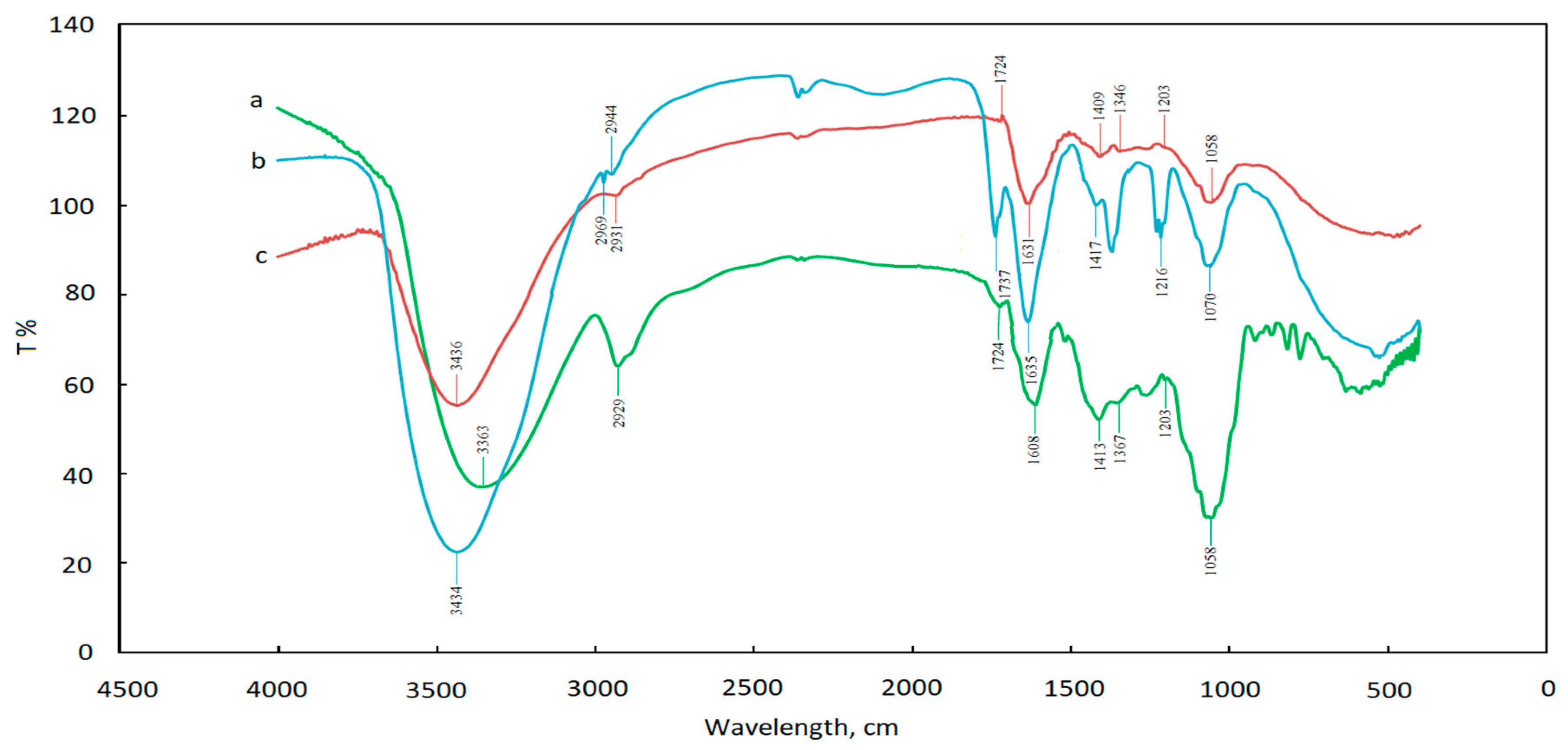
3.6. FT-IR Analysis of AuNPs
3.7. Stability of AuNPs at Room Temperature
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, K.X.; Shameli, K.; Miyake, M.; Kuwano, N.; Bt Ahmad Khairudin, N.B.; Bt Mohamad, S.E.; Yew, Y.P. Green synthesis of gold nanoparticles using aqueous extract of garcinia mangostana fruit peels. J. Nanomater. 2016, 2016, 8489094. [Google Scholar] [CrossRef]
- Islam, N.U.; Ahsan, F.; Khan, I.; Shah, M.R.; Shahid, M.; Khan, M.A. Green synthesis and biological activities of gold nanoparticles functionalized with Citrus reticulata, Citrus aurantium, Citrus sinensis and Citrus grandis. J. Chem. Soc. Pak. 2015, 37, 721–731. [Google Scholar]
- Chow, E. Gold Nanoparticles: Properties, Characterization and Fabrication; NOVA Science Publishers Inc.: New York, NY, USA, 2015. [Google Scholar]
- Trang, H.D.N.; Vardhanabhuti, B.; Lin, M.S.; Mustapha, A. Antibacterial properties of selenium nanoparticles and their toxicity to Caco-2 cells. Food Control 2017, 77, 17–24. [Google Scholar]
- Daniel, M.C.; Astruc, D. Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef] [PubMed]
- Freitas de Freitas, L.; Varca, G.H.C.; Dos Santos Batista, J.G.; Benevolo Lugao, A. An Overview of the Synthesis of Gold Nanoparticles Using Radiation Technologies. Nanomaterials 2018, 8, 939. [Google Scholar] [CrossRef] [PubMed]
- Veiko, V.P.; Tyurnina, A.E.; Shur, V.Y.; Kozin, R.V.; Kuznetsov, D.K.; Mingaliev, E.A.; Vartanyan, T.A. Synthesis of stable silver colloids by laser ablation in water. Int. Soc. Opt. Photonics 2013, 9065, 90650D. [Google Scholar]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D.J.; Whyman, R. Synthesis of thiol-derivatised gold nanoparticles in a two-phase liquid–liquid system. J. Chem. Soc. Chem. Commun. 1994, 7, 801–802. [Google Scholar] [CrossRef]
- Turner, M.; Golovko, V.B.; Vaughan, O.P.H.; Abdulkin, P.; Berenguer-Murcia, A.; Tikhov, M.S.; Johnson, B.F.G.; Lambert, R.M. Selective oxidation with dioxygen by gold nanoparticle catalysts derived from 55-atomclusters. Nature 2008, 454, 981–983. [Google Scholar] [CrossRef] [PubMed]
- Mandal, T.K.; Fleming, M.S.; Walt, D.R. Preparation of polymer coated gold nanoparticles by surface-confined living radical polymerization at ambient temperature. Nano Lett. 2002, 2, 3–7. [Google Scholar] [CrossRef]
- Browning, L.M.; Lee, K.J.; Huang, T.; Nallathamby, P.D.; Lowman, J.E.; Xu, X.H.N. Random walk of single gold nanoparticles inzebrafish embryos leading to stochastic toxic effects on embryonicdevelopments. Nanoscale 2009, 1, 138–152. [Google Scholar] [CrossRef]
- Leifert, A.; Pan-Bartnek, Y.; Simon, U.; Jahnen-Dechent, W. Molecularly stabilised ultrasmall gold nanoparticles: Synthesis, characterization and bioactivity. Nanoscale Res. Lett. 2013, 5, 6224–6242. [Google Scholar] [CrossRef] [PubMed]
- Deraedt, C.; Salmon, L.; Gatard, S.; Ciganda, R.; Hernandez, R.; Didier, J.R.; Astruc, D. Sodium borohydride stabilizes very active goldnanoparticle catalysts. Chem. Commun. 2014, 50, 14194–14196. [Google Scholar] [CrossRef] [PubMed]
- Tajammul Hussain, S.; Iqbal, M.; Mazhar, M. Size control synthesis of starch capped-gold nanoparticles. J. Nanopart. Res. 2009, 11, 1383–1391. [Google Scholar] [CrossRef]
- Eustis, S.; Hsu, H.Y.; EI-Sayed, M.A. Gold nanoparticle formation from photochemical reduction of Au3+ by continuous excitation in colloidal solutions: A proposed molecular mechanism. J. Phys. Chem. B 2005, 109, 4811–4815. [Google Scholar] [CrossRef] [PubMed]
- Djafari, J.; McConnell, M.T.; Santos, H.M.; Capelo, J.L.; Bertolo, E.; Harvey, S.C.; Lodeiro, C.; Fernandez-Lodeiro, J. Synthesis of Gold Functionalised Nanoparticles with the Eranthis hyemalis Lectin and Preliminary Toxicological Studies on Caenorhabditis elegans. Materials 2018, 11, 1363. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Fawcett, D.; Sharma, S.; Tripathy, S.K.; Poinern, G.E.J. Green Synthesis of Metallic Nanoparticles via Biological Entities. Materials 2015, 8, 7278–7308. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.J.; Gole, A.M.; Stone, J.W.; Sisco, P.N.; Alkilany, A.M.; Goldsmith, E.C.; Baxter, S.C. Gold nanoparticles in biology: Beyond toxicity to cellular imaging. Acc. Chem. Res. 2008, 41, 1721–1730. [Google Scholar] [CrossRef] [PubMed]
- Connor, E.E.; Mwamuka, J.; Gole, A.; Murphy, C.J.; Wyatt, M.D. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small 2005, 1, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Neuss, S.; Leifert, A.; Fischler, M.; Wen, F.; Simon, U.; Schmid, G.; Brandau, W.; Jahnen-Dechent, W. Size-dependent cytotoxicity of gold nanoparticles. Small 2007, 3, 1941–1949. [Google Scholar] [CrossRef]
- Ray, P.C.; Yu, H.; Fu, P.P. Toxicity and environmental risks of nanomaterials: Challenges and future needs. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2009, 27, 1–35. [Google Scholar] [CrossRef]
- Yang, B.; Chou, J.; Dong, X.Q.; Qu, C.T.; Yu, Q.S.; Lee, K.J.; Harvey, N. Size-controlled green synthesis of highly stable and uniform small to ultrasmall gold nanoparticles by controlling reaction steps and pH. J. Phys. Chem. C 2017, 121, 8961–8967. [Google Scholar] [CrossRef]
- Dauthal, P.; Mukhopadhyay, M. Phyto-synthesis and structural characterization of catalytically active gold nanoparticles biosynthesized using Delonix regia leaf extract. 3 Biotech 2016, 6, 118. [Google Scholar] [CrossRef] [PubMed]
- Chou, J.; Li, X.F.; Yin, Y.F.; Indrisek, N. Determination of antioxidant activities in fruit juices based on rapid colorimetric measurement and characterisation of gold nanoparticles. Int. J. Environ. Anal. Chem. 2015, 95, 531–541. [Google Scholar] [CrossRef]
- Dhanasekar, N.N.; Rahul, G.R.; Narayanan, K.B.; Raman, G.; Sakthivel, N. Green chemistry cpproach for the synthesis of gold nanoparticles using the fungus Alternaria sp. J. Microbiol. Biotechnol. 2015, 25, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Khanehzaei, H.; Ahmad, M.B.; Shameli, K.; Ajdari, Z.; Ghani, M.A.; Kalantari, K. Effect of seaweed Kappaphycus alvareziiin the synthesis of Cu@Cu2O core–shell nanoparticles prepared by chemical reduction method. Res. Chem. Int. 2015, 41, 7363–7376. [Google Scholar] [CrossRef]
- Marín, F.R.; Soler-Rivas, C.; Benavente-García, O.; Castillo, J.; Pérez-Alvarezc, J.A. By-products from different citrus processes as a source of customized functional fibres. Food Chem. 2007, 99, 736–741. [Google Scholar] [CrossRef]
- Braddock, R.J. Handbook Ofcitrus By-Products and Processing Technology; Wiley: New York, NY, USA, 1999. [Google Scholar]
- Balu, A.M.; Budarin, V.; Shuttleworth, P.S.; Pfaltzgraff, L.A.; Waldron, K.; Luque, R.; Clark, J.H. Valorisation of orange peel residues: Waste to biochemicals and nanoporous materials. ChemSusChem 2012, 5, 1694–1697. [Google Scholar] [CrossRef] [PubMed]
- Torrado, A.M.; Cortes, S.; Manuel Salgado, J.; Max, B.; Rodriguez, N.; Bibbins, B.P.; Converti, A.; Manuel Dominguez, J. Citric Acid production from orange peel wastes by solid-state fermentation. Braz. J. Microbiol. 2011, 42, 394–409. [Google Scholar] [CrossRef] [PubMed]
- Kahrilas, G.A.; Wally, L.M.; Fredrick, S.J.; Hiskey, M.; Prieto, A.L.; Owens, J.E. Microwave-assisted green synthesis of silver nanoparticles using orange peel extract. ACS Sustain. Chem. Eng. 2014, 2, 367–376. [Google Scholar] [CrossRef]
- Castro, L.; Blázquez, M.L.; González, F.; Muñoz, A.; Ballester, A. Gold, silver and platinum nanoparticles biosynthesized using orange peel extract. Adv. Mater. Res. 2013, 825, 556–559. [Google Scholar] [CrossRef]
- Singh, P.; Kim, Y.; Zhang, D.; Yang, D. Biological synthesis of nanoparticles from plants an microoranganisms. Trends Biotechnol. 2016, 3, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Socrates, G. Infrared Characteristic Group Frequencies; Wiley-Interscience Publication: New York, NY, USA, 1980. [Google Scholar]
- Rao, K.J.; Paria, S. Aegle marmelos leaf extract and plant surfactants mediated green synthesis of Au and Ag nanoparticles by optimizing process parameters using Taguchi method. ACS Sustain. Chem. Eng. 2015, 3, 438–491. [Google Scholar] [CrossRef]
- Shameli, K.; Ahmad, M.B.; Shabanzadeh, P.; Al-Mulla, E.A.J.; Zamanian, A.; Abdollahi, Y.; Jazayeri, S.D.; Eili, M.; Jalilian, F.A.; Haroun, R.Z. Effect of Curcuma longa tuber powder extract on size of silver nanoparticles prepared by green method. Res. Chem. Int. 2013, 40, 1313–1325. [Google Scholar] [CrossRef]
- Bogireddy, N.K.R.; Anand, K.K.H.; Mandal, B.K. Gold nanoparticles—Synthesis by sterculia acuminata extract and its catalytic efficiency in alleviating different organic dyes. J. Mol. Liq. 2015, 211, 868–875. [Google Scholar] [CrossRef]
- Lee, K.J.; Nallathamby, P.D.; Browning, L.M.; Osgood, C.J.; Xu, X.H.N. In vivo imaging of transport and biocompatibility of single silver nanoparticles in early development of zebrafish embryos. ACS Nano 2007, 1, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Browning, L.M.; Nallathamby, P.D.; Osgood, C.J.; Xu, X.H. Silver nanoparticles induce developmental stage-specific embryonic phenotypes in zebrafish. Nanoscale 2013, 5, 11625–11636. [Google Scholar] [CrossRef] [PubMed]
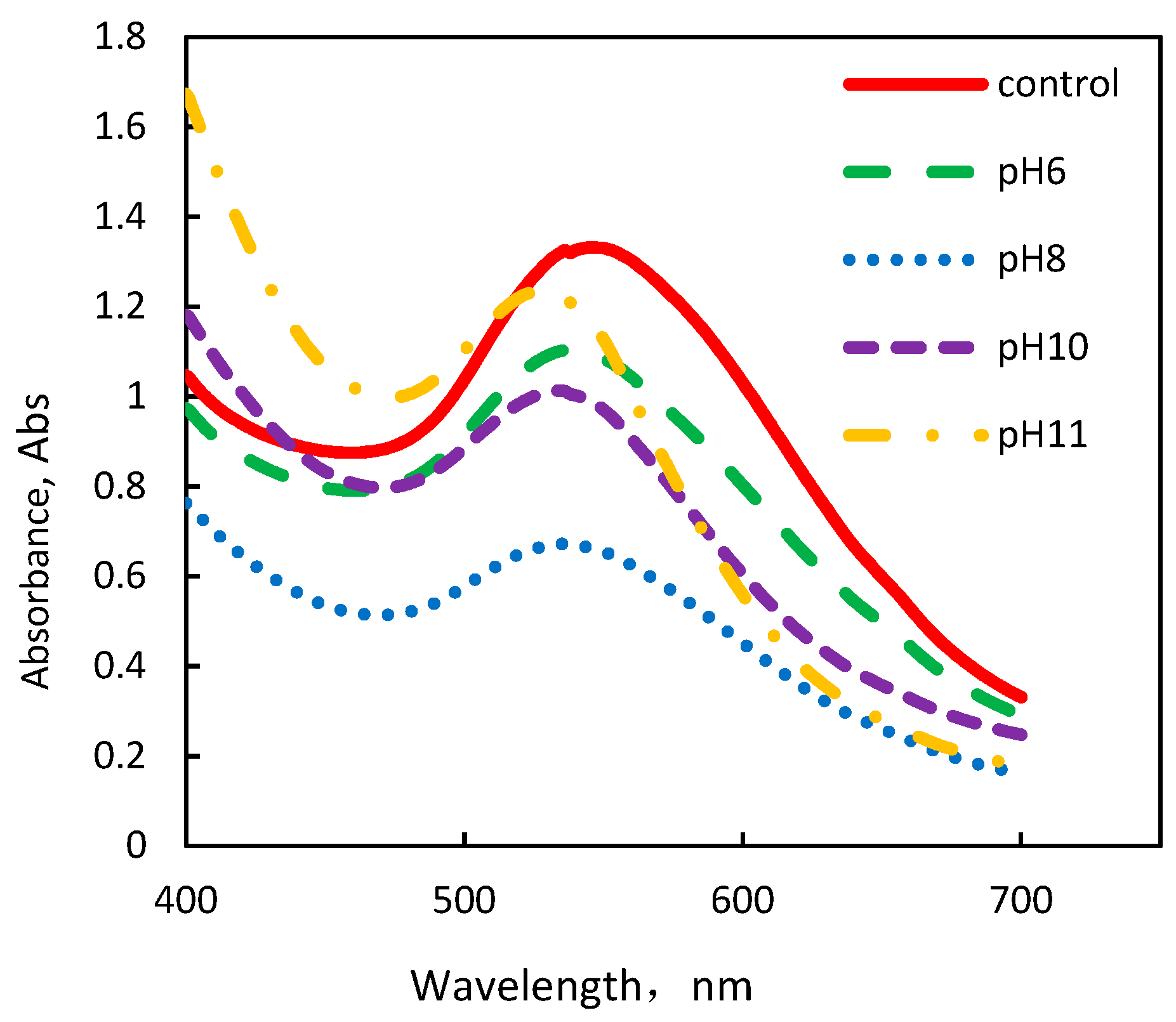
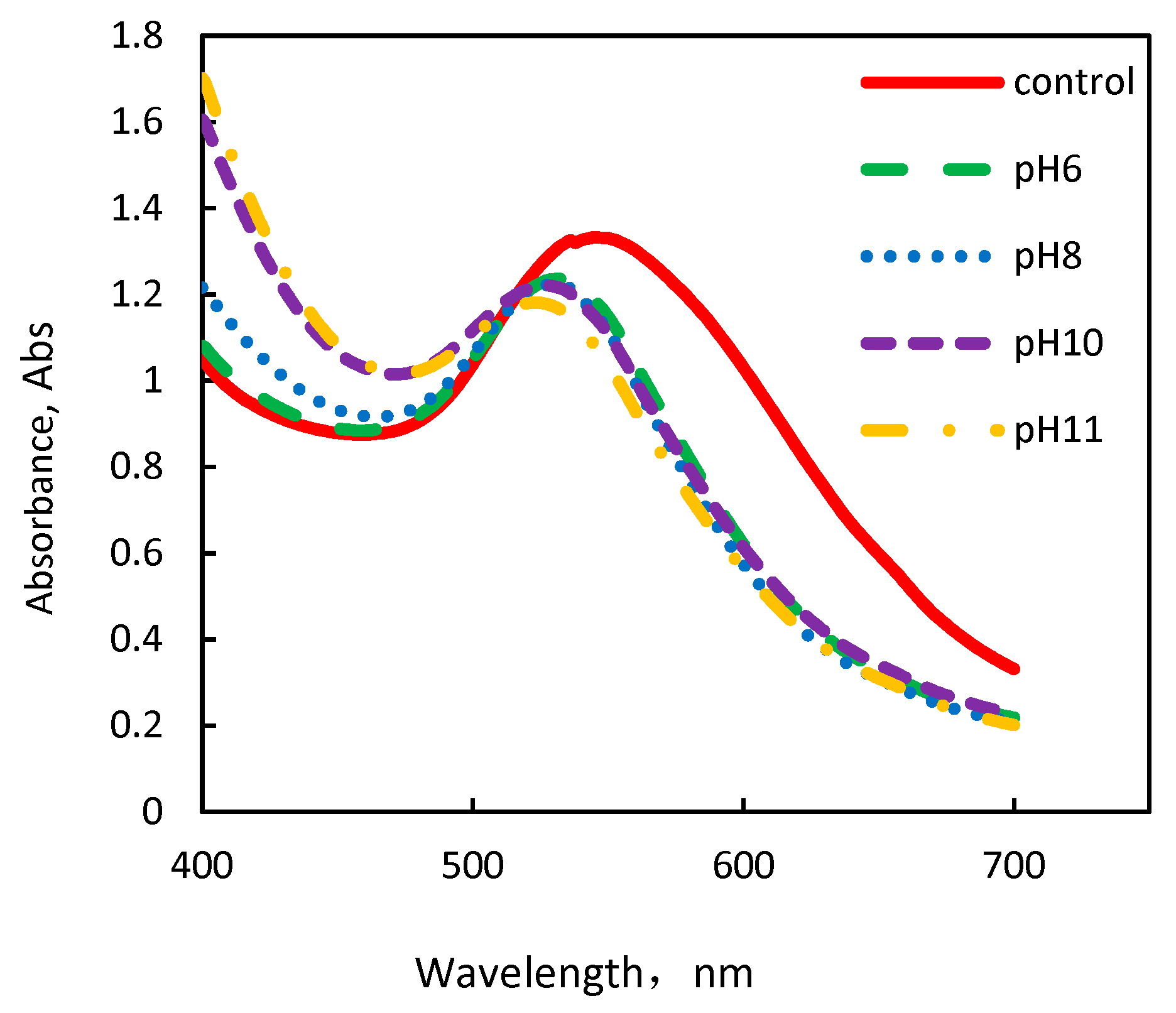
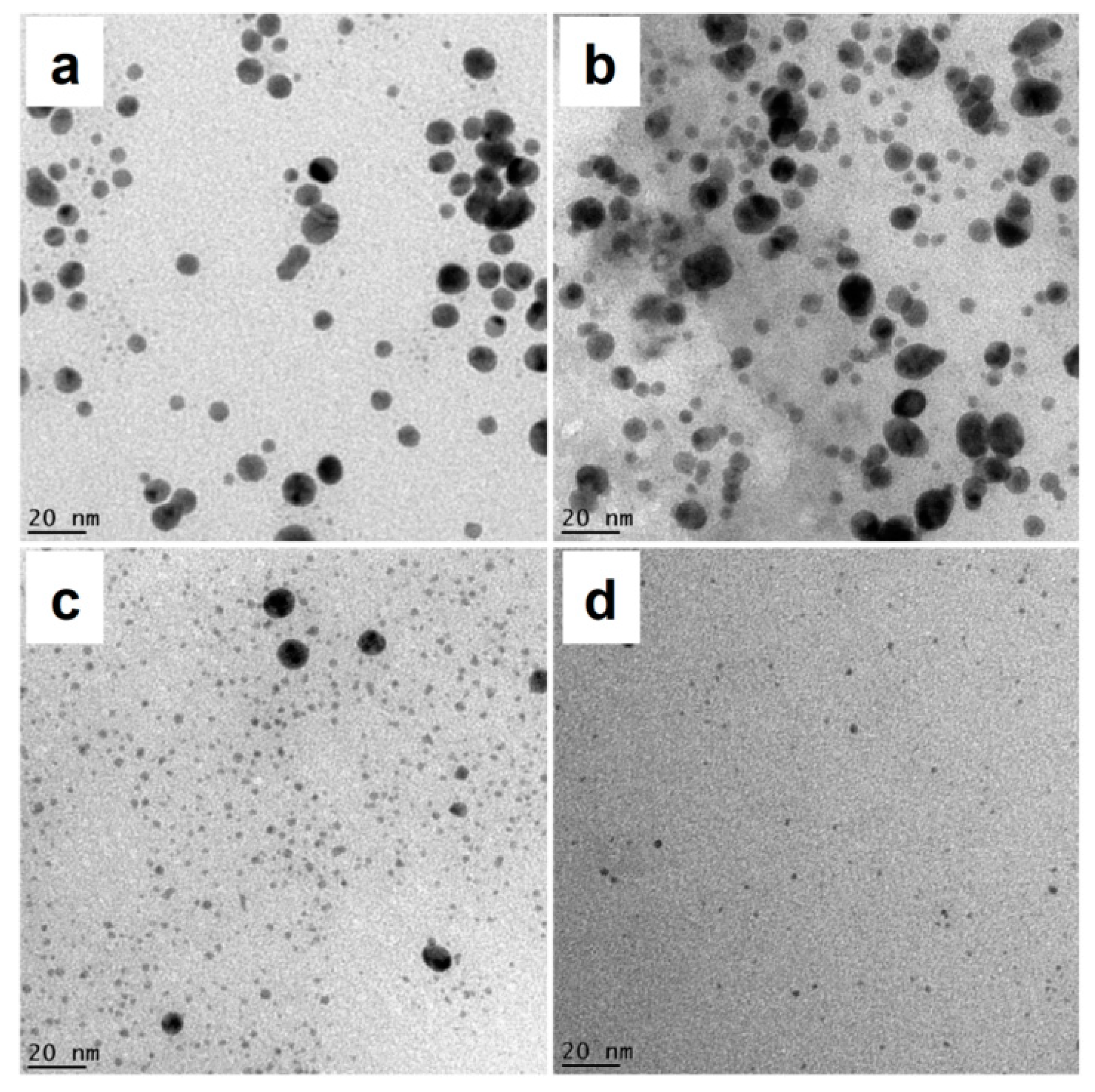
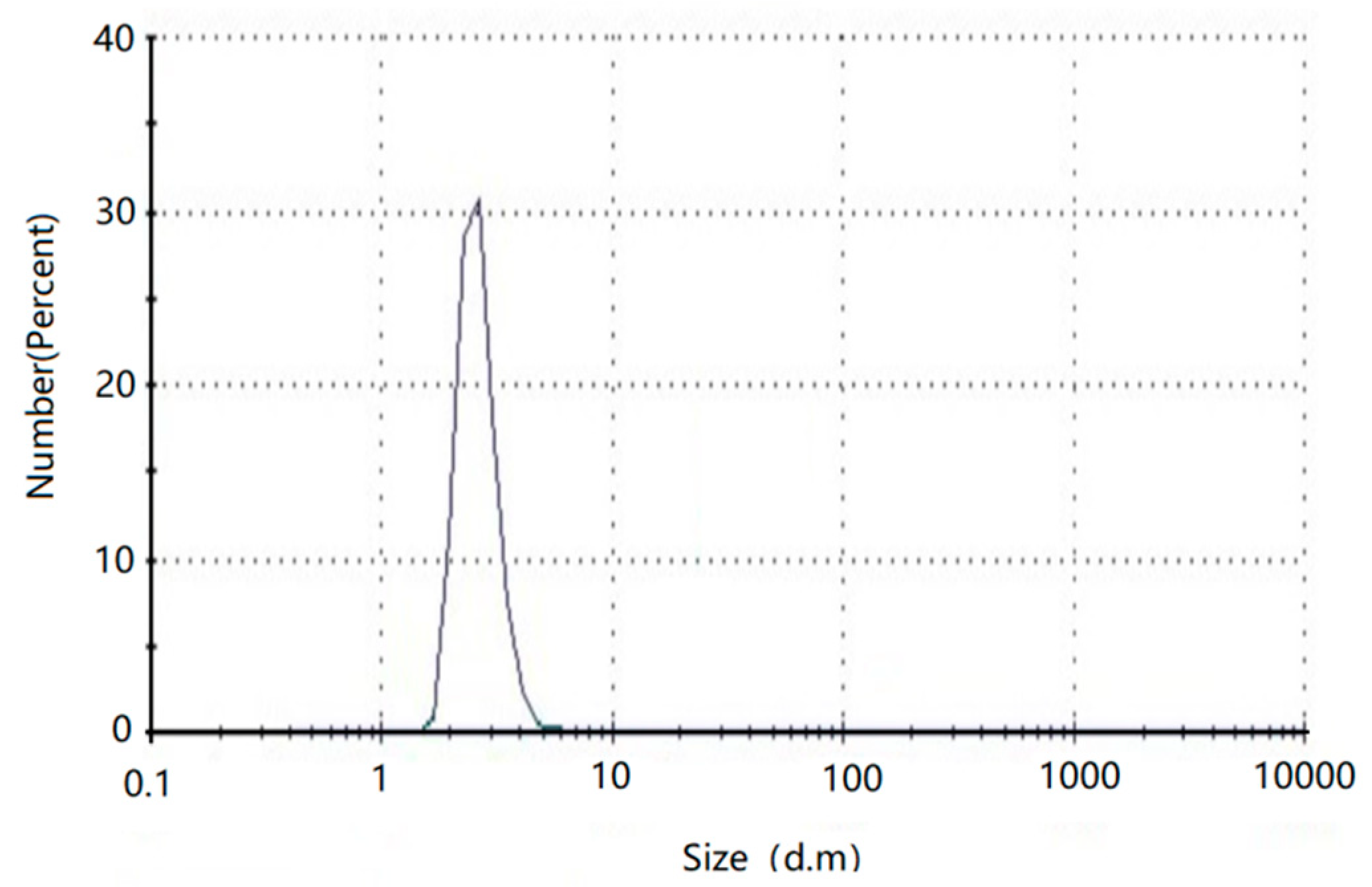
| Size (nm) | Absorbance | λmax (nm) | Size(nm) | Absorbance | λmax (nm) | |
|---|---|---|---|---|---|---|
| Control | 12.32 ± 4.56 | 1.33 | 546 | 12.32 ± 4.56 | 1.33 | 546 |
| Adjust pH before Reaction (nm) | Adjust pH after Reaction (nm) | |||||
| pH 6 | 9.33 ± 2.37 | 1.10 | 536 | 4.91 ± 3.03 | 1.24 | 531 |
| pH 8 | 7.15 ± 3.38 | 0.67 | 536 | 6.33 ± 2.56 | 1.22 | 530 |
| pH 10 | 3.07 ± 1.69 | 1.01 | 534 | 1.99 ± 0.84 | 1.22 | 526 |
| pH 11 | 2.79 ± 1.53 | 1.24 | 529 | 1.75 ± 0.86 | 1.18 | 522 |
| Bond | Type of Vibration | Control Spectrum (cm−1) | Adjust pH before Reaction (cm−1) | Adjust pH after Reaction (cm−1) |
|---|---|---|---|---|
| O-H | Alcohol, phenols, Carboxylic acids | 3363 | 3434 | 3436 |
| N-H | Amines | |||
| C-H | Alkanes | 2929 | 2969, 2944 | 2931 |
| C=O | Carboxylic acid or Ester | 1724 | 1737 | 1724 |
| N-C=O | Amide I bond of proteins | 1608 | 1635 | 1631 |
| CH2 | Alkanes | 1367 | 1363 | 1346 |
| C-O | Carboxylic acid, ester, or ether | 1203 | 1216 | 1203 |
| C-N | Aliphatic amines or alcohol/phenol | 1058 | 1070 | 1058 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; Qi, F.; Tan, J.; Yu, T.; Qu, C. Study of Green Synthesis of Ultrasmall Gold Nanoparticles Using Citrus Sinensis Peel. Appl. Sci. 2019, 9, 2423. https://doi.org/10.3390/app9122423
Yang B, Qi F, Tan J, Yu T, Qu C. Study of Green Synthesis of Ultrasmall Gold Nanoparticles Using Citrus Sinensis Peel. Applied Sciences. 2019; 9(12):2423. https://doi.org/10.3390/app9122423
Chicago/Turabian StyleYang, Bo, Feng Qi, Jingwen Tan, Tao Yu, and Chengtun Qu. 2019. "Study of Green Synthesis of Ultrasmall Gold Nanoparticles Using Citrus Sinensis Peel" Applied Sciences 9, no. 12: 2423. https://doi.org/10.3390/app9122423
APA StyleYang, B., Qi, F., Tan, J., Yu, T., & Qu, C. (2019). Study of Green Synthesis of Ultrasmall Gold Nanoparticles Using Citrus Sinensis Peel. Applied Sciences, 9(12), 2423. https://doi.org/10.3390/app9122423





