Microwave-Assisted Hydrothermal Synthesis of SrTiO3:Rh for Photocatalytic Z-scheme Overall Water Splitting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Rh-Doped SrTiO3 with Ru Cocatalyst for H2 Evolution
2.2. Synthesis of BiVO4 for O2 Evolution
2.3. Synthesis of [Co(bpy)3]SO4 for Electron Mediator
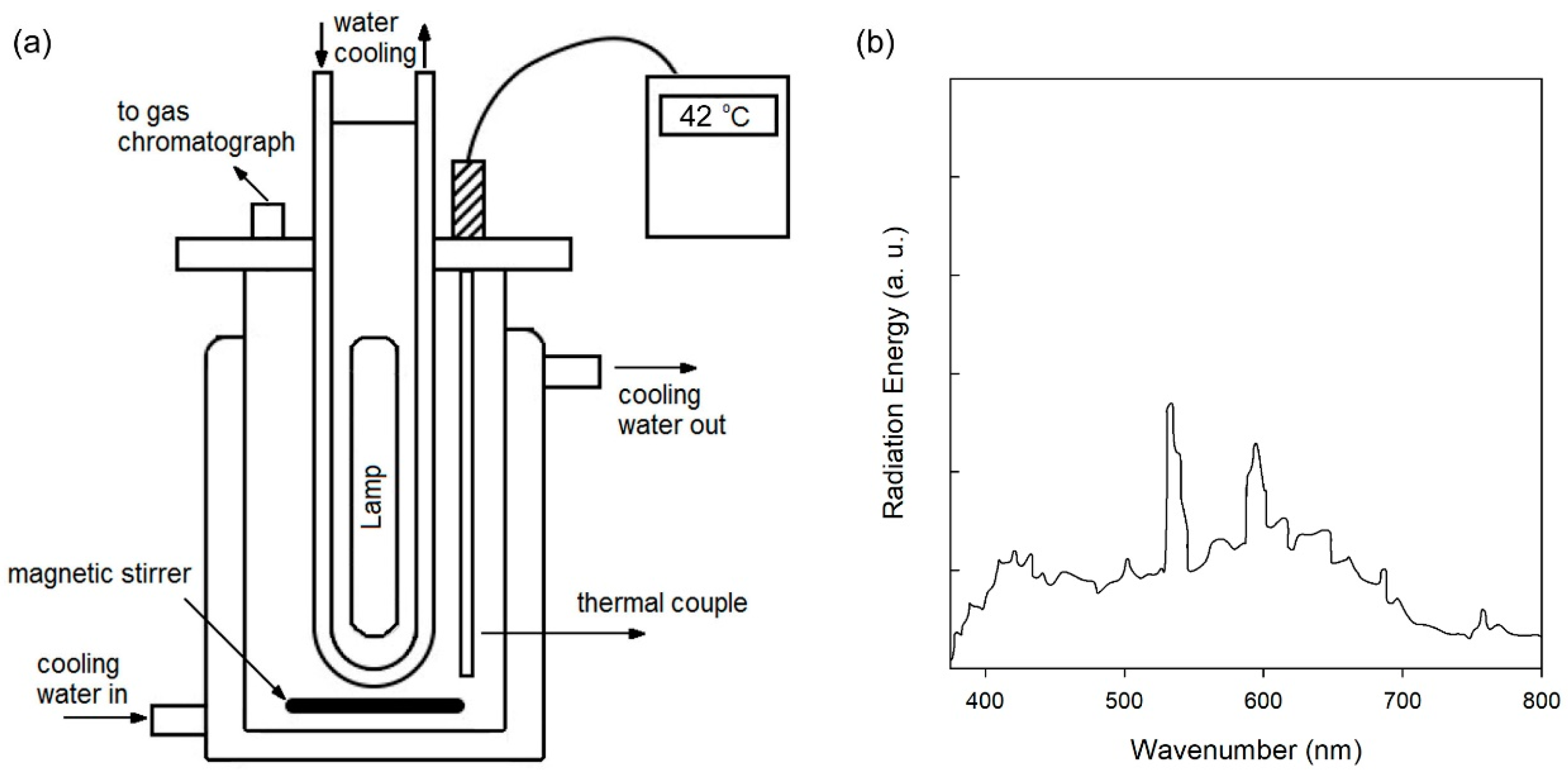
2.4. Characterization and Photocatalytic Reactions
3. Results and Discussion
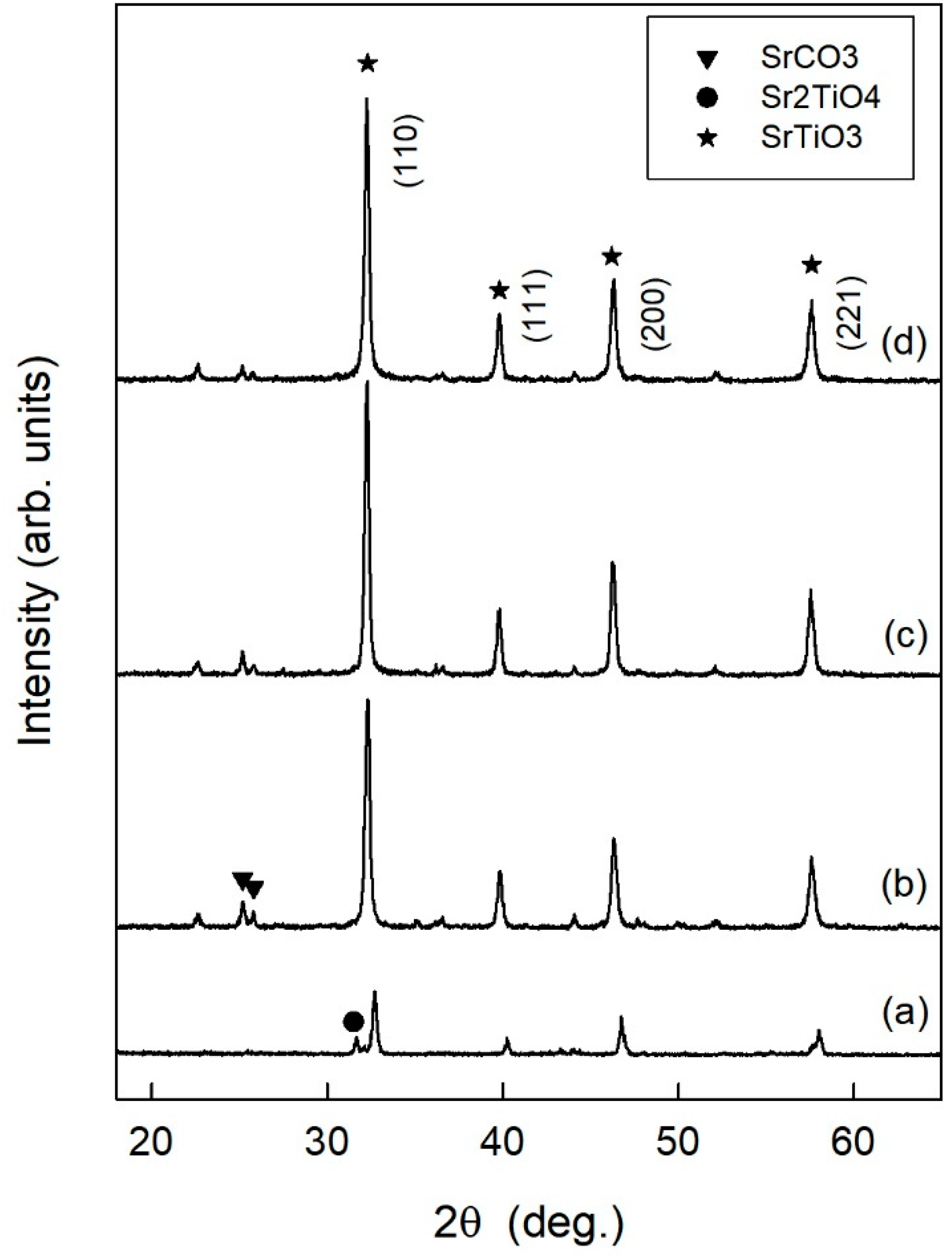
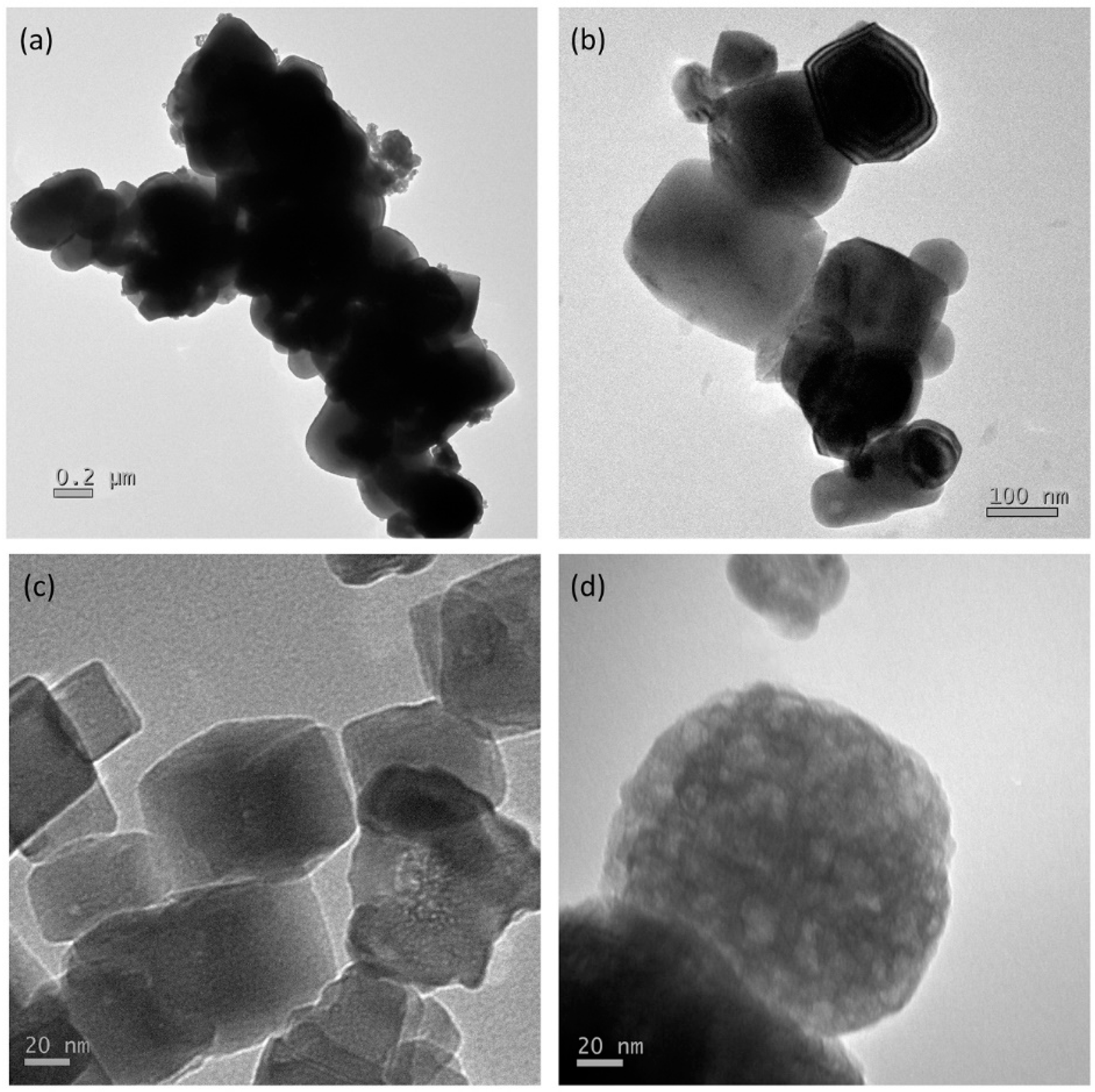
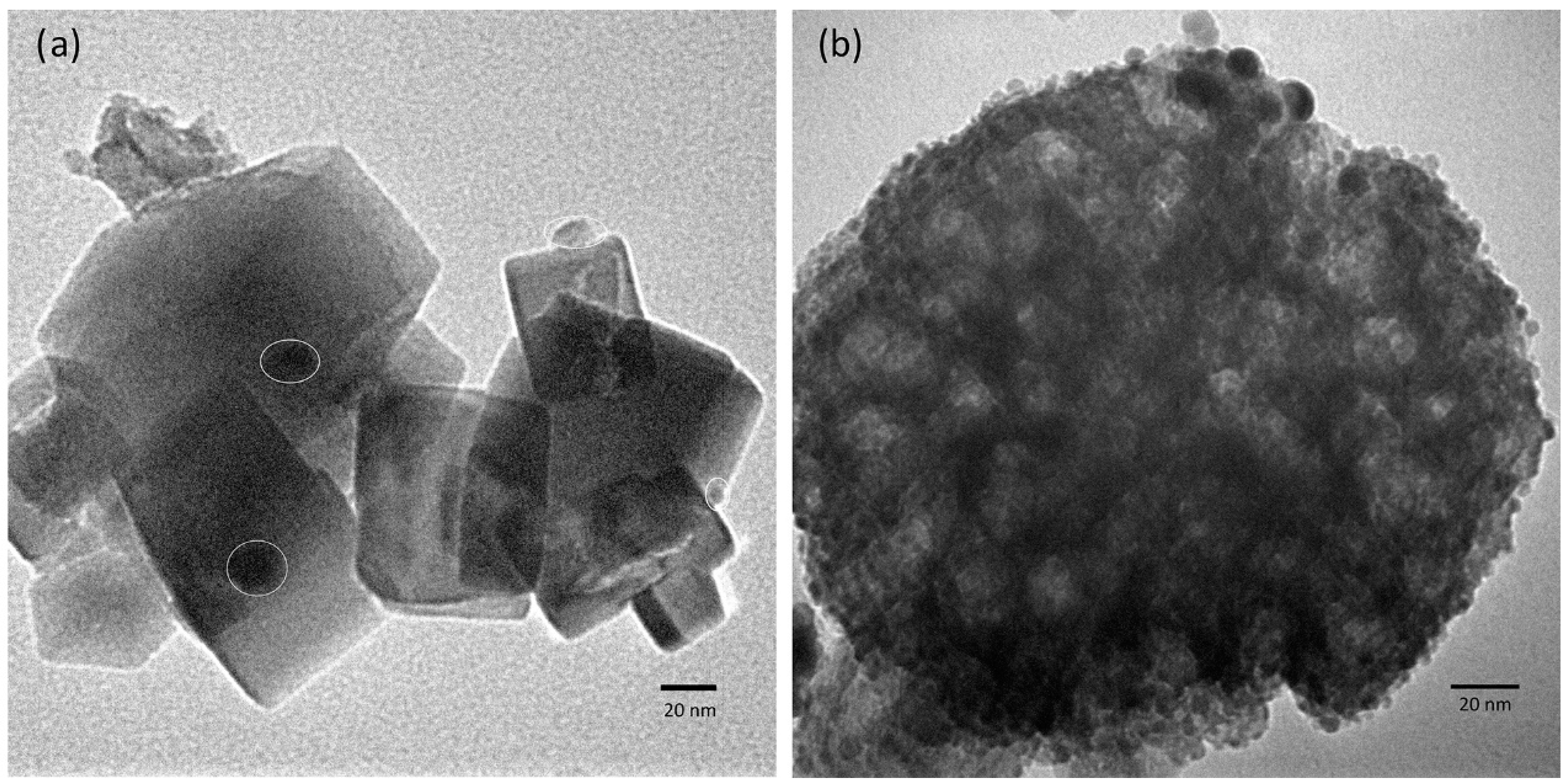
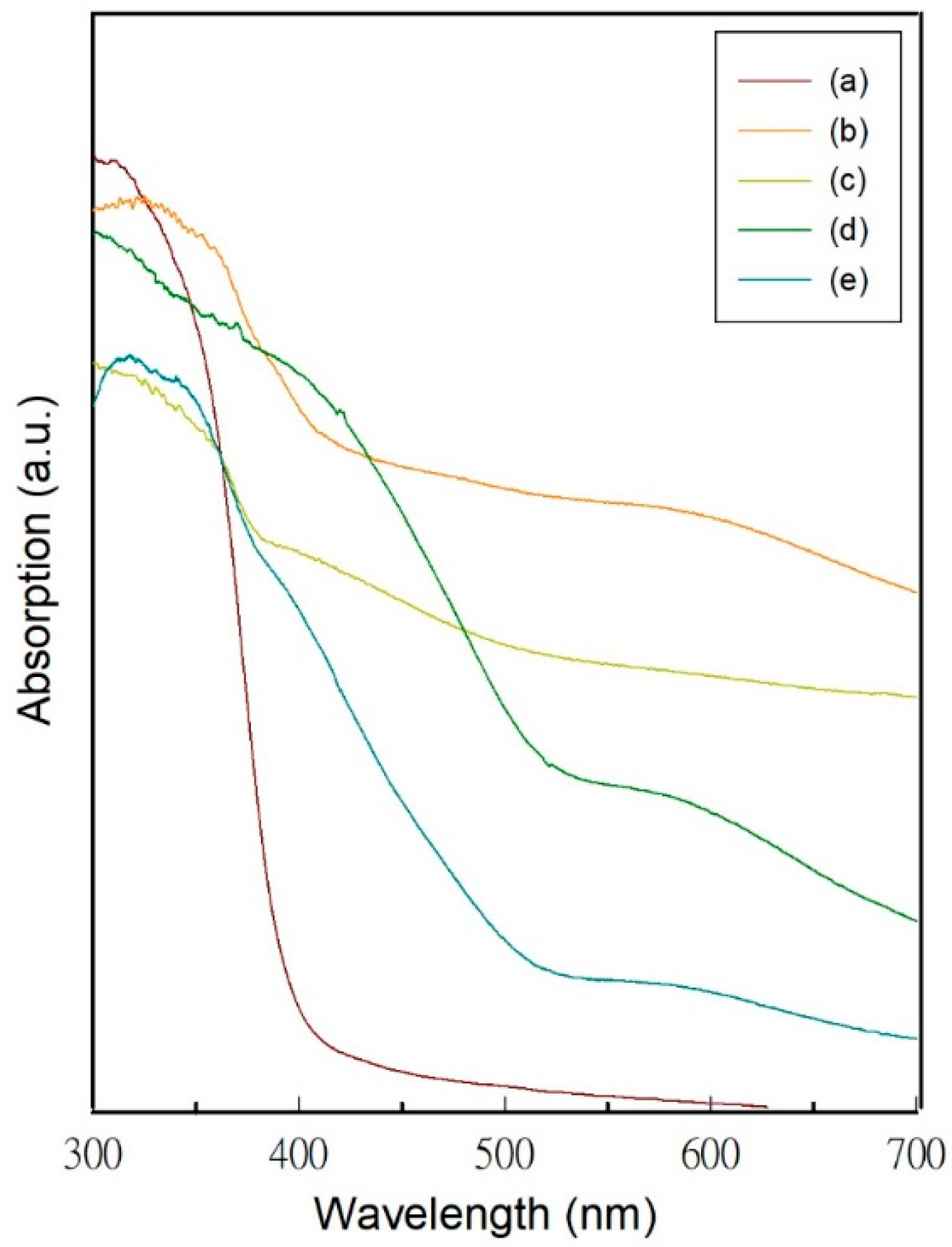
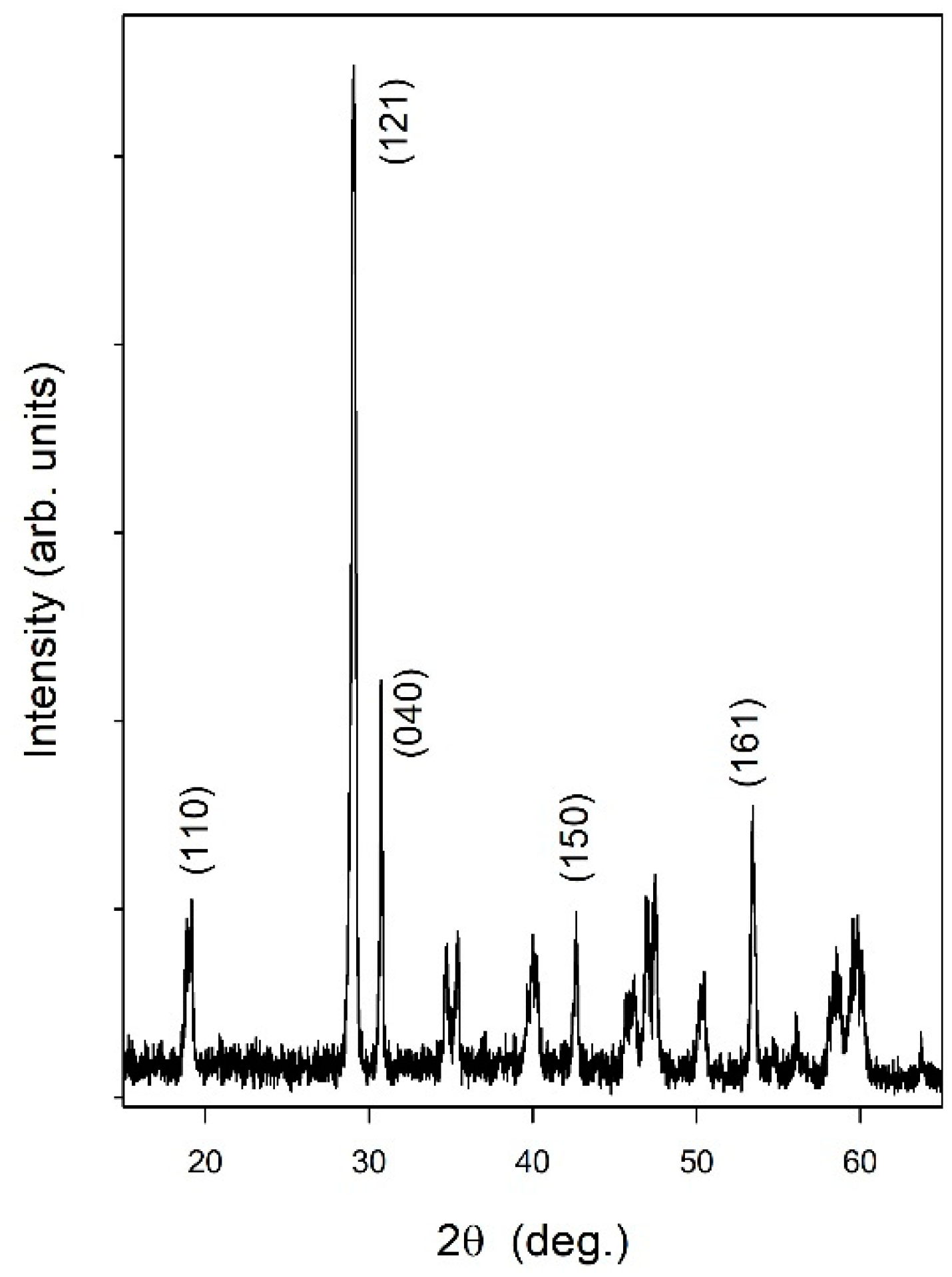
3.1. Characterization of the Hydrogen Evolution Catalyst: Rh-Doped SrTiO3
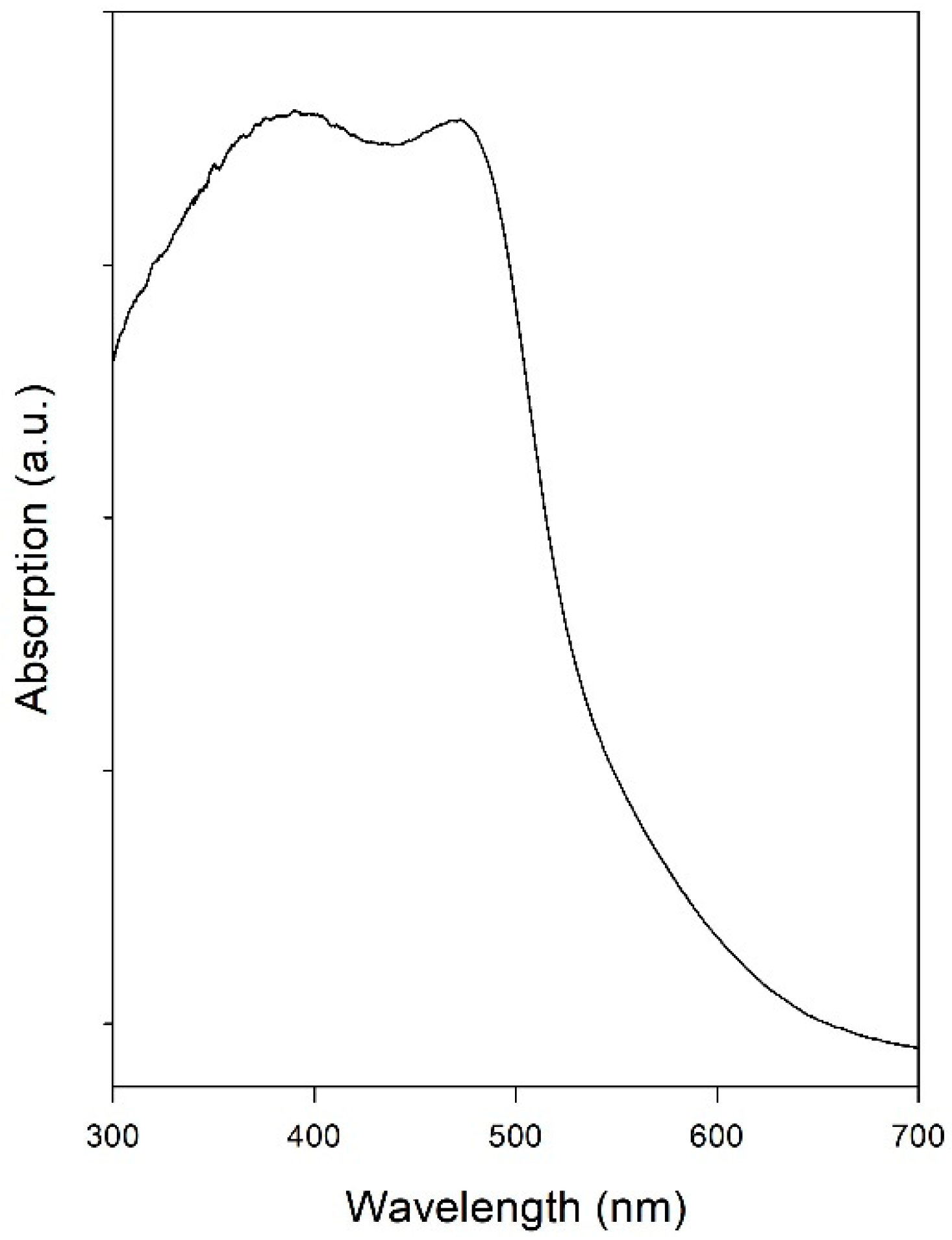
3.2. Characterization of the Oxygen Evolution Catalyst: BiVO4
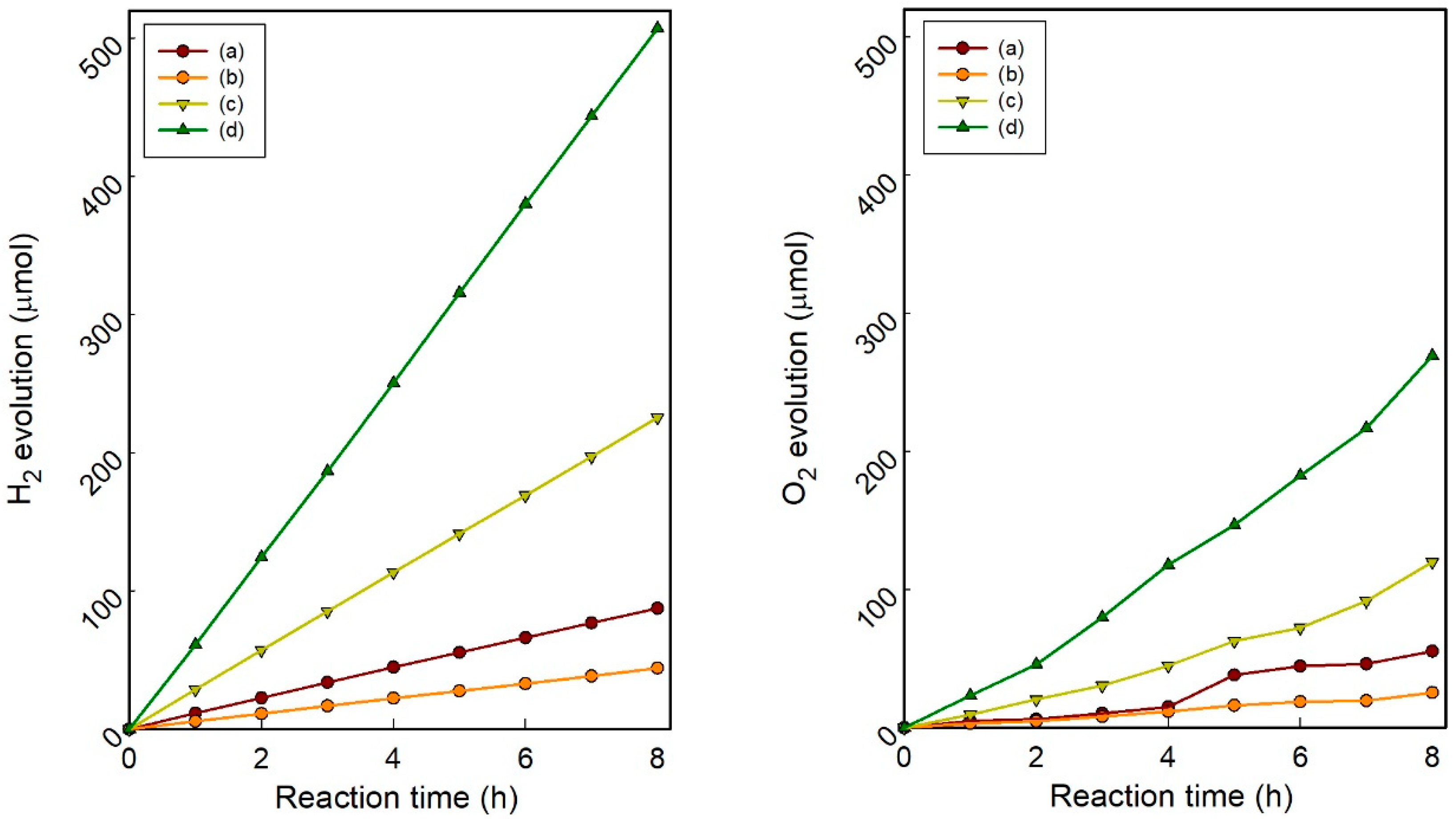
3.3. Photocatalytic Water-Splitting Activity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Asakura, K.; Kudo, A. Highly Efficient water splitting into H2 and O2 over lanthanum-doped NaTaO3 photocatalysts with high crystallinity and surface nanostructure. J. Am. Chem. Soc. 2003, 125, 3082–3089. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Lee, T.H.; Sie, C.Y. Photocatalytic hydrogen production with nickel oxide intercalated K4Nb6O17 under visible light irradiation. Int. J. Hydrogen Energy 2008, 33, 4055–4063. [Google Scholar] [CrossRef]
- Sato, J.; Saito, N.; Nishiyama, H.; Inoue, Y. Photocatalytic water decomposition by RuO2-loaded antimonates, M2Sb2O7 (M = Ca, Sr), CaSb2O6 and NaSbO3, with d10 configuration. J. Photochem. Photobiol. A 2002, 148, 85. [Google Scholar] [CrossRef]
- Takata, T.; Tanaka, A.; Hara, M.; Kondo, J.N.; Domen, K. Recent progress of photocatalysts for overall water splitting. Catal. Today 1998, 44, 17–26. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Low, J.; Fang, Y.; Xiao, J.; Chen, X. Engineering heterogeneous semiconductors for solar water splitting. J. Mater. Chem. A 2015, 3, 2485–2534. [Google Scholar] [CrossRef]
- Hisatomi, T.; Kubota, J.; Domen, K. Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem. Soc. Rev. 2014, 43, 7520–7535. [Google Scholar] [CrossRef]
- Suzuki, T.M.; Iwase, A.; Tanaka, H.; Sato, S.; Kudo, A.; Morikawa, T. Z-scheme water splitting under visible light irradiation over powdered metal-complex/semiconductor hybrid photocatalysts mediated by reduced graphene oxide. J. Mater. Chem. A 2015, 3, 13283–13290. [Google Scholar] [CrossRef]
- Lin, H.Y.; Shih, C.Y. Efficient one-pot microwave-assisted hydrothermal synthesis of M (M = Cr, Ni, Cu, Nb) and nitrogen co-doped TiO2 for hydrogen production by photocatalytic water splitting. J. Mol. Catal. A Chem. 2016, 411, 128–137. [Google Scholar] [CrossRef]
- Sasaki, Y.; Nemoto, H.; Saito, K.; Kudo, A. Solar Water Splitting Using Powdered Photocatalysts Driven by Z-Schematic Interparticle Electron Transfer without an Electron Mediator. J. Phys. Chem. C 2009, 113, 17536–17542. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Kato, H.; Kudo, A. [Co(bpy)3]3+/2+ and [Co(phen)3]3+/2+ Electron Mediators for Overall Water Splitting under Sunlight Irradiation Using Z-Scheme Photocatalyst System. J. Am. Chem. Soc. 2013, 135, 5441–5449. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Lu, D.; Domen, K. Solar-Driven Z-scheme Water Splitting Using Modified BaZrO3-BaTaO2N Solid Solutions as Photocatalysts. ACS Catal. 2013, 3, 1026–1033. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Y.; Hisatomi, T.; Nakabayashi, M.; Shibata, N.; Kubota, J.; Domen, K. Z-scheme water splitting using particulate semiconductors immobilized onto metal layers for efficient electron relay. J. Catal. 2015, 328, 308–315. [Google Scholar] [CrossRef]
- Kato, H.; Sasaki, Y.; Shirakura, N.; Kudo, A. Synthesis of highly active rhodium-doped SrTiO3 powders in Z-scheme systems for visible-light-driven photocatalytic overall water splitting. J. Mater. Chem. A 2013, 1, 12327–12333. [Google Scholar] [CrossRef]
- Tonda, S.; Kumar, S.; Anjaneyulu, O.; Shanker, V. Synthesis of Cr and La-codoped SrTiO3 nanoparticles for enhanced photocatalytic performance under sunlight irradiation. Phys. Chem. Chem. Phys. 2014, 16, 23819–23828. [Google Scholar] [CrossRef] [PubMed]
- Kalyani, V.; Vasile, B.S.; Ianculescu, A.; Testino, A.; Carino, A.; Buscaglia, M.T.; Buscaglia, V.; Nanni, P. Hydrothermal Synthesis of SrTiO3: Role of Interfaces. Cryst. Growth Des. 2015, 15, 5712–5725. [Google Scholar] [CrossRef]
- Zheng, J.-Q.; Zhu, Y.-J.; Xu, J.-S.; Lu, B.-Q.; Qi, C.; Chen, F.; Wu, J. Microwave-assisted rapid synthesis and photocatalytic activity of mesoporous Nd-doped SrTiO3 nanospheres and nanoplates. Mater. Lett. 2013, 100, 62–65. [Google Scholar] [CrossRef]
- Konta, R.; Ishii, T.; Kato, H.; Kudo, A. Photocatalytic activities of noble metal ion doped SrTiO3 under visible light irradiation. J. Phys. Chem. B 2004, 108, 8992–8995. [Google Scholar] [CrossRef]
- Kiss, B.; Manning, T.D.; Hesp, D.; Didier, C.; Taylor, A.; Pickup, D.M.; Chadwick, A.V.; Allison, H.E.; Dhanak, V.R.; Claridge, J.B.; et al. Nano-structured rhodium doped SrTiO3-Visible light activated photocatalyst for water decontamination. Appl. Catal. B-Environ. 2017, 206, 547–555. [Google Scholar] [CrossRef]
- Moreira, M.L.; Longo, V.M.; Avansi, W., Jr.; Ferrer, M.M.; Andres, J.; Mastelaro, V.R.; Varela, J.A.; Longo, E. Quantum Mechanics Insight into the Microwave Nucleation of SrTiO3 Nanospheres. J. Phys. Chem. C 2012, 116, 24792–24808. [Google Scholar] [CrossRef]
- Kawasaki, S.; Akagi, K.; Nakatsuji, K.; Yamamoto, S.; Matsuda, I.; Harada, Y.; Yoshinobu, J.; Komori, F.; Takahashi, R.; Lippmaa, M.; et al. Elucidation of Rh-Induced In-Gap States of Rh:SrTiO3 Visible-Light-Driven Photocatalyst by Soft X-ray Spectroscopy and First-Principles Calculations. J. Phys. Chem. C 2012, 116, 24445–24448. [Google Scholar] [CrossRef]
- Tan, H.L.; Tahini, H.A.; Wen, X.; Wong, R.J.; Tan, X.; Iwase, A.; Kudo, A.; Amal, R.; Smith, S.C.; Ng, Y.H. Interfacing BiVO4 with Reduced Graphene Oxide for Enhanced Photoactivity: A Tale of Facet Dependence of Electron Shuttling. Small 2016, 12, 5295–5302. [Google Scholar] [CrossRef] [PubMed]
- Iwase, A.; Ng, Y.H.; Ishiguro, Y.; Kudo, A.; Amal, R. Reduced graphene oxide as a solid-state electron mediator in Z-scheme photocatalytic water splitting under visible light. J. Am. Chem. Soc. 2011, 133, 11054–11057. [Google Scholar] [CrossRef] [PubMed]

| Catalyst | H2 Evolution Rate (μmol eg−1 h−1) | O2 Evolution Rate (μmol eg−1 h−1) |
|---|---|---|
| Ru/SS-STO2Rh | 56 | 35 |
| Ru/HT-STO2Rh | 28 | 16 |
| Ru/MW-STO2Rh-A | 141 | 75 |
| Ru/MW-STO2Rh-T | 317 | 168 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.-y.; Cian, L.-T. Microwave-Assisted Hydrothermal Synthesis of SrTiO3:Rh for Photocatalytic Z-scheme Overall Water Splitting. Appl. Sci. 2019, 9, 55. https://doi.org/10.3390/app9010055
Lin H-y, Cian L-T. Microwave-Assisted Hydrothermal Synthesis of SrTiO3:Rh for Photocatalytic Z-scheme Overall Water Splitting. Applied Sciences. 2019; 9(1):55. https://doi.org/10.3390/app9010055
Chicago/Turabian StyleLin, Hsin-yu, and Lyu-Ting Cian. 2019. "Microwave-Assisted Hydrothermal Synthesis of SrTiO3:Rh for Photocatalytic Z-scheme Overall Water Splitting" Applied Sciences 9, no. 1: 55. https://doi.org/10.3390/app9010055
APA StyleLin, H.-y., & Cian, L.-T. (2019). Microwave-Assisted Hydrothermal Synthesis of SrTiO3:Rh for Photocatalytic Z-scheme Overall Water Splitting. Applied Sciences, 9(1), 55. https://doi.org/10.3390/app9010055




