Chitosan Microbeads as Supporter for Pseudomonas putida with Surface Displayed Laccases for Decolorization of Synthetic Dyes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals, Bacterial Strains and Culture Conditions
2.2. Preparation of CTS-MBs
2.3. Surface Aldehyde Modification of the CTS-MBs and Immobilization of P. putida MB285 Cells
2.4. Assay of IBCC-B Enzymatic Activity
2.5. Dye Decolorization
2.6. Characterization of the CTS-MBs, aCTS-MBs and bacCTS-MBs
2.7. Data Analysis
3. Results and Discussion
3.1. Preparation and Characterization of the IBCC-B Complexes
3.2. Effects of Temperature, pH and Storage Time on the Laccase Activity of the bacCTS-MB1
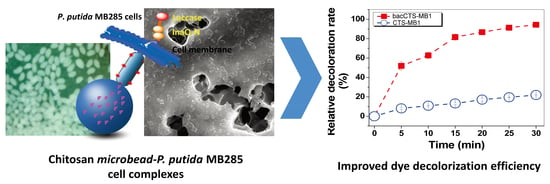
3.3. Dye Decolorization by the bacCTS-MB1 Complex
3.4. Effect of Repeated Use on AG25 Decolorization by the bacCTS-MB1s
3.5. Effect of Re-Culturing Time on Decolorization by the bacCTS-MB1s
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Husain, Q. Potential applications of the oxidoreductive enzymes in the decolorization and detoxification of textile and other synthetic dyes from polluted water: A review. Crit. Rev. Biotechnol. 2006, 26, 201–221. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, A.; Viraraghavan, T. Decolorization of dye wastewaters by biosorbents: A review. J. Environ. Manag. 2010, 91, 1915–1929. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S. Heavy metal pollution in China: Origin, pattern and control. Environ. Sci. Pollut. Res. Int. 2003, 10, 192–198. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, A.B.; Cervantes, F.J.; van Lier, J.B. Review paper on current technologies for decolourisation of textile wastewaters: Perspectives for anaerobic biotechnology. Bioresour. Technol. 2007, 98, 2369–2385. [Google Scholar] [CrossRef] [PubMed]
- Nidheesh, P.V.; Gandhimathi, R.; Ramesh, S.T. Degradation of dyes from aqueous solution by Fenton processes: A review. Environ. Sci. Pollut. Res. Int. 2013, 20, 2099–2132. [Google Scholar] [CrossRef] [PubMed]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Chitosan as a bioactive polymer: Processing, properties and applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef]
- Farzana, M.H.; Meenakshi, S. Photo-decolorization and detoxification of toxic dyes using titanium dioxide impregnated chitosan beads. Int. J. Biol. Macromol. 2014, 70, 420–426. [Google Scholar] [CrossRef]
- Bilal, M.; Asgher, M.; Iqbal, M.; Hu, H.; Zhang, X. Chitosan beads immobilized manganese peroxidase catalytic potential for detoxification and decolorization of textile effluent. Int. J. Biol. Macromol. 2016, 89, 181–189. [Google Scholar] [CrossRef]
- Lin, Y.H.; Lin, W.F.; Jhang, K.N.; Lin, P.Y.; Lee, M.C. Adsorption with biodegradation for decolorization of reactive black 5 by Funalia trogii 200800 on a fly ash-chitosan medium in a fluidized bed bioreactor-kinetic model and reactor performance. Biodegradation 2013, 24, 137–152. [Google Scholar] [CrossRef]
- Wang, J.; Chen, C. Chitosan-based biosorbents, modification and application for biosorption of heavy metals and radionuclides. Bioresour. Technol. 2014, 160, 129–141. [Google Scholar] [CrossRef]
- Philibert, T.; Lee, B.H.; Fabien, N. Current status and new perspectives on chitin and chitosan as functional biopolymers. Appl. Biochem. Biotechnol. 2017, 181, 1314–1337. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.K.; Voicu, S.I. Recent advances in cellulose and chitosan based membranes for water purification: A concise review. Carbohydr. Polym. 2016, 146, 148–165. [Google Scholar] [CrossRef]
- Yang, R.; Li, H.; Huang, M.; Yang, H.; Li, A. A review on chitosan-based flocculants and their applications in water treatment. Water Res. 2016, 95, 59–89. [Google Scholar] [CrossRef]
- LogithKumar, R.; KeshavNarayan, A.; Dhivya, S.; Chawla, A.; Saravanan, S.; Selvamurugan, N. A review of chitosan and its derivatives in bone tissue engineering. Carbohydr. Polym. 2016, 151, 172–188. [Google Scholar] [CrossRef] [PubMed]
- Muanprasat, C.; Chatsudthipong, V. Chitosan oligosaccharide: Biological activities and potential therapeutic applications. Pharmacol. Ther. 2017, 170, 80–97. [Google Scholar] [CrossRef] [PubMed]
- Canas, A.I.; Camarero, S. Laccases and their natural mediators: Biotechnological tools for sustainable eco-friendly processes. Biotechnol. Adv. 2010, 28, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Ba, S.; Vinoth Kumar, V. Recent developments in the use of tyrosinase and laccase in environmental applications. Crit. Rev. Biotechnol. 2017, 37, 819–832. [Google Scholar] [CrossRef] [PubMed]
- Claus, H. Laccases and their occurrence in prokaryotes. Arch. Microbiol. 2003, 179, 145–150. [Google Scholar] [CrossRef]
- Claus, H. Laccases: Structure, reactions, distribution. Micron 2004, 35, 93–96. [Google Scholar] [CrossRef]
- Bertrand, B.; Martinez-Morales, F.; Trejo-Hernandez, M.R. Upgrading laccase production and biochemical properties: Strategies and challenges. Biotechnol. Prog. 2017, 33, 1015–1034. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Goradia, B.; Saxena, A. Bacterial laccase: Recent update on production, properties and industrial applications. 3 Biotech 2017, 7, 323. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Stemple, B.; Kumar, M.; Wei, N. Cell surface display fungal laccase as a renewable biocatalyst for degradation of persistent micropollutants bisphenol A and sulfamethoxazole. Environ. Sci. Technol. 2016, 50, 8799–8808. [Google Scholar] [CrossRef] [PubMed]
- Couto, S.R.; Toca-Herrera, J.L. Industrial and biotechnological applications of laccass: A review. Biotechnol. Adv. 2006, 24, 500–513. [Google Scholar] [CrossRef] [PubMed]
- Peralta-Zamora, P.; Pereira, C.M.; Tiburtius, E.R.L.; Moraes, S.G.; Rosa, M.A.; Minussi, R.C.; Durán, N. Decolorization of reactive dyes by immobilized laccase. Appl. Catal. B Environ. 2003, 42, 131–144. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Z.; Ni, H.; Yang, X.; Li, Q.; Li, L. Decolorization of industrial synthetic dyes using engineered Pseudomonas putida cells with surface-immobilized bacterial laccase. Microb. Cell Fact. 2012, 11, 75. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Gao, Y.; Jiang, M.; Li, L. Deletion and site-directed mutagenesis of laccase from Shigella dysenteriae results in enhanced enzymatic activity and thermostability. Enzyme Microb. Technol. 2009, 44, 274–280. [Google Scholar] [CrossRef]
- Ramos, J.L.; Sol Cuenca, M.; Molina-Santiago, C.; Segura, A.; Duque, E.; Gomez-Garcia, M.R.; Udaondo, Z.; Roca, A. Mechanisms of solvent resistance mediated by interplay of cellular factors in Pseudomonas putida. FEMS Microbiol. Rev. 2015, 39, 555–566. [Google Scholar] [CrossRef]
- Kim, J.; Park, W. Oxidative stress response in Pseudomonas putida. Appl. Microbiol. Biotechnol. 2014, 98, 6933–6946. [Google Scholar] [CrossRef]
- El Kadib, A. Chitosan as a sustainable organocatalyst: A concise overview. ChemSusChem 2015, 8, 217–244. [Google Scholar] [CrossRef]
- Krajewska, B. Application of chitin- and chitosan-based materials for enzyme immobilizations: A review. Enzyme Microb. Technol. 2004, 35, 126–139. [Google Scholar] [CrossRef]
- Chiou, M.S.; Chuang, G.S. Competitive adsorption of dye metanil yellow and RB15 in acid solutions on chemically cross-linked chitosan beads. Chemosphere 2006, 62, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; Russell, D.W. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2001. [Google Scholar]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, Z.; Sun, X.; Yu, X.; Li, L. Chitosan Microbeads as Supporter for Pseudomonas putida with Surface Displayed Laccases for Decolorization of Synthetic Dyes. Appl. Sci. 2019, 9, 138. https://doi.org/10.3390/app9010138
Bai Z, Sun X, Yu X, Li L. Chitosan Microbeads as Supporter for Pseudomonas putida with Surface Displayed Laccases for Decolorization of Synthetic Dyes. Applied Sciences. 2019; 9(1):138. https://doi.org/10.3390/app9010138
Chicago/Turabian StyleBai, Zhiqiang, Xiaowen Sun, Xun Yu, and Lin Li. 2019. "Chitosan Microbeads as Supporter for Pseudomonas putida with Surface Displayed Laccases for Decolorization of Synthetic Dyes" Applied Sciences 9, no. 1: 138. https://doi.org/10.3390/app9010138
APA StyleBai, Z., Sun, X., Yu, X., & Li, L. (2019). Chitosan Microbeads as Supporter for Pseudomonas putida with Surface Displayed Laccases for Decolorization of Synthetic Dyes. Applied Sciences, 9(1), 138. https://doi.org/10.3390/app9010138