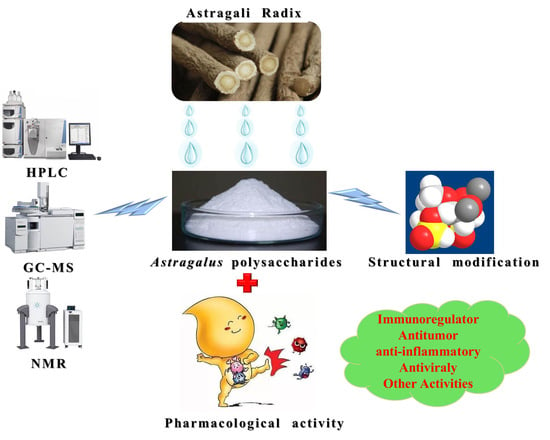
Extraction, Structure, and Pharmacological Activities of Astragalus Polysaccharides
Abstract
:Featured Application
Abstract
1. Introduction
2. Extraction of Astragalus Polysaccharides
2.1. Water Extraction Methods
2.2. Microwave-Assisted Extraction Methods
2.3. Ultrasonic Wave Extraction Methods
2.4. Enzymatic Hydrolysis Extraction Methods
3. Purification of Astragalus Polysaccharides
4. Chemical Composition and Structure of Astragalus Polysaccharides
5. Structural Modification of Astragalus Polysaccharides
6. Pharmacological Activities of Astragalus Polysaccharides
6.1. Immunoregulatory Effects
6.2. Antitumor Effects
6.3. Anti-Inflammatory Effects
6.4. Antiviral Effects
6.5. Other Activities
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pharmacopoeia Commission of PRC. Pharmacopoeia of the People’s Republic of China; China Medical Science Press: Beijing, China, 2015; Volume 1, p. 302. [Google Scholar]
- Shao, B.M.; Xu, W.; Dai, H.; Tu, P.; Li, Z.; Gao, X.M. A study on the immune receptors for polysaccharides from the roots of Astragalus membranaceus, a Chinese medicinal herb. Biochem. Biophys. Res. Commun. 2004, 4, 1103–1111. [Google Scholar] [CrossRef]
- Jiang, L.L.; Zhang, D.Z.; Hao, X.X.; Niu, L.L.; Ren-Gaowa, S.A.; Wang, L. Survey on Astragalus in Inner Mongolia. J. Dis. Monit. Control 2013, 3, 165–166. [Google Scholar]
- Wang, X.F.; Liang, Y.; Liu, D.W.; Yang, J.Y.; Jian, Y.; Hu, Z.L. Study on Genetic Diversity of Astragalus membranaceus var. mongholicus Populations in Inner Mongolia. Chin. J. Grassl. 2018, 1, 42–48. [Google Scholar]
- Cui, R.T.; He, J.C.; Wang, B.E.; Zhang, F.K.; Chen, G.Y.; Yin, S.S.; Shen, H. Suppressive effect of Astragalus membranaceus Bunge on chemical hepatocarcinogenesis in rats. Cancer Chemother. Pharmacol. 2003, 51, 75–80. [Google Scholar] [CrossRef]
- Li, Q.; Hu, J.H.; Bo, G.; Li, H.D.; Song, S.; Yang, L.X. Advances on Immunoregulation Effect of Astragalus Polysaccharides. Chin. J. Exp. Tradit. Med. Formulae 2017, 2, 199–206. [Google Scholar]
- Yang, Q.Z.; Liu, D.W.; Tian, Y.X.; Huang, L.F. Research Progress on Chemical Structure and Pharmacological Activity of Astragalus Polysaccharide. North. Hortic. 2015, 7, 168–175. [Google Scholar]
- Bai, D.Z.; Dong, F.; Tang, W.T.; Chen, X. Study on Process of Pharmacology of Astragalus Polysaccharide. Heilongjiang Med. J. 2014, 1, 103–106. [Google Scholar]
- Wang, S.P.; Li, X.J.; Zhang, G.Z. Study on optimization of the technology for extraction and purification polysaccharide from Astragalus membranaceus. J. Mol. Sci. 2008, 1, 60–64. [Google Scholar]
- Zhu, Z.Y.; Liu, R.Q.; Zhou, F.; Li, S.F.; Yuan, J. Extraction and purification of Astragalus polysaccharide and its anti-tumor activity. Mod. Food Sci. Technol. 2011, 4, 376–379. [Google Scholar]
- Li, H.M.; Huang, R.Q.; Wang, Y.Z. A technological study on enhancing the extraction rate of Astragalus polysaccharids. J. Northwest. Univ. 2000, 6, 509–510. [Google Scholar]
- Gong, S.Z.; Yang, Z.R. Investigation into the Microwave-assisted Extraction Technology for Astragalus Polysaccharide. J. South China Univ. Technol. 2004, 8, 93–96. [Google Scholar]
- Chen, Y.X.; Lin, F.; Mo, J.; Du, X.D. Comparison of two extraction methods of Astragalus polysaccharide. Res. Explor. Lab. 2015, 3, 20–22. [Google Scholar]
- Dong, L.L.; Huang, X.; Qi, Y.G.; Feng, H. Study on the enzymatic-microwave extraction of Astragalus polysaccharides. J. Zhejiang Univ. Technol. 2011, 3, 220–232. [Google Scholar]
- Jin, R.C.; Zhou, S.T.; Zhang, D.B. Study on Extraction of Astragalus membranaceus Polysaccharides by Optimized Ultrasonic Method with Uniform Design. J. Anhui Agric. Sci. 2009, 12, 5498–5499. [Google Scholar]
- Du, G.F.; Cai, Z.H.; Wang, G.; Dai, B.; He, L. Study on Extraction of Astragalus Polysaccharide by Ultrasonic-Microwave Synergistically Assisted Technique. Nat. Prod. Res. Dev. 2012, s1, 114–117. [Google Scholar]
- Bi, Y.G.; Wu, Z.C. Factors analysis of ultrasound combined with cellulose enzymatic extraction of total Astragalus polysaccharides. J. Guangdong Pharm. College 2010, 2, 134–137. [Google Scholar]
- Chen, X.W.; Ma, S.L. Extraction of Astragalus Iepsensis by Cellulase Degradation. Shanghai J. Tradit. Chin. Med. 2005, 1, 56–58. [Google Scholar]
- Zheng, L.Y.; Wei, Y.M.; Chen, L. Extraction of effective component from Radix astragli with cellulase. J. Gansu Agric. Univ. 2005, 1, 94–96. [Google Scholar]
- Du, J.P.; Jia, C.Z.; Xue, Y.P.; Zhang, W.J.; Li, H.Q. Purification and Sulfating of Radix Astragali Polysacharides and Analysis with Infrared Spectroscopy. Hubei Agric. Sci. 2015, 1, 154–158. [Google Scholar]
- Zhao, F.C. Extraction, separation and purification of polysaccharides from Astragalus. Master’s Thesis, Guangxi University, Nanning, China, 2007. [Google Scholar]
- Liu, Y.L.; Zhang, G.Z.; Fan, K.F.; Zhu, Z.H. Study on the technologies for extraction and purification of Astragalus polysaccharides. J. Henan Agric. Sci. 2010, 3, 199–207. [Google Scholar]
- Liu, F.G.; Yang, Y.; Xiao, G.S.; Xu, X.H. Study on Methods of Astragalus Polysaccharides Extraction and Purification. Henan Sci. 2008, 6, 662–664. [Google Scholar]
- Li, H.Q.; Zhao, W.G.; Lv, X.H. Analysis on chemical components and structure of Astraglus polysaccharides. J. Tradition. Chin. Vet. Med. 2008, 5, 5–9. [Google Scholar]
- Chen, H.H.; Sun, J.; Yang, J.Y.; Gong, S.X.; Zhang, T.J. Determination of monosaccharide compositions in Astragalus mongholicus var. mongholicus polysaccharides by pre-column derivatization-HPLC. Drugs Clin. 2012, 5, 468–470. [Google Scholar]
- Yao, D.; Wang, H.J. Monosaccharide composition in Radix Astragali polysaccharides by gas chromatography. Med. Plant 2012, 5, 36–38. [Google Scholar]
- Chen, Y.R.; Mao, X.Y.; Jin, W.W.; Luo, H.J.; Li, T. Study on Astragalus Polysaccharide Structure and Monosaccharide Constituents by Gas Chromatography-Mass Spectrometry. Prog. Mod. Biomed. 2011, s1, 4632–4635. [Google Scholar]
- Qu, J. The Isolation, Purification and Structural Characterization of Low molecular Polysaccharide Purified from Radix Astragali. Master’s Thesis, Northeast Normal University, Changchun, China, 2010. [Google Scholar]
- Weng, L.; Liu, X.Y.; Liu, Y. Effect of Astragalus polysaccharide (APS-P) on the proliferation and mobilization of murine hematopoietic stem cells. Basic Med. Sci. Clin. 2003, 6, 306–309. [Google Scholar]
- Fu, J.; Huang, L.; Zhang, H.; Yang, S.h.; Chen, S.L. Structural features of a polysaccharide from Astragalus membranaceus (Fisch.) Bge. var. mongholicus (Bge.) Hsiao. J. Asian Nat. Prod. Res. 2013, 6, 687–692. [Google Scholar] [CrossRef]
- Niu, Y.G.; Wang, H.Y.; Xie, Z.H.; Whent, M.; Gao, X.D.; Zhang, X.; Zou, S.; Yao, W.B.; Yu, L.L. Structural analysis and bioactivity of a polysaccharide from the roots of Astragalus membranaceus (Fisch) Bge. var mongolicus (Bge.) Hsiao. Food Chem. 2011, 3, 620–626. [Google Scholar] [CrossRef]
- Yin, J.Y.; Chan, C.L.; Yu, H.; Lau, Y.K.; Han, X.Q.; Cheng, S.W.; Wong, C.K.; Lau, B.S.; Xie, M.Y.; Fung, K.P.; et al. Separation, structure characterization, conformation and immunomodulating effect of a hyperbranched heteroglycan from Radix Astragali. Carbohydr. Polym. 2012, 1, 667–675. [Google Scholar] [CrossRef]
- Shimizu, N.; Tomoda, M.; Kanari, M.; Gonda, R. An acidic polysaccharide having activity on the reticuloendothelial system from the root of Astragalus mongholicus. Chem. Pharm. Bull. 1991, 11, 2969. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.M.; Zhang, Q.F.; Qiao, S.Y.; Qi, C.H.; Zhang, Y.X. Isolation and structure elucidation of novel glucan from Astragalus mongholicus. Chin. Tradition. Herbal Drugs 2001, 11, 962–964. [Google Scholar]
- Li, H.Q.; Zhao, W.G.; Lv, X.H.; Li, X.H. Physical and Chemical Analysis of a New Heteropolysaccharide from Radix Astragalus. Chin. Pharma. J. 2009, 9, 654–657. [Google Scholar]
- Lin, M.G.; Yang, Y.F.; Xu, H.Y. Chemical structure of Astragalus Polysaccharides MAPS-5 and its proliferative activity in vitro. Chin. Herbal Med. 2009, 12, 1865–1868. [Google Scholar]
- Liu, R.Q. Structural Analysis of Polysaccharide from Astragalus and its Anti-tumor Activity. Master’s Thesis, Tianjin University of Science & Technology, Tianjin, China, 2011. [Google Scholar]
- Liu, X.J.; Wang, M.Y.; Wu, H.S.; Zhao, X.F.; Li, H. studies on isolation of astragalan and its immunological activities. Nat. Prod. Res. Dev. 1994, 1, 23–31. [Google Scholar]
- Li, S.G.; Zhang, Y.Q. Characterization and renal protective effect of a polysaccharide from Astragalus membranaceus. Carbohydr. Polym. 2009, 2, 343–348. [Google Scholar] [CrossRef]
- Shan, J.J.; Wang, Y.; Weng, Y.Q.; Xie, C.Y.; Liu, D.; Hu, Z.B. Comparing compositions and immunoactivities of polysaccharide in hair root of Astragalus membranaceus and cultivated A. membranaceus. Chin. Tradition. Herbal Drugs 2002, 12, 1096–1099. [Google Scholar]
- Liao, J.Z.; Li, C.Y.; Huang, J.; Liu, W.P.; Chen, H.C.; Liao, S.Y.; Chen, H.Y.; Rui, W. Structure Characterization of Honey-Processed Astragalus Polysaccharides and Its Anti-Inflammatory Activity in Vitro. Molecules 2018, 23, 168. [Google Scholar] [CrossRef]
- Pu, X.Y.; Ma, X.L.; Lu, L.; Jing, R.; Li, H.B.; Li, X.Y.; Yu, S.; Zhang, W.J.; Fan, W.B. Structural characterization and antioxidant activity in vitro of polysaccharides from Angelica and Astragalus. Carbohydr. Polym. 2016, 137, 154. [Google Scholar] [CrossRef]
- Lu, X.Y.; Mo, X.Y.; Guo, H.; Zhang, Y.L. Sulfation modification and anticoagulant activity of the polysaccharides obtained from persimmon (Diospyros kaki L.) fruits. Int. J. Biol. Macromol. 2012, 5, 1189–1195. [Google Scholar] [CrossRef]
- Wang, X.F. Study on anti-inflammatory activities of Astragalus polysaccharides and their sulphation modification products in vitro and in vivo. Ph.D. Thesis, Northwest A&F University, Xianyang, China, 2014. [Google Scholar]
- Takano, R.; Nagai, T.; Wu, X.F.; Wu, X.Y.; Huy, N.T.; Kamei, K.; Hara, S. Sulfation of Polysaccharides using Monomethyl Sulfate. J. Carbohydr. Chem. 2000, 9, 1185–1190. [Google Scholar] [CrossRef]
- Wang, X.F.; Wang, S.Y.; Li, Y.L.; Wang, F.; Yang, X.J.H.; Yao, J. Sulfated Astragalus polysaccharide can regulate the inflammatory reaction induced by LPS in Caco2 cells. Int. J. Biol. Macromol. 2013, 6, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.F.; Shen, J.; Li, S.Z.; Zhi, L.H.; Yang, X.J.; Yao, J.H. Sulfated Astragalus polysaccharide regulates the inflammatory reaction in LPS-infected broiler chicks. Int. J. Biol. Macromol. 2014, 8, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Jin, Y.M.; Zhang, Y.H.; Li, C.Y.; Zhang, X.Y. Effects of Eight polysaccharides in Chinese herbal and sulfated polysaccharides on ND. Chin. J. Vet. Med. 2012, 8, 42–45. [Google Scholar]
- Huang, X.Y.; Hu, Y.L.; Lu, Y.; Zhang, F.; Guo, Z.H. Sulfated modification can enhance the adjuvant activity of Astragalus polysaccharide for ND vaccine. Carbohydr. Polym. 2008, 2, 303–308. [Google Scholar] [CrossRef]
- Shi, Z.Y.; Huang, X.Y.; Li, D.P.; Sun, J.L.; Ju, Y.; Hu, Y.L. Effects of sulfated Astragalus polysaccharides on immune responses of chickens inoculated with IBD vaccine. Anim. Husb. Vet. Med. 2009, 2, 6–8. [Google Scholar]
- Jung, H.Y.; Bae, I.Y.; Lee, S.Y.; Lee, H.G. Effect of the degree of sulfation on the physicochemical and biological properties of Pleurotus eryngii polysaccharides. Food Hydrocoll. 2011, 5, 1291–1295. [Google Scholar] [CrossRef]
- Liu, J.G.; Wang, Y.X.; Chen, Y.; Xiong, W.; Wang, D.Y.; Wu, Y.; Hu, Y.L. Phosphorylation Molecular Modification of Astragalus Polysaccharide against Duck Viral Hepatitis. CN Patent CN105560352A, 11 May 2016. [Google Scholar]
- Guo, J.Z.; Liang, J.P.; Tao, L.; Chu, G.P.; Zhao, F.W.; Jia, Z.; Li, C.H.; Zhang, M. A preparation method of Astragalus Polysaccharide phosphate. CN Patent CN104877036A, 2 September 2015. [Google Scholar]
- Gong, X.Z.; Ouyang, Z. Investigation of selenoastragalans preparation conditions and structure determination. Nat. Prod. Res. Dev. 1998, 2, 26–32. [Google Scholar]
- Zhang, Y.F.; Wu, F.L. Inhibitory Effects of Se-Astragalus Membranaceus Polysaccharide on Tumor in Mice. Chin. J. Public Health 1997, 4, 229–230. [Google Scholar]
- Zhang, Y.F.; Wu, F.L.; Zhang, M.Z. Interaction between Vitamin E and Se-Astragalus Membranaceus Polysaccharide on the effects of tumor inhibition on S180 in mice. Chin. J. Cancer 1996, 6, 290–300. [Google Scholar]
- Chen, H.H. Selenium modification of Astragalus polysaccharide and its antioxidant acticitu in vitro. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2010. [Google Scholar]
- Yang, X.J.; Wang, S.Y.; Yao, J.H.; Wang, X.F. Preparation of Astragalus Polysaccharide by Carboxymethylation Molecular Modification. CN Patent CN103030704A, 10 April 2013. [Google Scholar]
- Liu, P.; Zhao, H.P.; Luo, Y.M. Anti-Aging Implications of Astragalus membranaceus (Astragali Radix): A Well-Known Chinese Tonic. Aging Dis. 2017, 6, 868–886. [Google Scholar] [CrossRef]
- Li, S.P.; Zhao, X.J.; Wang, J.Y. Synergy of Astragalus polysaccharides and probiotics (Lactobacillus and Bacillus cereus) on immunity and intestinal microbiota in chicks. Poult. Sci. 2009, 3, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.J.; Wang, Z.X.; Long, T.T.; Zhou, X.; Bao, Y.X. Roles of Astragalus polysaccharides in coculture system of breast cancer cells and macrophages in vitro. Immunol. J. 2017, 6, 469–476. [Google Scholar]
- Hou, Y.C.; Wu, J.M.; Wang, M.Y.; Wu, M.H.; Chen, K.Y.; Yeh, S.L.; Lin, M.T. Modulatory Effects of Astragalus Polysaccharides on T-Cell Polarization in Mice with Polymicrobial Sepsis. Mediat. Inflamm. 2015, 26, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zahran, E.; Risha, E.; Abdelhamid, F.; Mahgoub, H.A.; Ibrahim, T. Effects of dietary Astragalus polysaccharides (APS) on growth performance, immunological parameters, digestive enzymes, and intestinal morphology of Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2014, 1, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Li, J.F.; Zhong, Y.G.; Li, H.R.; Zhang, N.W.; Ma, W.R.; Cheng, G.L.; Liu, F.Q.; Liu, F.H.; Xu, J.Q. Enhancement of Astragalus polysaccharide on the immune responses in pigs inoculated with foot-and-mouth disease virus vaccine. Int. J. Biol. Macromol. 2011, 3, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Chen, W.C.; Wang, W.P.; Tian, W.Y.; Zhang, X.G. Extraction, characterization of Astragalus polysaccharides and its immune modulating activities in rats with gastric cancer. Carbohydr. Polym. 2009, 4, 738–742. [Google Scholar] [CrossRef]
- Yin, X.L.; Chen, L.; Liu, Y.; Yang, J.L.; Ma, C.Q.; Yao, Z.Y.; Yang, L.J.; Wei, L.; Li, M.Y. Enhancement of the innate immune response of bladder epithelial cells by Astragalus polysaccharides through upregulation of TLR4 expression. Biochem. Biophys. Res. Commun. 2010, 2, 232–238. [Google Scholar] [CrossRef]
- Yao, J.F.; Wu, C.L.; Chen, H.X.; Zhang, R.F. Effect of Astragalus Polysaccharide on Telomerase of HL-60 Cell. Henan J. Oncol. 2005, 4, 247–248. [Google Scholar]
- Xu, D.J.; Chen, M.Z. Antitumor activity of APS and its mechanism of action. Chin. J. Hosp. Pharm. 2005, 10, 923–925. [Google Scholar]
- Liu, X.; Yang, Y.; Zhang, X.X.; Xu, S.X.; He, S.F.; Huang, W.J.; Roberts, M.S. Compound Astragalus and Salvia miltiorrhiza extract inhibits cell invasion by modulating transforming growth factor-beta/Smad in HepG2 cell. J. Gastroenterol. Hepatol. 2010, 2, 420–426. [Google Scholar] [CrossRef]
- Wang, J.H.; Dong, J.C.; Zhao, J.X.; Jin, S.Z.; Zhang, H.Y.; Zhang, S.Y. Effects of different molecule weight Astragalus polysacharin isolated from annual Astragalus membra-neaceus on experssions of inflammatory cytokines in RAW264.7 cells. J. Jilin Univ. (Med. Ed.) 2011, 6, 1051–1056. [Google Scholar]
- Zhang, S.; Zhang, T.; Teng, K.D.; Wang, A.R.; Yu, Y.F.; Zhang, H. Effect of Astragalus polysaccharide powder injection on the density of microvessels and mast cells in ovalbulmin-sensitized rat skin. J. China Agric. Univ. 2010, 1, 67–71. [Google Scholar]
- Zhang, Y.; Li, J.T.; Liu, Y.Q.; Li, J.; Su, W.; Yan, C.L.; Nie, L. Effects of Astragalus polysaccharides on immune balance and the expression of nitric oxide in pulmonary fibrosis rats. Chin. J. Gerontol. 2009, 10, 1185–1187. [Google Scholar]
- Yang, M.; Lin, H.B.; Gong, S.T.; Chen, P.Y.; Geng, L.L.; Zeng, Y.M.; Li, D.Y. Effect of Astragalus polysaccharides on expression of TNF-α, IL-1β and NFATc4 in a rat model of experimental colitis. Cytokine 2014, 2, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Du, X.G.; Zhao, B.; Li, J.Y.; Cao, X.H.; Diao, M.K.; Feng, H.B.; Chen, X.B.; Chen, Z.Y.; Zeng, X.Y. Astragalus polysaccharides enhance immune responses of HBV DNA vaccination via promoting the dendritic cell maturation and suppressing Treg frequency in mice. Int. Immunopharmacol. 2012, 4, 463–470. [Google Scholar] [CrossRef]
- Zhang, N.W.; Li, J.F.; Hu, Y.X.; Cheng, G.L.; Zhu, X.Y.; Liu, F.Q.; Zhang, Y.J.; Liu, Z.J.; Xu, J.Q. Effects of Astragalus polysaccharide on the immune response to foot-and-mouth disease vaccine in mice. Carbohydr. Polym. 2010, 3, 680–686. [Google Scholar] [CrossRef]
- Löndt, B.Z.; Brookes, S.M.; Kelly, M.D.; Nash, B.J.; Brown, I.H. Failure to infect pigs co-housed with ducks or chickens infected experimentally with A/turkey/Turkey/1/2005 (H5N1) highly pathogenic avian influenza virus. Vet. Microbiol. 2013, 2–4, 944–948. [Google Scholar]
- Xue, H.X.; Gan, F.; Zhang, Z.Q.; Hu, J.F.; Chen, X.X.; Huang, K.H. Astragalus polysaccharides inhibits PCV2 replication by inhibiting oxidative stress and blocking NF-κB pathway. Int. J. Biol. Macromol. 2015, 7, 22–30. [Google Scholar] [CrossRef]
- Guo, Q.W.; Sun, X.S.; Zhang, Z.B.; Zhang, L.; Yao, G.; Li, F.; Yang, X.; Song, L.; Jiang, G. The effect of Astragalus polysaccharide on the Epstein-Barr virus lytic cycle. Acta Virol. 2014, 1, 76–80. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, Y.; Liu, Z.R.; Tian, Z.Q.; Xia, P.Y. Protective Effects of Astragali Radix Polysaccharides Combined with Astragaloside IV against Radiation Injury Model Mice. China Pharm. 2014, 3, 211–214. [Google Scholar]
- Jia, R.; Cao, L.P.; Xu, P.; Jeney, G.; Yin, G.J. In vitro and in vivo hepatoprotective and antioxidant effects of Astragalus polysaccharides against carbon tetrachloride-induced hepatocyte damage in common carp (Cyprinus carpio). Fish Physiol. Biochem. 2012, 3, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gu, J.Y.; Chen, Z.S.; Xing, K.C.; Sun, B. Astragalus polysaccharide suppresses palmitate-induced apoptosis in human cardiac myocytes: The role of Nrf1 and antioxidant response. Int. J. Clin. Exp. Pathol. 2015, 3, 2515–2524. [Google Scholar]
- Li, R.; Chen, W.C.; Wang, W.P.; Tian, W.Y.; Zhang, X.G. Antioxidant activity of Astragalus polysaccharides and antitumour activity of the polysaccharides and siRNA. Carbohydr. Polym. 2010, 2, 240–244. [Google Scholar] [CrossRef]
- Huang, W.M.; Liang, Y.Q.; Tang, L.J.; Ding, Y.; Wang, X.H. Antioxidant and anti-inflammatory effects of Astragalus polysaccharide on EA.hy926 cells. Exp. Ther. Med. 2013, 1, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Li, X.T.; Zhang, Y.K.; Kuang, H.X.; Jin, F.X.; Liu, D.W.; Gao, M.B.; Liu, Z.; Xin, X.J. Mitochondrial protection and anti-aging activity of Astragalus polysaccharides and their potential mechanism. Int. J. Mol. Sci. 2012, 13, 1747–1761. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Wang, Z.F.; Wen, D.J. Study on anti-aging effect of Astragalus Polysaccharide. Chin. J. Appl. Physiol. 2013, 4, 350–352. [Google Scholar]
- Zhao, D.W.; Ou, Q.; Wei, X.D.; Wang, M.F. Study on the Effect of Astragalus Polysaccharide on the Activity of Aging HDF-β-galactosidase. Chin. J. Gerontol. 2006, 10, 1361–1362. [Google Scholar]
- Cao, Y.; Shen, T.; Huang, X.Q.; Lin, Y.J.; Chen, B.D.; Pang, J.; Li, G.P.; Wang, Q.; Zohrabian, S.; Duan, C.; et al. Astragalus polysaccharide restores autophagic flux and improves cardiomyocyte function in doxorubicin-induced cardiotoxicity. Oncotarget 2017, 3, 4837–4848. [Google Scholar] [CrossRef]
- Wu, D.G.; Li, Q.X.; Wang, Y.F.; Liu, K.; Shi, S.W.; Li, S. Protection of Astragalus polysaccharides in atherosclerosis. J. Clin. Cardiol. 2012, 2, 147–152. [Google Scholar]
- Zhang, J.; Wang, H.X.; Yang, J.; Lu, M.L.; Li, S.T.; Yu, X.C. Astragalus polysaccharides inhibites cardiomyocyte hypertrophy induced by isoproterenol via Toll-like receptor 4/nuclear factor-κB signal pathway. Chin. J. Hypertens. 2014, 2, 157–162. [Google Scholar]
- Chen, L.X.; Zhu, H.Y.; Zhu, L.Q.; Xie, L.D.; Lin, Q.; Zhang, Y. Effect of Astragalus Polysaccharides on Expression of ICAM-1 and VCAM-1 in Human Cardiac Microvascular Endothelial Cells after Hypoxia and Reoxygenation. Liaoning J. Tradition. Chin. Med. 2008, 27, 5382–5386. [Google Scholar]
- Liu, M.; Wu, K.; Mao, X.Q.; Wu, Y.; Ouyang, J.P. Astragalus polysaccharide improves insulin sensitivity in KKAy mice: Regulation of PKB/GLUT4 signaling in skeletal muscle. J. Ethnopharmacol. 2010, 1, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhang, D.L.; Mao, X.Q.; Zou, F.; Jin, H.; Ouyang, J.P. Astragalus polysaccharides decreased the expression of PTP1B through relieving ER stress induced activation of ATF6 in a rat model of type 2 diabetes. Mol. Cell. Endocrinol. 2009, 1–2, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Li, Y.M.; Yu, M.H. Astragalus Polysaccharides: An Effective Treatment for Diabetes Prevention in NOD Mice. Exp. Clin. Endocrinol. Diabetes 2008, 8, 468–474. [Google Scholar] [CrossRef]
- Liang, J.H.; Zheng, K.W.; Sun, L.Q. Explore the Regulative Action of Astragalus Polysaccharide for Intestinal Dysbacteriosis in Ulcerative Colitis Rat Models. Stud. Trace Elements Health 2013, 2, 1–3. [Google Scholar]
- Zhao, H.M.; Wang, Y.; Huang, X.Y.; Huang, M.F.; Xu, R.; Yue, H.Y.; Zhou, B.G.; Huang, H.Y.; Sun, Q.M.; Liu, D.Y. Astragalus polysaccharide attenuates rat experimental colitis by inducing regulatory T cells in intestinal Peyer’s patches. World J. Gastroenterol. 2016, 11, 3175–3185. [Google Scholar] [CrossRef]
- Sun, S.Y.; Zheng, K.; Zhao, H.Y.; Lu, C.; Liu, B.; Yu, C.Y.; Zhang, G.; Bian, Z.X.; Lu, A.P.; He, X.J. Regulatory Effect of Astragalus Polysaccharides on Intestinal Intraepithelial γδT Cells of Tumor Bearing Mice. Molecules 2014, 19, 15224–15236. [Google Scholar] [CrossRef]
- Yan, F.; Zhang, Q.Y.; Jiao, L.; Han, T.; Zhang, H.; Qin, L.P.; Khalid, R. Synergistic hepatoprotective effect of Schisandrae lignans with Astragalus polysaccharides on chronic liver injury in rats. Phytomed. Int. 2009, 9, 805–813. [Google Scholar] [CrossRef]
- Liu, W.; Gao, F.F.; Li, Q.; Lv, J.W.; Wang, Y.; Hu, P.C.; Xiang, Q.M.; Wei, L. Protective Effect of Astragalus Polysaccharideson Liver Injury Induced with Different Chemotherapeutics in Mice. Asian Pac. J. Cancer Prev. 2014, 23, 10413–10420. [Google Scholar]
- Pu, X.Y.; Fan, W.B.; Yu, S.; Li, Y..; Ma, X.L.; Liu, L.; Ren, J.; Zhang, W.J. Polysaccharides from Angelica and Astragalus exert hepatoprotective effects against carbon-tetrachloride-induced intoxication in mice. Can. J. Physiol. Pharmacol. 2015, 1, 39–43. [Google Scholar] [CrossRef]
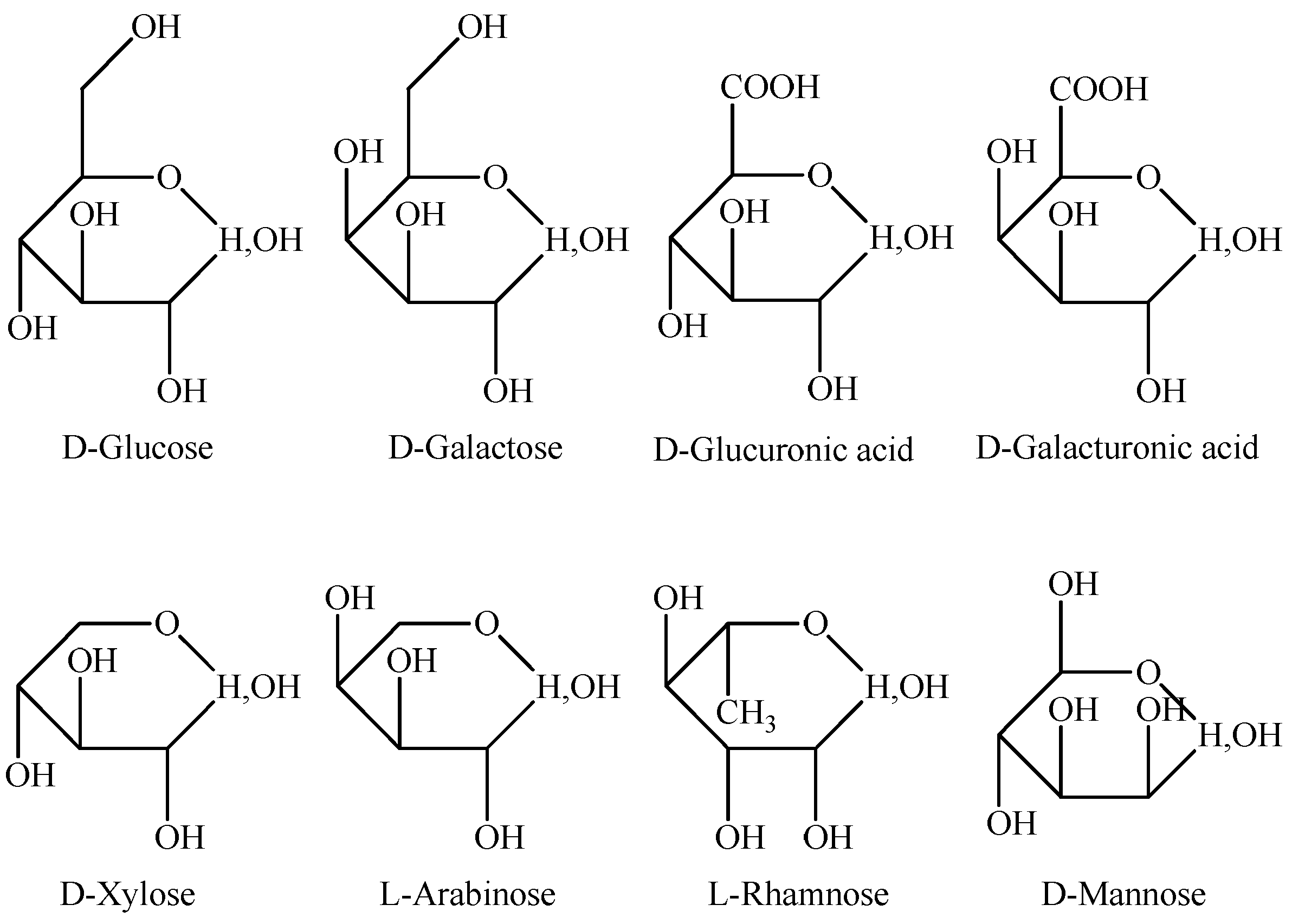

| Name | Molecular Mass (Da) | Monosaccharide Composition | Molar Ratio | Method | Reference |
|---|---|---|---|---|---|
| APS | 3.0 × 105 | L-Rhamnose, D-Xylose, D-Glucose, D-Galactose | 1:4:5:1.5 | GC; H-1, C-13 NMR | Fu et al. [30] |
| LMw-APS | 5.6 × 103 | Glucose, Galactose, Arabinose, Galactoside acid, Xylose | 10:1.3:1.7:0.95:1 | HPLC; GC | Qu [28] |
| APS | 2.1 × 104 | 1,4 Glucose backbone, 1,6 Glucose branched | NMR | Niu et al. [31] | |
| Rap-APS | 1.3 × 106 | Rhamnose, Arabinose, Glucose, galactose, Galactoside acid | 0.03:1.00:0.27:0.36:0.30 | GC-MS; 1H, 13C NMR | Yin et al. [32] |
| APS | 7.6 × 106 | L-Arabinose, D-Galactose, D-Galacturonic acid, D-Glucuronic acid | 18:18:1:1 | Electrophoresis; GC | Shimizu et al. [33] |
| APS | 3.6 × 105 | α-D-(1→4) glucose | GC-MS; 13CNMR | Wang et al. [34] | |
| 1.1 × 104 | Rhamnose, Glucose, Galactose, Arabinoser | 1.19:72.01:5.85:20.95 | HPLC; IR; 1HNME | Li et al. [35] | |
| MAPS-5 | 1.3 × 104 | α-D-(1→4) glucose | GC-MS; IR; NMR | Lin et al. [36] | |
| APS I | 4.8 × 106 | Arabinose, Xylose, Glucose | 0.54:1.00:18.14 | HPLC; GC; IR; NMR | Liu [37] |
| APS II | 8.7 × 103 | Arabinose, Xylose, Glucose | 0.23:1.00:29.39 | HPLC; GC; IR; NMR | Liu [37] |
| APS | 3.8 × 104 | Glucose, Galactose, Arabinose | 1.00:0.95:0.70 | HPLC | Liu et al. [38] |
| APS | 3.6 × 104 | α-(1→4)-D-glucose | FTIR; AMLC; GLC- MS; NMR | Li et al. [39] | |
| HAPS | Rhamnose, Arabinose, Xylose, Mannose, Galactose, Glucose | 1.00:2.26:0.21:0.74:2.49:19.47 | HPLC; GC | Shan et al. [40] | |
| APS | Rhamnose, Arabinose, Xylose, Mannose, Galactose, Glucose | 1.00:4.34:3.92:1.95:11.41:20.52 | HPLC; GC | Shan et al. [40] | |
| HAPS | 1.7 × 106 | Mannose, Glucose, Xylose, Arabinose, Glucuronic acid, Rhamnose | 0.06:28.34:0.58:0.24:0.33:0.21 | UPLC/ESI-Q-TOF-MS; FT-IR and NMR | Liao et al. [41] |
| APS | 2.0 × 106 | Mannose, Glucose, Xylose, Arabinose, Glucuronic acid, Rhamnose | 0.27:12.83:1.63:0.71:1.04:0.56 | UPLC/ESI-Q-TOF-MS; FT-IR and NMR | Liao et al. [41] |
| APP-2A | 2.3 × 106 | Rhamnose, Galactose, Arabinose, Glucose | 1.00:2.13:3.22:6.18 | FT-IR; GC; NMR | Pu et al. [42] |
| Pharmacological | Experimental Model | Dosage | Mechanism | Reference |
|---|---|---|---|---|
| Antioxidant | Radiation injury model mice | 80 mg/kg | Significantly increased peripheral blood leucocyte count and DNA content in marrow cells, and the activities of SOD in serum. | Liu et al. [79] |
| Carbon tetrachloride-induced hepatocyte | 200, 400, 800 μg/mL in vitro; 1.5, 3 g/kg in vivo | Inhibited the elevation of GPT, GOT, LDH and MDA; significantly increased the level of SOD | Jia et al. [80] | |
| Human cardiac myocytes | 200 μg/mL | Significantly inhibited generation of ROS | Zhang et al. [81] | |
| Subcutaneously inoculated viable B16-F10 cells male mice | Significantly inhibited the NBT reduction index | Li et al. [82] | ||
| BPD cell model | 2.5 mg/mL | Down-regulated the expression of IL-8, ICAM-1, and NF-κB p65 | Huang et al. [83] | |
| Anti-aging | Mouse liver | 100, 200, 300 mg/kg | Scavenging reactive oxygen species (ROS); inhibiting mitochondrial PT; increasing the activities of antioxidases | Li et al. [84] |
| Aging model of D-galactose mice | 50, 100, 200 mg/kg | Increased the spleen and thymus indexes, and decreased MDA content and increased SOD, GSH-Px, and CAT activity. | Zhong et al. [85] | |
| HDF cell | 1.0 mg/mL | Enhanced cell viability and decreased the number of SAβ-gal positive cells | Zhao et al. [86] | |
| Cardiovascular protection | Rat cardiomyocyte injury model; mouse heart failure model | 1.5 g/kg | Restored normal autophagic flux; regulated the AMPK/mTOR pathway | Cao et al. [87] |
| THP-1 derived foam cells | 25, 50, 100 mg/L | Protected ABCA1 against the lesion of TNF-α in THP-1-derived foam cells | Wu et al. [88] | |
| SD neonatal rat | 10 mg/L | Decreased the expression of ANP mRNA, TNF-α, and IL-6 in extracellular fluid | Zhang et al. [89] | |
| Human cardiac microvascular endothelial cells (HCMEC) | 2.5, 5, 10 mg/mL | Reduced the expression of ICAM-1 and VCAM-1 in HCMEC, inhibiting leukocytes infiltration | Chen et al. [90] | |
| Diabetes treatment | KKAy female mice | 700 mg/kg | Restored insulin-induced protein kinase B Ser-473 phosphorylation; translocate glucose transporter 4 in skeletal muscle | Liu et al. [91] |
| Rat model of type II diabetes mellitus | 700 mg/kg | Restored the glucose homeostasis; reduced the ER stress in the rat model of T2DM | Wang et al. [92] | |
| NOD mice | 2.0 g/kg | Correcting the imbalance between the Th1/Th2 cytokines | Chen et al. [93] | |
| Intestinal protection | Ulcerative colitis rat | 200 mg/mL | Increased the volatile fatty acids; and liver bacterial translocation was in effective control; effectively control bacterial translocation in liver | Liang et al. [94] |
| 2, 4, 6-trinitrobenzene sulfonic acid-induced colitis rat model | 400 mg/kg | Restoring the number of Treg cells; Inhibiting interleukin IL-17 | Zhao et al. [95] | |
| Tumor-bearing mice; γδT cells | 150 and 300 mg/kg | Improved proliferation and function of intestinal intraepithelial γδT cells | Sun et al. [96] | |
| Hepatoprotective | Chronic liver injury male SD rats | 450 mg/kg | Lowered the levels of serum ALT, AST, ALP, and hepatic MDA concentration; higher SOD, CAT activities, and GSH concentration | Yan et al. [97] |
| Liver injury mice | 100 mg/kg | Anti-apoptosis pathway | Liu et al. [98] | |
| CCl4 induced liver damage mice | 1.0 g/kg | Scavenge free radicals to ameliorate oxidative stress and to inhibit lipid peroxidation | Pu et al. [99] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Jia, J.; Song, L.; Gong, X.; Xu, J.; Yang, M.; Li, M. Extraction, Structure, and Pharmacological Activities of Astragalus Polysaccharides. Appl. Sci. 2019, 9, 122. https://doi.org/10.3390/app9010122
Wang J, Jia J, Song L, Gong X, Xu J, Yang M, Li M. Extraction, Structure, and Pharmacological Activities of Astragalus Polysaccharides. Applied Sciences. 2019; 9(1):122. https://doi.org/10.3390/app9010122
Chicago/Turabian StyleWang, Jia, Junying Jia, Li Song, Xue Gong, Jianping Xu, Min Yang, and Minhui Li. 2019. "Extraction, Structure, and Pharmacological Activities of Astragalus Polysaccharides" Applied Sciences 9, no. 1: 122. https://doi.org/10.3390/app9010122
APA StyleWang, J., Jia, J., Song, L., Gong, X., Xu, J., Yang, M., & Li, M. (2019). Extraction, Structure, and Pharmacological Activities of Astragalus Polysaccharides. Applied Sciences, 9(1), 122. https://doi.org/10.3390/app9010122