Determination of Relative Ionization Cross Sections for Resonance Enhanced Multiphoton Ionization of Polycyclic Aromatic Hydrocarbons
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup
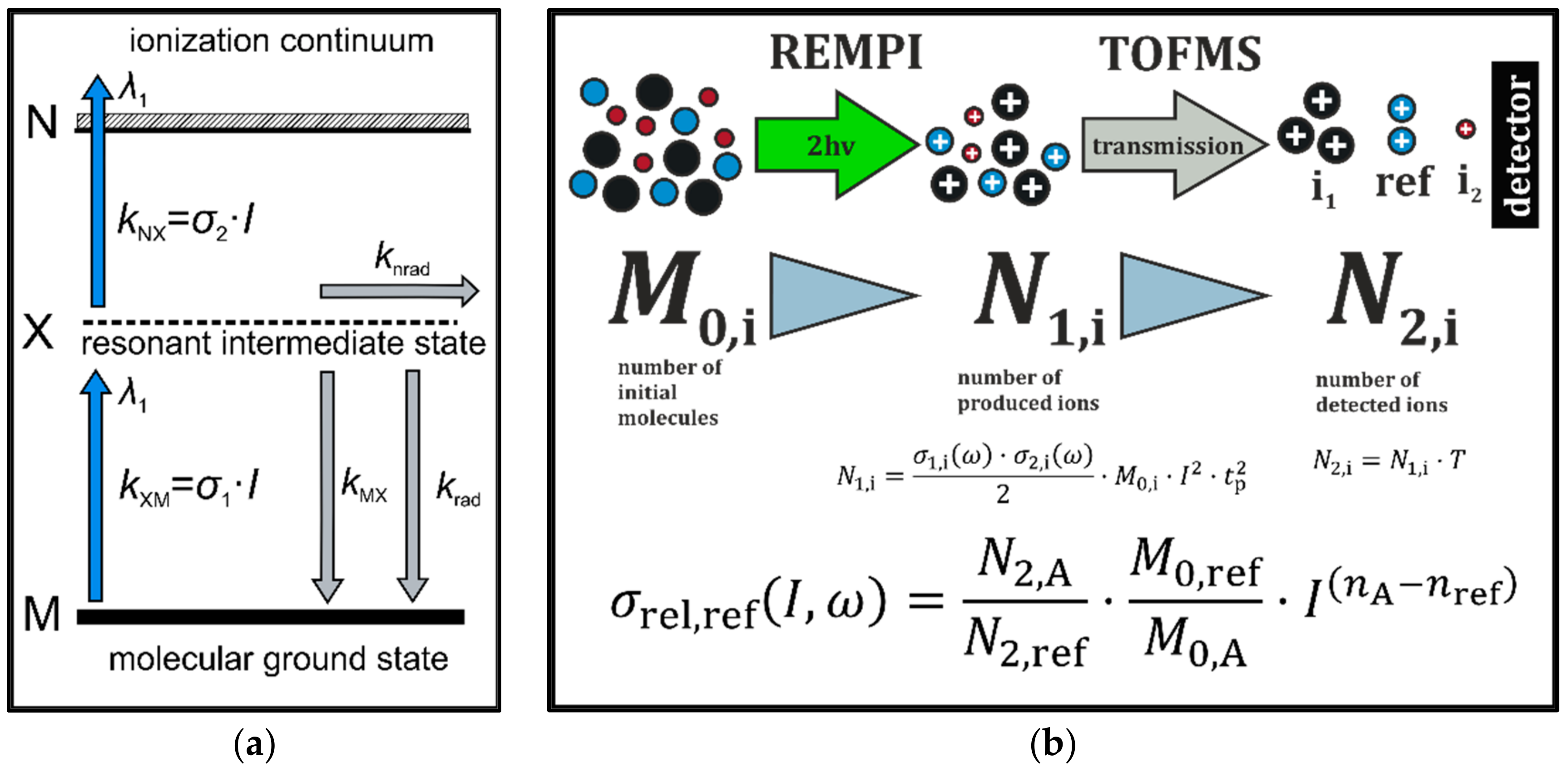
2.1.1. Mass Spectrometric Systems
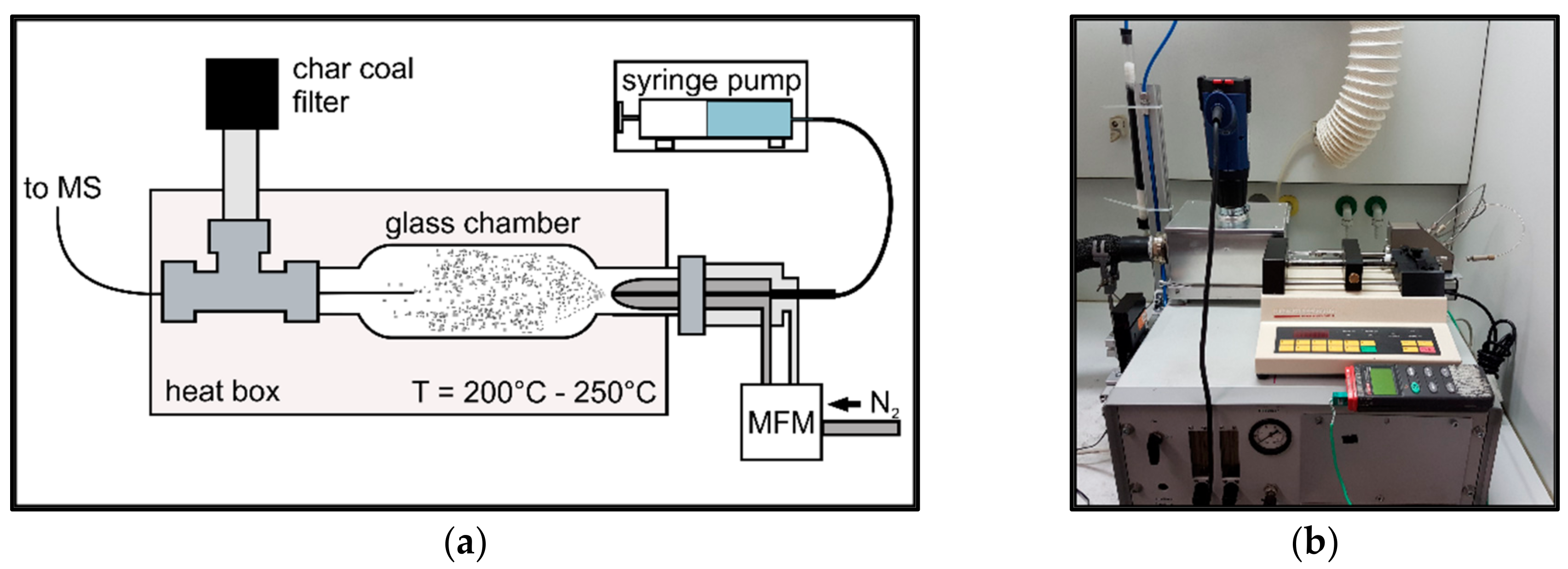
2.1.2. Sample Introduction
2.2. Standard Mixtures
2.3. Data Handling
3. Results
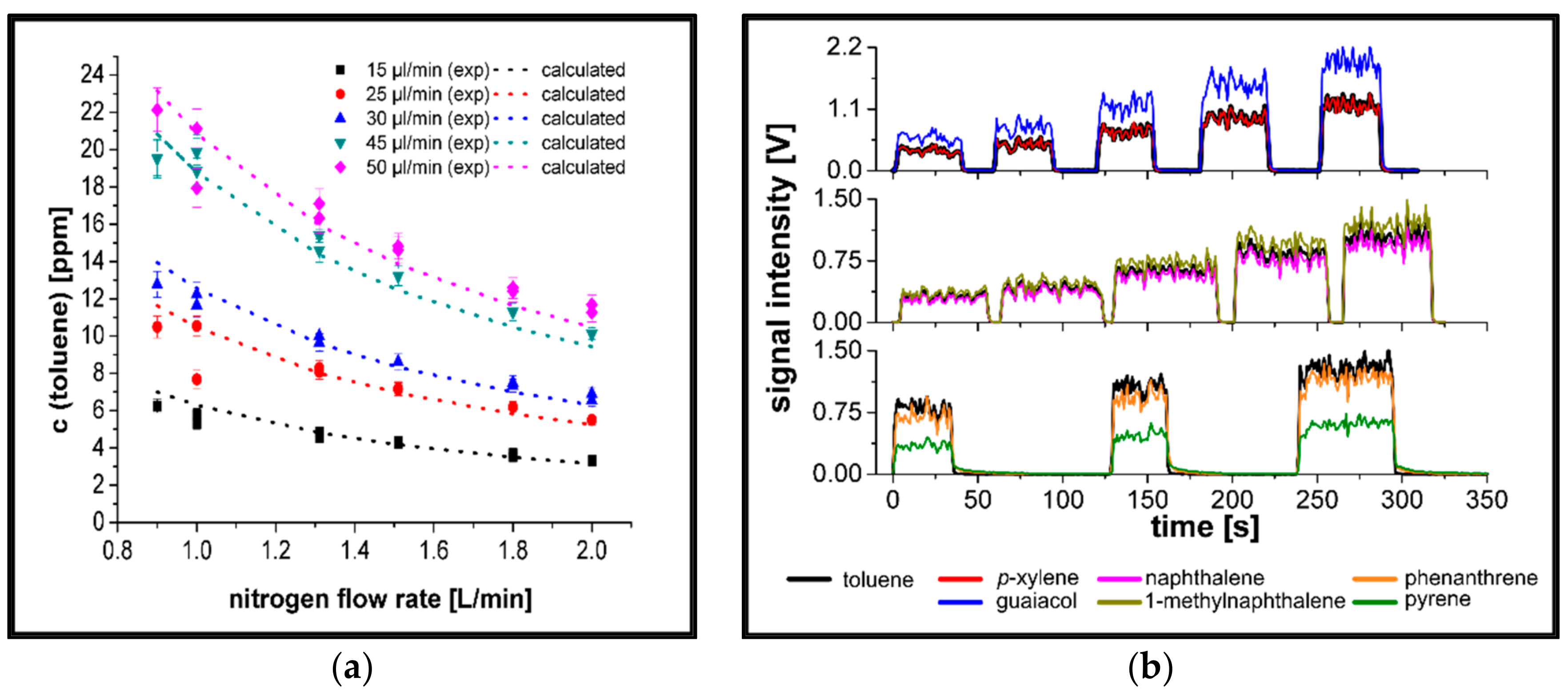
3.1. Evaluation of the Sample Introduction System
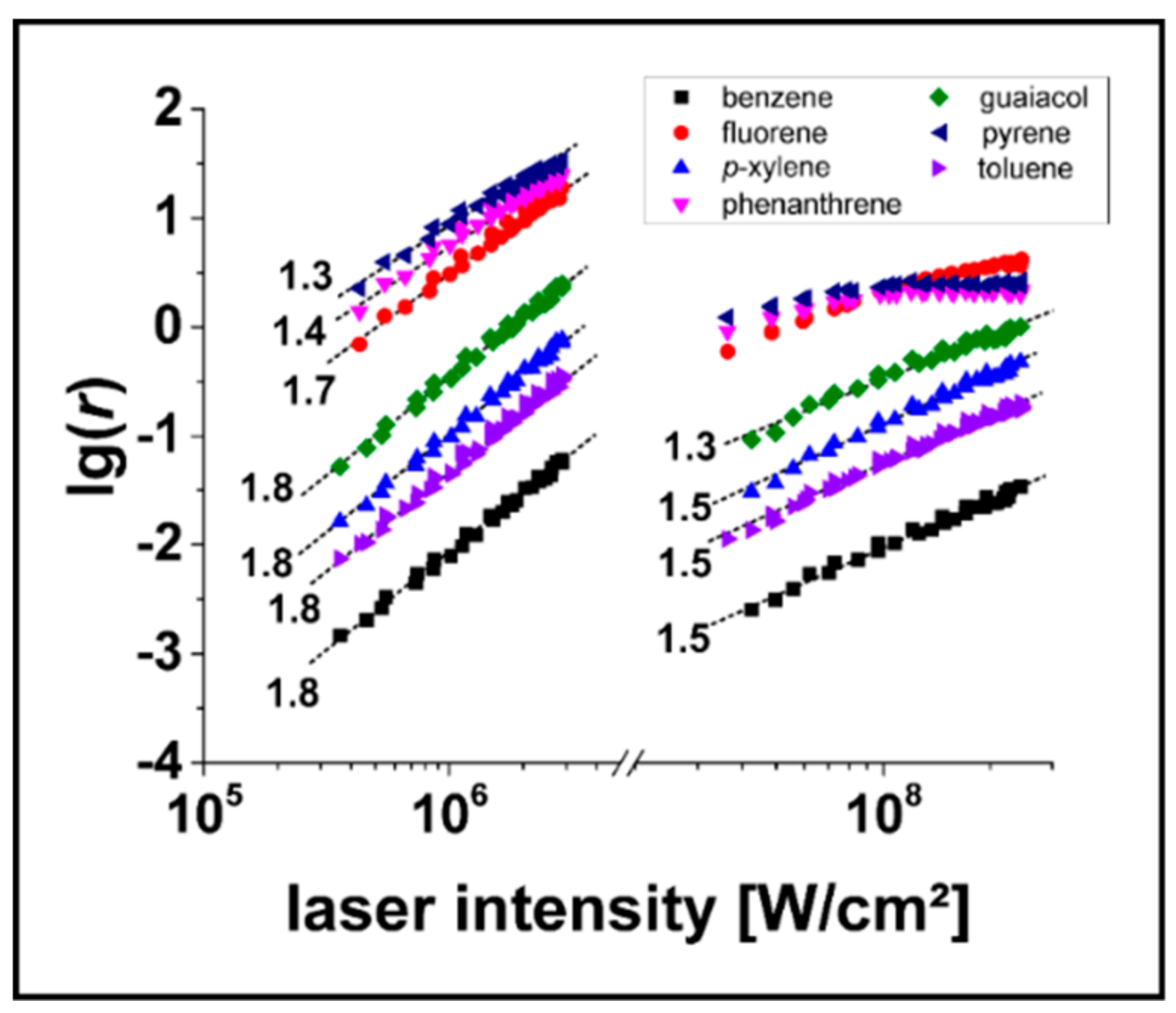
3.2. Determination of relPICS for 266 nm
3.3. Determination of relPICS for 248 nm
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boesl, U.; Neusser, H.J.; Schlag, E.W. Two-photon spectroscopy in the gas phase: First excited state of naphthalene, 1B3u. Chem. Phys. 1976, 15, 167–178. [Google Scholar] [CrossRef]
- Boesl, U.; Neusser, H.J.; Schlag, E.W. Two Photon Ionization of Polyatomic Molecules in a Mass Spectrometer. Z. Naturforsch. 1978, 33, 1546–1548. [Google Scholar] [CrossRef]
- Boesl, U.; Neusser, H.J.; Schlag, E.W. Lifetime measurements of two-photon excited vibronic levels in the low pressure limit: Naphthalene. Chem. Phys. Lett. 1976, 42, 16–21. [Google Scholar] [CrossRef]
- Zimmermann, R. Laser ionisation mass spectrometry for on-line analysis of complex gas mixtures and combustion effluents. Anal. Bioanal. Chem. 2005, 381, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Boesl, U.; Heger, H.J.; Zimmermann, R.; Nagel, H.; Püffel, P. Laser Mass Spectrometry in Trace Analysis. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2006. [Google Scholar]
- Streibel, T.; Zimmermann, R. Resonance-enhanced multiphoton ionization mass spectrometry (REMPI-MS): Applications for process analysis. Annu. Rev. Anal. Chem. 2014, 7, 361–381. [Google Scholar] [CrossRef] [PubMed]
- Kohse-Höinghaus, K.; Schocker, A.; Kasper, T.; Kamphus, M.; Brockhinke, A. Combination of Laser- and Mass-Spectroscopic Techniques for the Investigation of Fuel-Rich Flames. Z. Phys. Chem. 2005, 219, 583–599. [Google Scholar] [CrossRef]
- Kamphus, M.; Braun-Unkhoff, M.; Kohse-Höinghaus, K. Formation of small PAHs in laminar premixed low-pressure propene and cyclopentene flames: Experiment and modeling. Combust. Flame 2008, 152, 28–59. [Google Scholar] [CrossRef]
- Ahrens, J.; Kovacs, R.; Shafranovskii, E.A.; Homann, K.H. On-line multi-photon ionization mass spectrometry applied to PAH and fullerenes in flames. Ber. Bunsengesellschaft für Physikalische Chemie 1994, 98, 265–268. [Google Scholar] [CrossRef]
- Ahrens, J.; Keller, A.; Kovacs, R.; Homann, K. Large molecules, radicals, ions, and small soot particles in fuel-rich hydrocarbon flames: Part III: REMPI mass spectrometry of large flame PAHs and fullerenes and their quantitative calibration through sublimation. Berichte Bunsenges. Phys. Chem. 1998, 102, 1823–1839. [Google Scholar] [CrossRef]
- Otto, S.; Streibel, T.; Erdmann, S.; Klingbeil, S.; Schulz-Bull, D.; Zimmermann, R. Pyrolysis–gas chromatography–mass spectrometry with electron-ionization or resonance-enhanced-multi-photon-ionization for characterization of polycyclic aromatic hydrocarbons in the Baltic Sea. Mar. Pollut. Bull. 2015, 99, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Antonov, V.S.; Letokhov, V.S. Laser multiphoton and multistep photoionization of molecules and mass spectrometry. Appl. Phys. 1981, 24, 89–106. [Google Scholar] [CrossRef]
- Boesl, U.; Zimmermann, R.; Weickhardt, C.; Lenoir, D.; Schramm, K.W.; Kettrup, A.; Schlag, E.W. Resonance-enhanced multi-photon ionization: A species-selective ion source for analytical time-of-flight mass spectroscopy. Chemosphere 1994, 29, 1429–1440. [Google Scholar] [CrossRef]
- Boesl, U. Laser mass spectrometry for environmental and industrial chemical trace analysis. J. Mass Spectrom. 2000, 35, 289–304. [Google Scholar] [CrossRef]
- Zakheim, D.S.; Johnson, P.M. Rate equation modelling of molecular multiphoton ionization dynamics. Chem. Phys. 1980, 46, 263–272. [Google Scholar] [CrossRef]
- Letokhov, V.S.; Mishin, V.I.; Puretzky, A.A. Selective photoionization of atoms by laser radiation and its applications. Prog. Quantum Electron. 1979, 5, 139–203. [Google Scholar] [CrossRef]
- Dietz, W.; Neusser, H.J.; Boesl, U.; Schlag, E.W.; Lin, S.H. A model for multiphoton ionisation mass spectroscopy with application to benzene. Chem. Phys. 1982, 66, 105–127. [Google Scholar] [CrossRef]
- Brophy, J.H.; Rettner, C.T. Laser two-photon ionization of aniline in a molecular beam and the bulk gas phase. Chem. Phys. Lett. 1979, 67, 351–355. [Google Scholar] [CrossRef]
- Rettner, C.T.; Brophy, J.H. Resonance enhanced laser ionisation mass spectrometry of four aromatic molecules. Chem. Phys. 1981, 56, 53–61. [Google Scholar] [CrossRef]
- Reilly, J.P.; Kompa, K.L. Laser induced multiphoton ionization mass spectrum of benzene. J. Chem. Phys. 1980, 73, 5468. [Google Scholar] [CrossRef]
- Boesl, U.; Neusser, H.J.; Schlag, E.W. Visible and UV multiphoton ionization and fragmentation of polyatomic molecules. J. Chem. Phys. 1980, 72, 4327. [Google Scholar] [CrossRef]
- Boesl, U.; Neusser, H.J.; Schlag, E.W. Multi-photon ionization in the mass spectrometry of polyatomic molecules: Cross sections. Chem. Phys. 1981, 55, 193–204. [Google Scholar] [CrossRef]
- Adam, T.W.; Clairotte, M.; Streibel, T.; Elsasser, M.; Pommeres, A.; Manfredi, U.; Carriero, M.; Martini, G.; Sklorz, M.; Krasenbrink, A.; et al. Real-time analysis of aromatics in combustion engine exhaust by resonance-enhanced multiphoton ionisation time-of-flight mass spectrometry (REMPI-TOF-MS): A robust tool for chassis dynamometer testing. Anal. Bioanal. Chem. 2012, 404, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Czech, H.; Schepler, C.; Klingbeil, S.; Ehlert, S.; Howell, J.; Zimmermann, R. Resolving Coffee Roasting-Degree Phases Based on the Analysis of Volatile Compounds in the Roasting Off-Gas by Photoionization Time-of-Flight Mass Spectrometry (PI-TOFMS) and Statistical Data Analysis: Toward a PI-TOFMS Roasting Model. J. Agric. Food Chem. 2016, 64, 5223–5231. [Google Scholar] [CrossRef] [PubMed]
- Radischat, C.; Sippula, O.; Stengel, B.; Klingbeil, S.; Sklorz, M.; Rabe, R.; Streibel, T.; Harndorf, H.; Zimmermann, R. Real-time analysis of organic compounds in ship engine aerosol emissions using resonance-enhanced multiphoton ionisation and proton transfer mass spectrometry. Anal. Bioanal. Chem. 2015, 407, 5939–5951. [Google Scholar] [CrossRef] [PubMed]
- Heger, H.J.; Zimmermann, R.; Dorfner, R.; Beckmann, M.; Griebel, H.; Kettrup, A.; Boesl, U. On-Line Emission Analysis of Polycyclic Aromatic Hydrocarbons down to pptv Concentration Levels in the Flue Gas of an Incineration Pilot Plant with a Mobile Resonance-Enhanced Multiphoton Ionization Time-of-Flight Mass Spectrometer. Anal. Chem. 1999, 71, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Oser, H.; Coggiola, M.J.; Young, S.E.; Crosley, D.R.; Hafer, V.; Grist, G. Membrane introduction/laser photoionization time-of-flight mass spectrometry. Chemosphere 2007, 67, 1701–1708. [Google Scholar] [CrossRef] [PubMed]
- Soni, M.H.; Baronavski, A.P.; McElvany, S.W. Trace analysis of polyaromatic hydrocarbons in water using multiphoton ionization-membrane introduction mass spectrometry. Rapid Commun. Mass Spectrom. 1998, 12, 1635–1638. [Google Scholar] [CrossRef]
- Gehm, C.; Streibel, T.; Passig, J.; Schulz-Bull, D.; Zimmermann, R. Development and optimization of a membrane introduction photoionization mass spectrometer for the real time analysis of (poly)aromatic compounds in aquatic systems. Rapid Commun. Mass Spectrom. 2018. Submitted. [Google Scholar]
- Kruth, C.; Czech, H.; Sklorz, M.; Passig, J.; Ehlert, S.; Cappiello, A.; Zimmermann, R. Direct Infusion Resonance-Enhanced Multiphoton Ionization Mass Spectrometry of Liquid Samples under Vacuum Conditions. Anal. Chem. 2017, 89, 10917–10923. [Google Scholar] [CrossRef] [PubMed]
- Passig, J.; Schade, J.; Oster, M.; Fuchs, M.; Ehlert, S.; Jäger, C.; Sklorz, M.; Zimmermann, R. Aerosol Mass Spectrometer for Simultaneous Detection of Polyaromatic Hydrocarbons and Inorganic Components from Individual Particles. Anal. Chem. 2017, 89, 6341–6345. [Google Scholar] [CrossRef] [PubMed]
- Mühlberger, F.; Hafner, K.; Kaesdorf, S.; Ferge, T.; Zimmermann, R. Comprehensive On-Line Characterization of Complex Gas Mixtures by Quasi-Simultaneous Resonance-Enhanced Multiphoton Ionization, Vacuum-UV Single-Photon Ionization, and Electron Impact Ionization in a Time-of-Flight Mass Spectrometer: Setup and Instrument Characterization. Anal. Chem. 2004, 76, 6753–6764. [Google Scholar] [CrossRef] [PubMed]
- Etzkorn, T.; Klotz, B.; Sørensen, S.; Patroescu, I.V.; Barnes, I.; Becker, K.H.; Platt, U. Gas-phase absorption cross sections of 24 monocyclic aromatic hydrocarbons in the UV and IR spectral ranges. Atmos. Environ. 1999, 33, 525–540. [Google Scholar] [CrossRef]
- Carpentier, Y.; Pino, T.; Bréchignac, P. R2PI Spectroscopy of Aromatic Molecules Produced in an Ethylene-Rich Flame. J. Phys. Chem. A 2013, 117, 10092–10104. [Google Scholar] [CrossRef] [PubMed]
- Haefliger, O.P.; Zenobi, R. Laser mass spectrometric analysis of polycyclic aromatic hydrocarbons with wide wavelength range laser multiphoton ionization spectroscopy. Anal. Chem. 1998, 70, 2660–2665. [Google Scholar] [CrossRef] [PubMed]
- Boesl, U. Multiphoton excitation and mass-selective ion detection for neutral and ion spectroscopy. J. Phys. Chem. 1991, 95, 2949–2962. [Google Scholar] [CrossRef]
- Sippula, O.; Stengel, B.; Sklorz, M.; Streibel, T.; Rabe, R.; Orasche, J.; Lintelmann, J.; Michalke, B.; Abbaszade, G.; Radischat, C.; et al. Particle emissions from a marine engine: Chemical composition and aromatic emission profiles under various operating conditions. Environ. Sci. Technol. 2014, 48, 11721–11729. [Google Scholar] [CrossRef] [PubMed]
- Opsal, R.B.; Reilly, J.P. Ionization of alkylbenzenes studied by gas chromatography/laser ionization mass spectrometry. Anal. Chem. 2002, 60, 1060–1065. [Google Scholar] [CrossRef]
- Linstrom, P.J.; Mallard, W.G. (Eds.) NIST Chemistry WebBook, NIST Standard Reference Database Number 69. Available online: http://webbook.nist.gov (accessed on 1 August 2018).
- Ławicki, A.; Holm, A.I.S.; Rousseau, P.; Capron, M.; Maisonny, R.; Maclot, S.; Seitz, F.; Johansson, H.A.B.; Rosén, S.; Schmidt, H.T.; et al. Multiple ionization and fragmentation of isolated pyrene and coronene molecules in collision with ions. Phys. Rev. A 2011, 83, 22704. [Google Scholar] [CrossRef]
- Gotkis, Y.; Oleinikova, M.; Naor, M.; Lifshitz, C. Time-dependent mass spectra and breakdown graphs. 17. Naphthalene and phenanthrene. J. Phys. Chem. 1993, 97, 12282–12290. [Google Scholar] [CrossRef]
- Speiser, S.; Jortner, J. The power law for high order multiphoton processes. Chem. Phys. Lett. 1976, 44, 399–403. [Google Scholar] [CrossRef]
- Dufour, A.; Weng, J.; Jia, L.; Tang, X.; Sirjean, B.; Fournet, R.; Le Gall, H.; Brosse, N.; Billaud, F.; Mauviel, G.; et al. Revealing the chemistry of biomass pyrolysis by means of tunable synchrotron photoionisation-mass spectrometry. RSC Adv. 2013, 3, 4786–4792. [Google Scholar] [CrossRef]
- Weickhardt, C.; Boesl, U.; Schlag, E.W. Laser Mass Spectrometry for Time-Resolved Multicomponent Analysis of Exhaust Gas. Anal. Chem. 2002, 66, 1062–1069. [Google Scholar] [CrossRef]
- Salama, F.; Joblin, C.; Allamandola, L.J. Electronic absorption spectroscopy of matrix-isolated polycyclic aromatic hydrocarbon cations. II. The phenanthrene cation (C14H10+) and its 1-methyl derivative. J. Chem. Phys. 1998, 101, 10252–10262. [Google Scholar] [CrossRef]
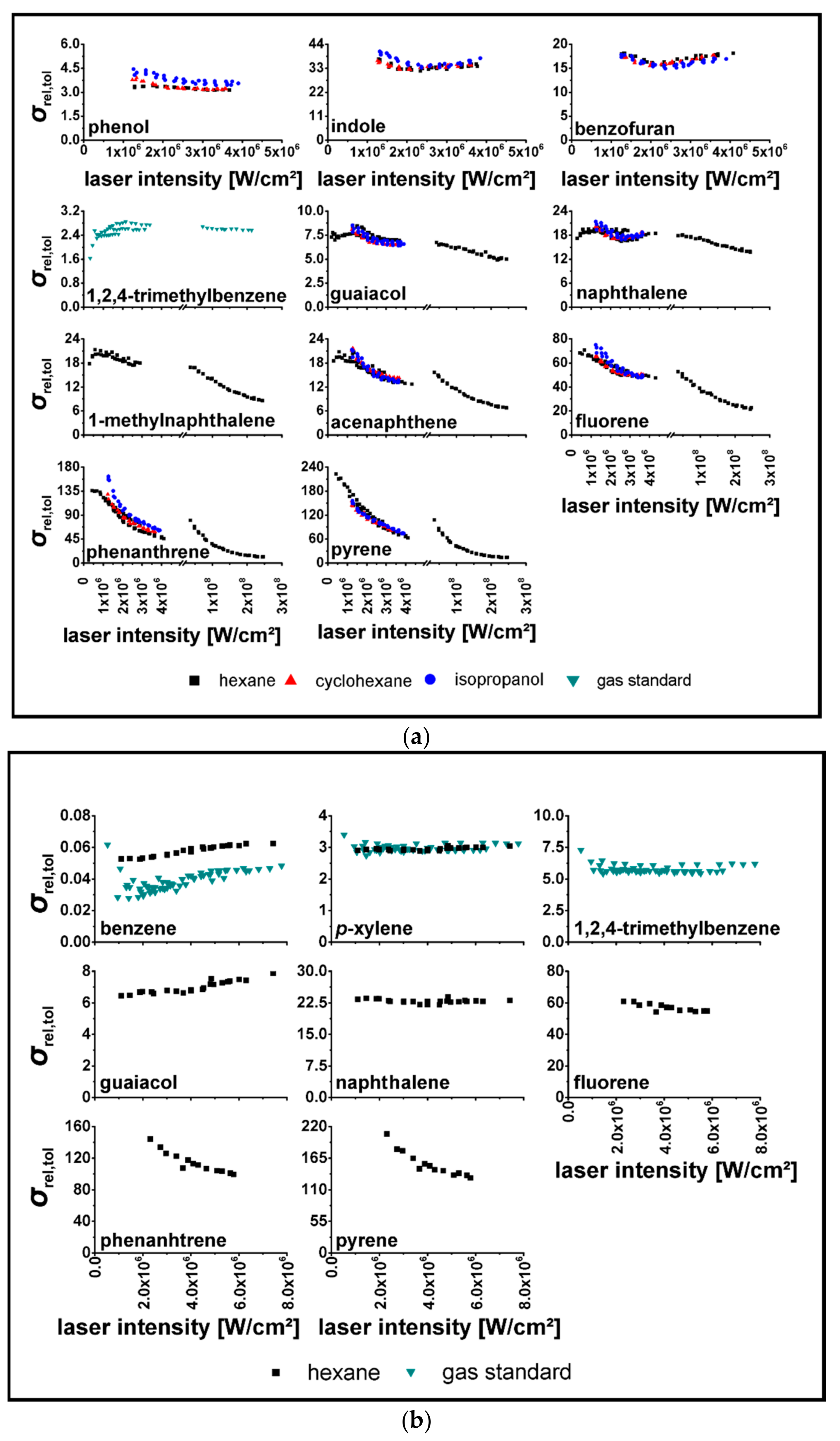

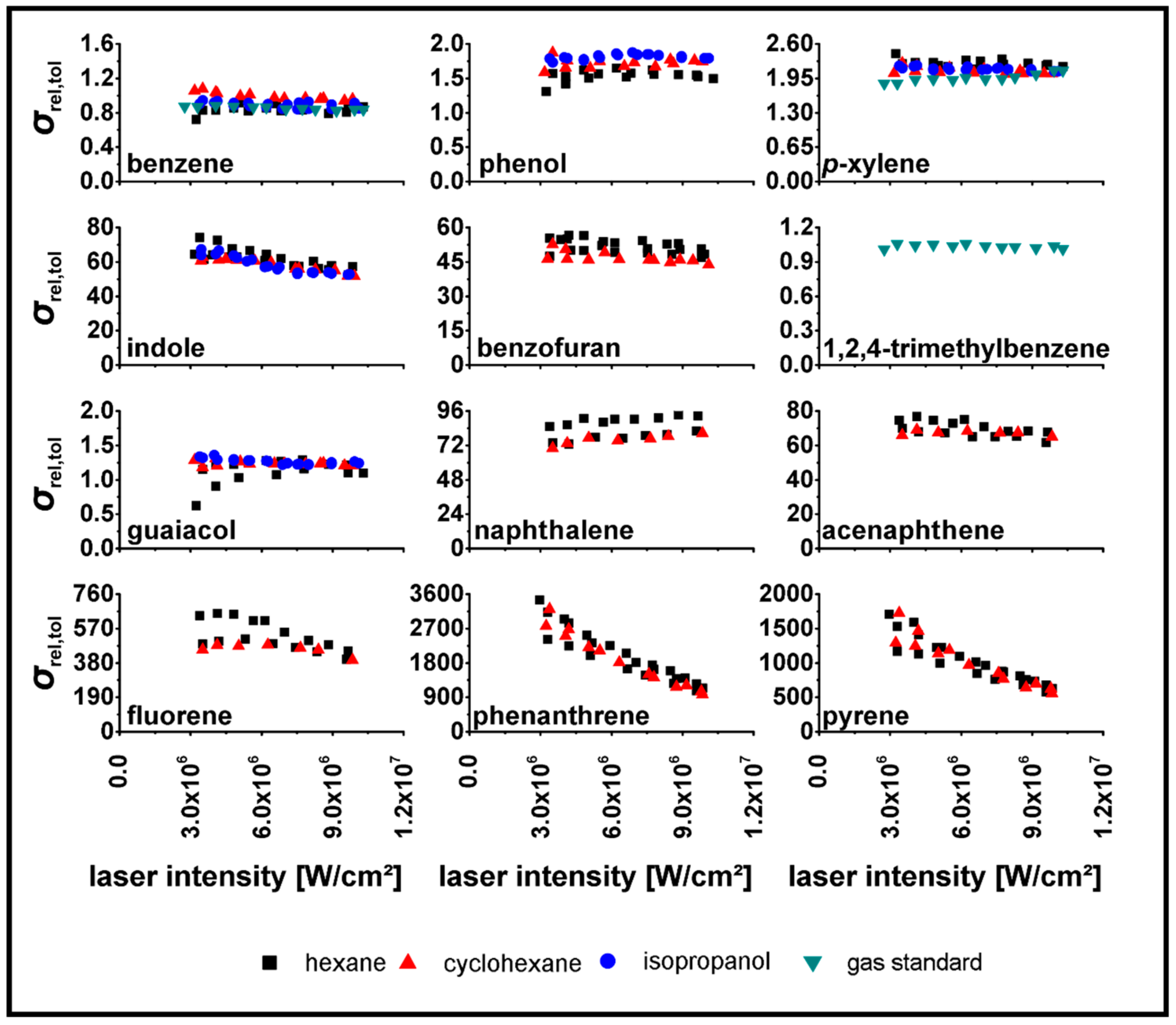
| Substance | |||||
|---|---|---|---|---|---|
| m/z | PITOF 1 (266 nm) | PITOF 2 (266 nm) | PITOF 2 Used in Reference [17] (266 nm) | PITOF 1 (248 nm) | |
| benzene | 78 | 0.11 * (0,15 *) | 0.05 * | 0.06 | 0.91 * (0.86 *) |
| phenol | 94 | 3.6 * | - | 1.66 | 1.70 |
| o-xylene | 106 | 2.0 * | - | 2.1 | - |
| p-xylene | 106 | 2.5 * (2.4 *) | 3.0* | 2.3 * (3.1 *) | 2.2 * (1.96 *) |
| indole | 117 | 32.2–40.7 | - | 31.1 | 51.8–74.2 |
| benzofuran | 118 | 15.0–18.1 | - | 13.6 | 49.3 |
| 1,2,4-trimethylbenzene | 120 | (2.6 *) | (5.8 *) | (5.13 *) | (1.03 *) |
| guaiacol | 124 | 4.9–8.5 | 6.5–7.9 | 5.45 | 1.2 * |
| naphthalene | 128 | 13.8–21.4 | 22.9 | 25.5 | 59.4–93.3 |
| 1-methylnaphthalene | 142 | 9.0–20 | - | 24.1 | - |
| acenaphthene | 154 | 6.8–21.6 | - | 28.5 | 40.4–76.8 |
| fluorene | 166 | 21.3–75.0 | 54.2–61.0 | 86.8 | 270–654 |
| phenanthrene | 178 | 11.5–163 | 99.4–144 | 179 | 976–3451 |
| pyrene | 202 | 14.0–223 | 130–207 | 196 | 555–1730 |
| Substance | Ionization Energy (eV) | Absolute REMPI Cross Sections at 266 nm (cm4) (I = 3 × 106 W/cm2) | Absolute REMPI Cross Sections at 248 nm (cm4) (I = 6 × 106 W/cm2) |
|---|---|---|---|
| toluene | 8.83 a | 5.4 × 10−35 | 2.7 × 10−35 |
| benzene | 9.24 a | 4.9 × 10−36 | 2.4 × 10−35 |
| phenol | 8.49 a | 1.9 × 10−34 | 4.6 × 10−35 |
| o-xylene | 8.56 a | 1.1 × 10−34 | n.d. |
| p-xylene | 8.44 a | 1.5 ×10−34 | 5.7 × 10−35 |
| indole | 7.76 a | 1.8 × 10−33 | 1.6 × 10−33 |
| benzofuran | 8.36 a | 9.1 × 10−34 | 1.3 × 10−33 |
| 1,2,4-trimethylbenzene | 8.27 a | 2.3 × 10−34 | 2.8 × 10−35 |
| guaiacol | 7,99 b | 3.9 × 10−34 | 3.2 × 10−35 |
| naphthalene | 8.14 a | 1.1 × 10−33 | 2.2 × 10−33 |
| 1-methylnaphthalene | 7.96 a | 9.8 × 10−34 | n.d. |
| acenaphthene | 7.75 a | 7.7 × 10−34 | 1.8 × 10−33 |
| fluorene | 7.91 a | 2.8 × 10−33 | 1.4 × 10−32 |
| phenanthrene | 7,89 a | 4.4 × 10−33 | 7.9 × 10−32 |
| pyrene | 7.43 a | 6.1 × 10−33 | 2.8 × 10−32 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gehm, C.; Streibel, T.; Passig, J.; Zimmermann, R. Determination of Relative Ionization Cross Sections for Resonance Enhanced Multiphoton Ionization of Polycyclic Aromatic Hydrocarbons. Appl. Sci. 2018, 8, 1617. https://doi.org/10.3390/app8091617
Gehm C, Streibel T, Passig J, Zimmermann R. Determination of Relative Ionization Cross Sections for Resonance Enhanced Multiphoton Ionization of Polycyclic Aromatic Hydrocarbons. Applied Sciences. 2018; 8(9):1617. https://doi.org/10.3390/app8091617
Chicago/Turabian StyleGehm, Christian, Thorsten Streibel, Johannes Passig, and Ralf Zimmermann. 2018. "Determination of Relative Ionization Cross Sections for Resonance Enhanced Multiphoton Ionization of Polycyclic Aromatic Hydrocarbons" Applied Sciences 8, no. 9: 1617. https://doi.org/10.3390/app8091617
APA StyleGehm, C., Streibel, T., Passig, J., & Zimmermann, R. (2018). Determination of Relative Ionization Cross Sections for Resonance Enhanced Multiphoton Ionization of Polycyclic Aromatic Hydrocarbons. Applied Sciences, 8(9), 1617. https://doi.org/10.3390/app8091617




