Effect of Temperature, pH, and Reaction Duration on Microbially Induced Calcite Precipitation
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms
2.2. Experimental Procedures
3. Test Results
4. Conclusions
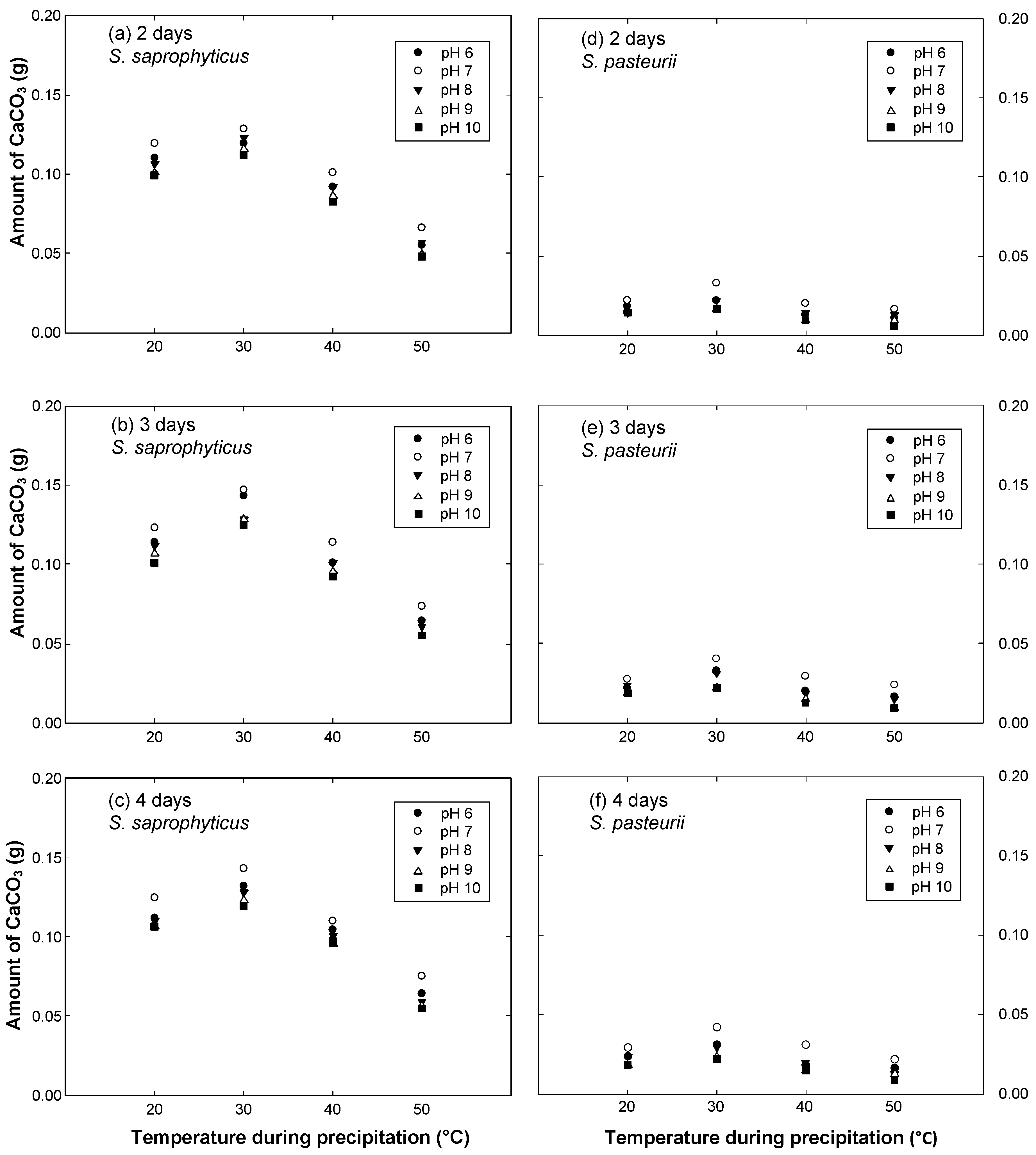
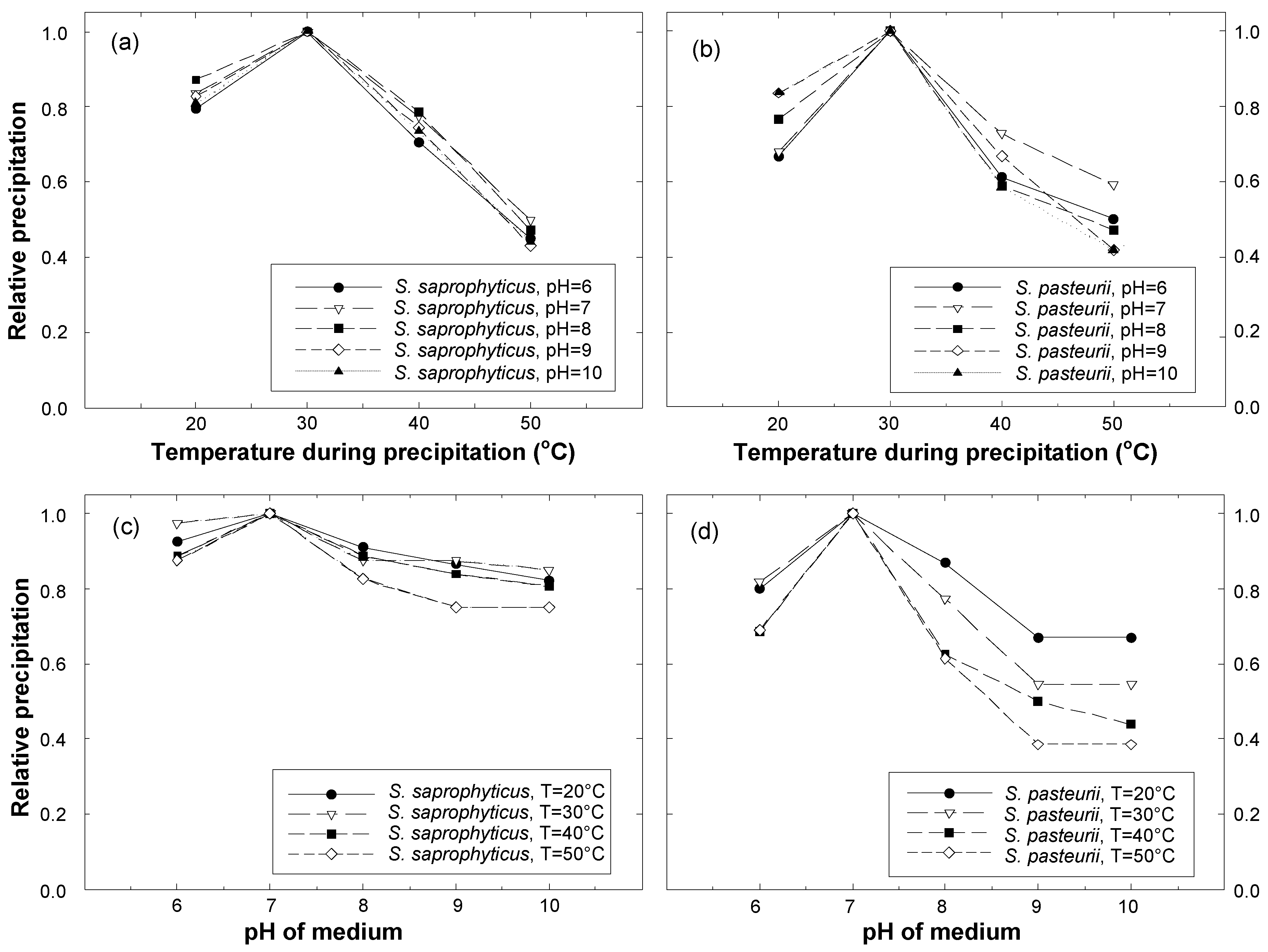
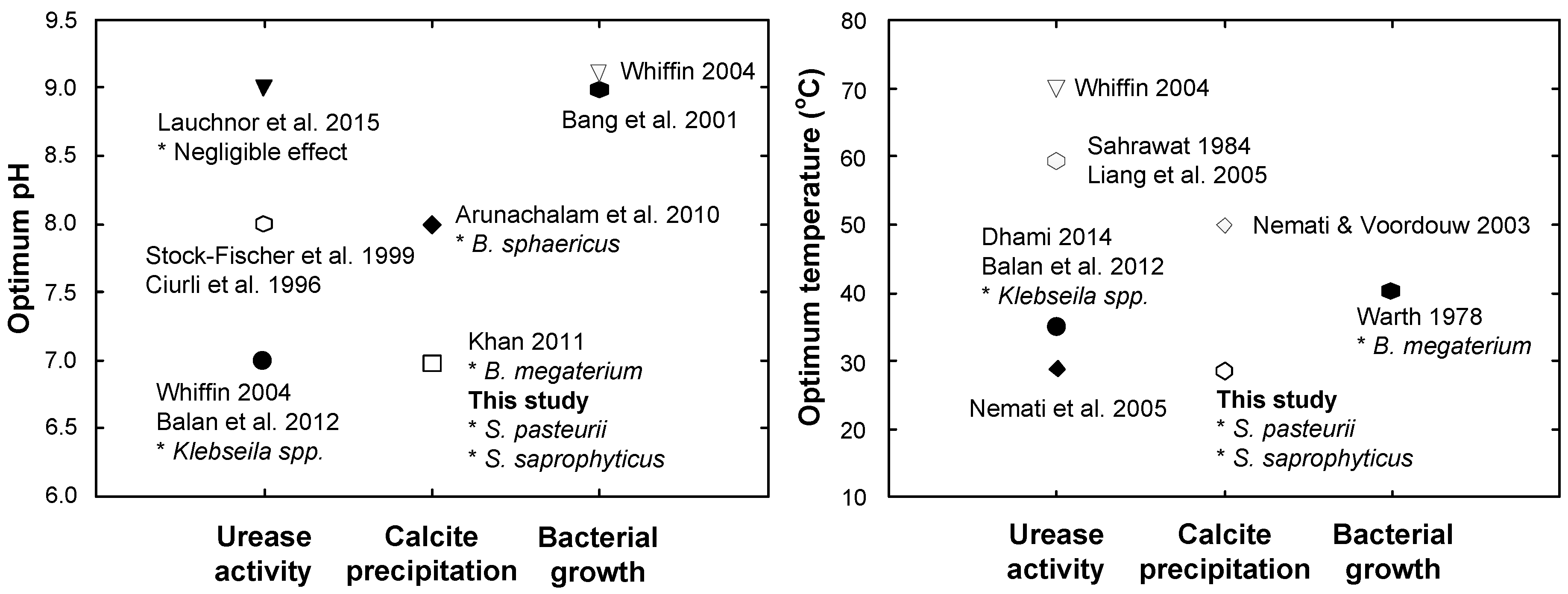
- The optimal conditions for calcite precipitation for both the species were identical: the optimum pH of the urea-CaCl2 medium was 7 and the optimum temperature during precipitation was 30 °C. The precipitation of S. saprophyticus was five times more than that of S. pasteurii under identical conditions.
- Both microbial species were strongly influenced by the temperature of the environment during precipitation, showing a significant decrease with varying temperatures. The least precipitation was measured at a temperature of 50 °C, which was as little as 30–40% of that seen at the optimal temperature (30 °C).
- S. saprophyticus was found to be less sensitive to a change in pH of the urea-CaCl2 medium than S. pasteurii; the decrease in precipitation by varying the pH was less than 20% for S. saprophyticus. On the other hand, pH significantly affected the precipitation of S. pasteurii, reducing it by 60%.
- For S. saprophyticus, a meaningful increase in precipitation was observed between the reaction durations of 2 and 3 days, but little difference was measured for longer durations, indicating that maximum precipitation occurred within 3 days. Likewise, most of the precipitation occurred within 3 days for S. pasteurii.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mitchell, J.K.; Santamarina, J.C. Biological considerations in geotechnical engineering. J. Geotech. Geoenviron. Eng. 2005, 131, 1222–1233. [Google Scholar] [CrossRef]
- Paul, E.; Clark, F. Soil Microbiology and Biochemistry; Academic Press: New York, NY, USA, 1996; p. 254. [Google Scholar]
- Chu, J.; Ivanov, V.; Naeimi, M.; Stabnikov, V.; Liu, H.L. Optimization of calcium-based bioclogging and biocementation of sand. Acta Geotech. 2014, 9, 277–285. [Google Scholar] [CrossRef]
- Harkes, M.P.; van Paassen, L.A.; Booster, J.L.; Whiffin, V.S.; van Loosdrecht, M. Fixation and distribution of bacterial activity in sand to induce carbonate precipitation for ground reinforcement. Ecol. Eng. 2010, 36, 112–117. [Google Scholar] [CrossRef]
- Achal, V.; Pan, X. Influence of calcium sources on microbially induced calcium carbonate precipitation by Bacillus sp. CR2. Appl. Biochem. Biotechnol. 2014, 173, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Shahrokhi-Shahraki, R.; Zomorodian, S.M.A.; Niazi, A.; O’Kelly, B.C. Improving sand with microbial-induced carbonate precipitation. Proc. Inst. Civ. Eng. Improv. 2015, 168, 217–230. [Google Scholar] [CrossRef]
- Chang, I.; Im, J.; Cho, G.C. Introduction of microbial biopolymers in soil treatment for future environmentally-friendly and sustainable geotechnical engineering. Sustainability 2016, 8, 251. [Google Scholar] [CrossRef]
- Al Qabany, A.; Soga, K.; Santamarina, C. Factors affecting efficiency of microbially induced calcite precipitation. J. Geotech. Geoenviron. Eng. 2011, 138, 992–1001. [Google Scholar] [CrossRef]
- Ng, W.S.; Lee, M.L.; Hii, S.L. An overview of the factors affecting microbial-induced calcite precipitation and its potential application in soil improvement. World Acad. Sci. Eng. Technol. 2012, 62, 723–729. [Google Scholar]
- Mujah, D.; Shahin, M.A.; Cheng, L. State-of-the-art review of biocementation by microbially induced calcite precipitation (MICP) for soil stabilization. Geomicrobiol. J. 2017, 34, 524–537. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, N.; Cai, G.; Jin, Y.; Ding, N.; Shen, D. Review of ground improvement using microbial induced carbonate precipitation (MICP). Mar. Georesour. Geotechnol. 2017, 35, 1135–1146. [Google Scholar] [CrossRef]
- Cheng, L.; Shahin, M.; Mujah, D. Influence of key environmental conditions on microbially induced cementation for soil stabilization. J. Geotech. Geoenviron. Eng. 2017, 143, 04016083. [Google Scholar] [CrossRef]
- Soon, N.W.; Lee, L.M.; Khun, T.C.; Ling, H.S. Factors affecting improvement in engineering properties of residual soil through microbial-induced calcite precipitation. J. Geotech. Geoenviron. Eng. 2014, 140, 04014006. [Google Scholar] [CrossRef]
- Umar, M.; Kassim, K.A.; Chiet, K.T.P. Biological process of soil improvement in civil engineering: A review. J. Rock Mech. Geotech. Eng. 2016, 8, 767–774. [Google Scholar] [CrossRef]
- Mahawish, A.; Bouazza, A.; Gates, W.P. Factors affecting the bio-cementing process of coarse sand. Proc. Inst. Civ. Eng. Improv. 2018, 1–45. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, L.; Li, C.; Li, M.; Amini, F.; Zhang, H. Factors affecting improvement of engineering properties of MICP-treated soil catalyzed by bacteria and urease. J. Mater. Civ. Eng. 2014, 26, 04014094. [Google Scholar] [CrossRef]
- Wang, R.; Qian, C.; Wang, J. Study on microbiological precipitation of CaCO3. J. Southeast Univ. 2005, 35, 95–191. [Google Scholar]
- Castanier, S.; Le Métayer-Levrel, G.; Perthuisot, J.P. Ca-carbonates precipitation and limestone genesis—the microbiogeologist point of view. Sediment. Geol. 1999, 126, 9–23. [Google Scholar] [CrossRef]
- Sahrawat, K. Effects of temperature and moisture on urease activity in semi-arid tropical soils. Plant Soil 1984, 78, 401–408. [Google Scholar] [CrossRef]
- Liang, Z.P.; Feng, Y.Q.; Meng, S.X.; Liang, Z.Y. Preparation and properties of urease immobilized onto glutaraldehyde cross-linked chitosan beads. Chin. Chem. Lett. 2005, 16, 135–138. [Google Scholar]
- Nemati, M.; Greene, E.; Voordouw, G. Permeability profile modification using bacterially formed calcium carbonate: comparison with enzymic option. Process Biochem. 2005, 40, 925–933. [Google Scholar] [CrossRef]
- Nemati, M.; Voordouw, G. Modification of porous media permeability, using calcium carbonate produced enzymatically in situ. Enzyme Microb. Technol. 2003, 33, 635–642. [Google Scholar] [CrossRef]
- Rebata-Landa, V. Microbial Activity in Sediments: Effects on Soil Behavior. Ph.D. Thesis, Georgia Institute of Technology, Atlanta, GA, USA, 2007. [Google Scholar]
- Cheng, L.; Shahin, M.; Cord-Ruwisch, R.; Addis, M.; Hartanto, T.; Elms, C. In Soil stabilisation by microbial-induced calcite precipitation (micp): Investigation into some physical and environmental aspects. In Proceedings of the 7th International Congress on Environmental Geotechnics, Melbourne, Australia, 10–14 November 2014; p. 1105. [Google Scholar]
- Jones, T.; Detwiler, R. In Fracture-aperture alteration induced by calcite precipitation. In Proceedings of the 49th US Rock Mechanics/Geomechanics Symposium, San Francisco, CA, USA, 28 June–1 July 2015. [Google Scholar]
- Ferris, F.; Stehmeier, L.; Kantzas, A.; Mourits, F. Bacteriogenic mineral plugging. J. Can. Pet. Technol. 1996, 35. [Google Scholar] [CrossRef]
- Stocks-Fischer, S.; Galinat, J.K.; Bang, S.S. Microbiological precipitation of CaCO3. Soil Biol. Biochem. 1999, 31, 1563–1571. [Google Scholar] [CrossRef]
- Evans, D.; Evans, D.G.; Takemura, T.; Nakano, H.; Lampert, H.C.; Graham, D.Y.; Granger, D.N.; Kvietys, P.R. Characterization of a Helicobacter pylori neutrophil-activating protein. Infect. Immun. 1995, 63, 2213–2220. [Google Scholar] [PubMed]
- Arunachalam, K.D.; Sathyanarayanan, K.; Darshan, B.; Raja, R.B. Studies on the characterisation of Biosealant properties of Bacillus sphaericus. Int. J. Eng. Sci. Technol. 2010, 2, 270–277. [Google Scholar]
- Mobley, H.; Island, M.D.; Hausinger, R.P. Molecular biology of microbial ureases. Microbiol. Rev. 1995, 59, 451–480. [Google Scholar] [PubMed]
- Ciurli, S.; Marzadori, C.; Benini, S.; Deiana, S.; Gessa, C. Urease from the soil bacterium Bacillus pasteurii: immobilization on Ca-polygalacturonate. Soil Biol. Biochem. 1996, 28, 811–817. [Google Scholar] [CrossRef]
- Dupraz, S.; Ménez, B.; Gouze, P.; Leprovost, R.; Bénézeth, P.; Pokrovsky, O.S.; Guyot, F. Experimental approach of CO2 biomineralization in deep saline aquifers. Chem. Geol. 2009, 265, 54–62. [Google Scholar] [CrossRef]
- Fujita, Y.; Redden, G.D.; Ingram, J.C.; Cortez, M.M.; Ferris, F.G.; Smith, R.W. Strontium incorporation into calcite generated by bacterial ureolysis1. Geochim. Cosmochim. Acta 2004, 68, 3261–3270. [Google Scholar] [CrossRef]
- Ferris, F.; Phoenix, V.; Fujita, Y.; Smith, R. Kinetics of calcite precipitation induced by ureolytic bacteria at 10 to 20 °C in artificial groundwater. Geochim. Cosmochim. Acta 2004, 68, 1701–1710. [Google Scholar] [CrossRef]
- Khan, J.A. Biodegradation of azo dye by moderately halotolerant Bacillus megaterium and study of enzyme azoreductase involved in degradation. Adv. Biotech. 2011, 10, 21–27. [Google Scholar]
- DeJong, J.T.; Mortensen, B.M.; Martinez, B.C.; Nelson, D.C. Bio-mediated soil improvement. Ecol. Eng. 2010, 36, 197–210. [Google Scholar] [CrossRef]
- Ehrlich, H.L. Geomicrobiology: its significance for geology. Earth Sci. Rev. 1998, 45, 45–60. [Google Scholar] [CrossRef]
- Akiyama, M.; Kawasaki, S. Microbially mediated sand solidification using calcium phosphate compounds. Eng. Geol. 2012, 137, 29–39. [Google Scholar] [CrossRef]
- Stabnikov, V.; Jian, C.; Ivanov, V.; Li, Y. Halotolerant, alkaliphilic urease-producing bacteria from different climate zones and their application for biocementation of sand. World J. Microbiol. Biotechnol. 2013, 29, 1453–1460. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, B.; DeJong, J. Strength and stiffness of MICP treated sand subjected to various stress paths. In Proceedings of the Geo-Frontiers 2011, Advances in Geotechnical Engineering, Dallas, TX, USA, 13–16 March 2011; pp. 4012–4020. [Google Scholar]
- Kim, G.; Youn, H. Microbially induced calcite precipitation employing environmental isolates. Materials 2016, 9, 468. [Google Scholar] [CrossRef] [PubMed]
- ASTM-D4373. Standard Test Method for Rapid Determination of Carbonate Content of Soils; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- Frankenberger, W.; Dick, W. Relationships between enzyme activities and microbial growth and activity indices in soil1. Soil Sci. Soc. Am. J. 1983, 47, 945–951. [Google Scholar] [CrossRef]
- Mobley, H.; Hausinger, R. Microbial ureases: Significance, regulation, and molecular characterization. Microbiol. Rev. 1989, 53, 85–108. [Google Scholar] [PubMed]
- Lauchnor, E.G.; Topp, D.; Parker, A.; Gerlach, R. Whole cell kinetics of ureolysis by S porosarcina pasteurii. J. Appl. Microbiol. 2015, 118, 1321–1332. [Google Scholar] [CrossRef] [PubMed]
- Bang, S.S.; Galinat, J.K.; Ramakrishnan, V. Calcite precipitation induced by polyurethane-immobilized Bacillus pasteurii. Enzyme Microb. Technol. 2001, 28, 404–409. [Google Scholar] [CrossRef]
- Whiffin, V.S. Microbial CaCO3 Precipitation for the Production of Biocement. Ph.D. Thesis, Murdoch University, Murdoch, Australia, 2004. [Google Scholar]
- Dhami, N.K.; Reddy, M.S.; Mukherjee, A. Synergistic role of bacterial urease and carbonic anhydrase in carbonate mineralization. Appl. Biochem. Biotechnol. 2014, 172, 2552–2561. [Google Scholar] [CrossRef] [PubMed]
- Warth, A. Relationship between the heat resistance of spores and the optimum and maximum growth temperatures of Bacillus species. J. Bacteriol. 1978, 134, 699–705. [Google Scholar] [PubMed]
- Balan, S.S.; Fathima, F.; Jayalakshmi, S. Characterization of urease enzyme from marine bacterium Klebsiella species. Afr. J. Microbiol. Res. 2012, 6, 5914–5923. [Google Scholar]


| Reaction Duration (Day) | Amount of Calcite Precipitation (g) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Staphylococcus saprophyticus | Sporosarcina pasteurii | ||||||||||
| pH Temp. | 6 | 7 | 8 | 9 | 10 | 6 | 7 | 8 | 9 | 10 | |
| 2 | 20 | 0.1102 | 0.1193 | 0.1065 | 0.1010 | 0.0991 | 0.0184 | 0.0220 | 0.0147 | 0.0147 | 0.0147 |
| 30 | 0.1193 | 0.1285 | 0.1230 | 0.1157 | 0.1120 | 0.0220 | 0.0330 | 0.0220 | 0.0165 | 0.0165 | |
| 40 | 0.0918 | 0.1010 | 0.0918 | 0.0863 | 0.0826 | 0.0129 | 0.0202 | 0.0147 | 0.0092 | 0.0092 | |
| 50 | 0.0551 | 0.0661 | 0.0569 | 0.0496 | 0.0477 | 0.0110 | 0.0165 | 0.0129 | 0.0092 | 0.0055 | |
| 3 | 20 | 0.1138 | 0.1230 | 0.1120 | 0.1065 | 0.1010 | 0.0220 | 0.0275 | 0.0239 | 0.0184 | 0.0184 |
| 30 | 0.1432 | 0.1469 | 0.1285 | 0.1285 | 0.1249 | 0.0330 | 0.0404 | 0.0312 | 0.0220 | 0.0220 | |
| 40 | 0.1010 | 0.1138 | 0.1010 | 0.0955 | 0.0918 | 0.0202 | 0.0294 | 0.0184 | 0.0147 | 0.0129 | |
| 50 | 0.0643 | 0.0734 | 0.0606 | 0.0551 | 0.0551 | 0.0165 | 0.0239 | 0.0147 | 0.0092 | 0.0092 | |
| 4 | 20 | 0.1120 | 0.1249 | 0.1102 | 0.1065 | 0.1065 | 0.0239 | 0.0294 | 0.0239 | 0.0184 | 0.0184 |
| 30 | 0.1322 | 0.1432 | 0.1285 | 0.1230 | 0.1193 | 0.0312 | 0.0422 | 0.0294 | 0.0239 | 0.0220 | |
| 40 | 0.1047 | 0.1102 | 0.1010 | 0.0955 | 0.0973 | 0.0184 | 0.0312 | 0.0202 | 0.0147 | 0.0147 | |
| 50 | 0.0643 | 0.0753 | 0.0588 | 0.0569 | 0.0551 | 0.0165 | 0.0220 | 0.0129 | 0.0129 | 0.0092 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, G.; Kim, J.; Youn, H. Effect of Temperature, pH, and Reaction Duration on Microbially Induced Calcite Precipitation. Appl. Sci. 2018, 8, 1277. https://doi.org/10.3390/app8081277
Kim G, Kim J, Youn H. Effect of Temperature, pH, and Reaction Duration on Microbially Induced Calcite Precipitation. Applied Sciences. 2018; 8(8):1277. https://doi.org/10.3390/app8081277
Chicago/Turabian StyleKim, Gunjo, Janghwan Kim, and Heejung Youn. 2018. "Effect of Temperature, pH, and Reaction Duration on Microbially Induced Calcite Precipitation" Applied Sciences 8, no. 8: 1277. https://doi.org/10.3390/app8081277
APA StyleKim, G., Kim, J., & Youn, H. (2018). Effect of Temperature, pH, and Reaction Duration on Microbially Induced Calcite Precipitation. Applied Sciences, 8(8), 1277. https://doi.org/10.3390/app8081277





