Effect of Temperature, pH, and Reaction Duration on Microbially Induced Calcite Precipitation
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms
2.2. Experimental Procedures
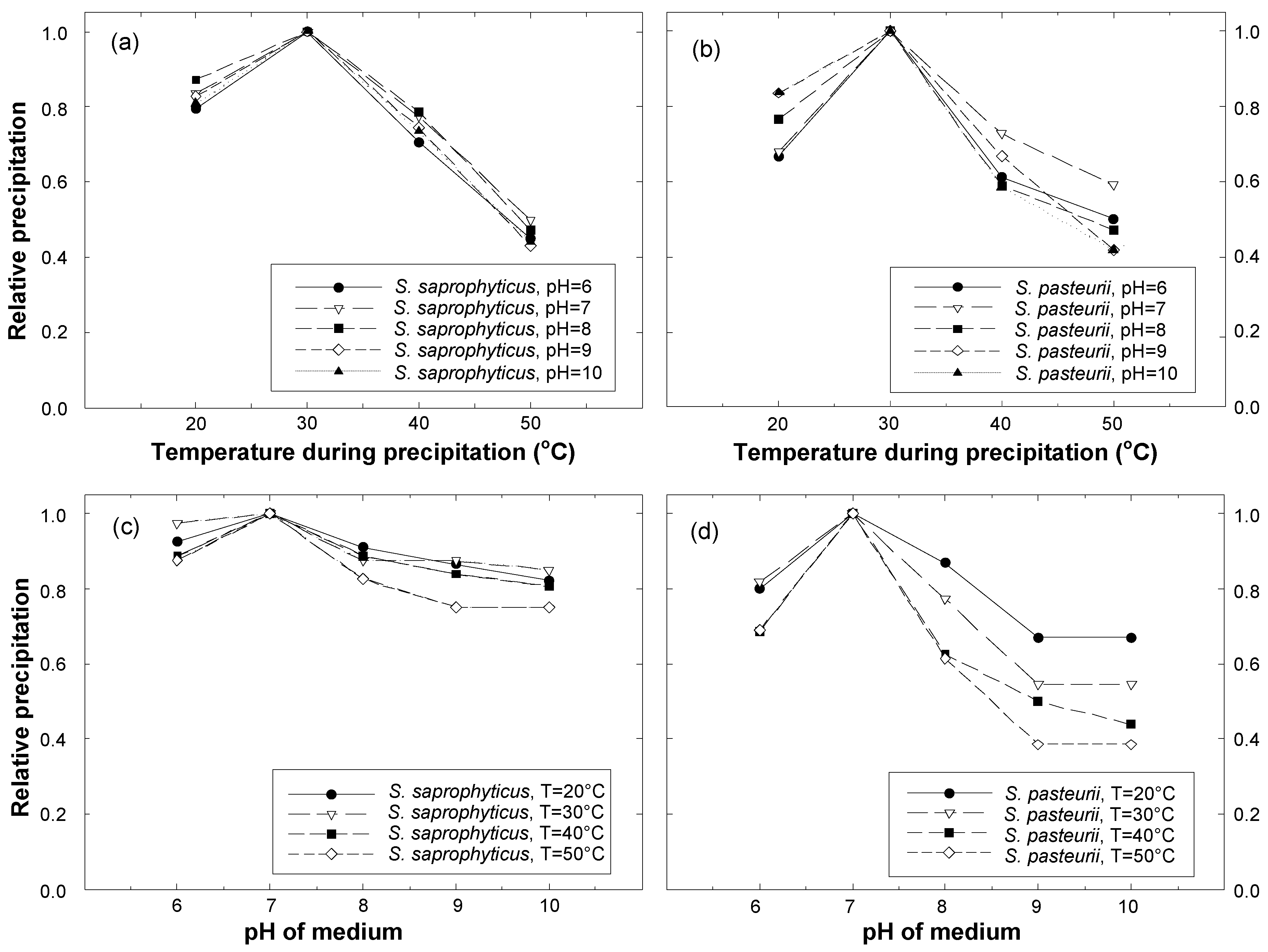
3. Test Results
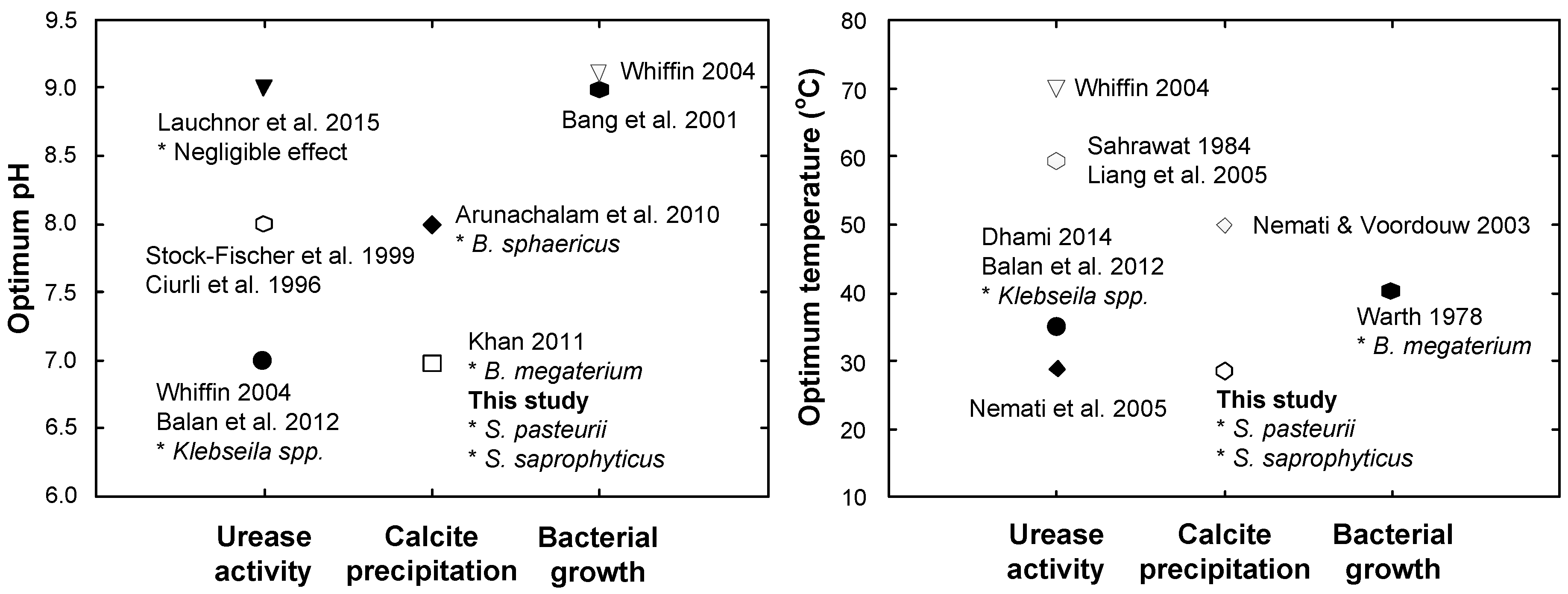
4. Conclusions
- The optimal conditions for calcite precipitation for both the species were identical: the optimum pH of the urea-CaCl2 medium was 7 and the optimum temperature during precipitation was 30 °C. The precipitation of S. saprophyticus was five times more than that of S. pasteurii under identical conditions.
- Both microbial species were strongly influenced by the temperature of the environment during precipitation, showing a significant decrease with varying temperatures. The least precipitation was measured at a temperature of 50 °C, which was as little as 30–40% of that seen at the optimal temperature (30 °C).
- S. saprophyticus was found to be less sensitive to a change in pH of the urea-CaCl2 medium than S. pasteurii; the decrease in precipitation by varying the pH was less than 20% for S. saprophyticus. On the other hand, pH significantly affected the precipitation of S. pasteurii, reducing it by 60%.
- For S. saprophyticus, a meaningful increase in precipitation was observed between the reaction durations of 2 and 3 days, but little difference was measured for longer durations, indicating that maximum precipitation occurred within 3 days. Likewise, most of the precipitation occurred within 3 days for S. pasteurii.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mitchell, J.K.; Santamarina, J.C. Biological considerations in geotechnical engineering. J. Geotech. Geoenviron. Eng. 2005, 131, 1222–1233. [Google Scholar] [CrossRef]
- Paul, E.; Clark, F. Soil Microbiology and Biochemistry; Academic Press: New York, NY, USA, 1996; p. 254. [Google Scholar]
- Chu, J.; Ivanov, V.; Naeimi, M.; Stabnikov, V.; Liu, H.L. Optimization of calcium-based bioclogging and biocementation of sand. Acta Geotech. 2014, 9, 277–285. [Google Scholar] [CrossRef]
- Harkes, M.P.; van Paassen, L.A.; Booster, J.L.; Whiffin, V.S.; van Loosdrecht, M. Fixation and distribution of bacterial activity in sand to induce carbonate precipitation for ground reinforcement. Ecol. Eng. 2010, 36, 112–117. [Google Scholar] [CrossRef]
- Achal, V.; Pan, X. Influence of calcium sources on microbially induced calcium carbonate precipitation by Bacillus sp. CR2. Appl. Biochem. Biotechnol. 2014, 173, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Shahrokhi-Shahraki, R.; Zomorodian, S.M.A.; Niazi, A.; O’Kelly, B.C. Improving sand with microbial-induced carbonate precipitation. Proc. Inst. Civ. Eng. Improv. 2015, 168, 217–230. [Google Scholar] [CrossRef]
- Chang, I.; Im, J.; Cho, G.C. Introduction of microbial biopolymers in soil treatment for future environmentally-friendly and sustainable geotechnical engineering. Sustainability 2016, 8, 251. [Google Scholar] [CrossRef]
- Al Qabany, A.; Soga, K.; Santamarina, C. Factors affecting efficiency of microbially induced calcite precipitation. J. Geotech. Geoenviron. Eng. 2011, 138, 992–1001. [Google Scholar] [CrossRef]
- Ng, W.S.; Lee, M.L.; Hii, S.L. An overview of the factors affecting microbial-induced calcite precipitation and its potential application in soil improvement. World Acad. Sci. Eng. Technol. 2012, 62, 723–729. [Google Scholar]
- Mujah, D.; Shahin, M.A.; Cheng, L. State-of-the-art review of biocementation by microbially induced calcite precipitation (MICP) for soil stabilization. Geomicrobiol. J. 2017, 34, 524–537. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, N.; Cai, G.; Jin, Y.; Ding, N.; Shen, D. Review of ground improvement using microbial induced carbonate precipitation (MICP). Mar. Georesour. Geotechnol. 2017, 35, 1135–1146. [Google Scholar] [CrossRef]
- Cheng, L.; Shahin, M.; Mujah, D. Influence of key environmental conditions on microbially induced cementation for soil stabilization. J. Geotech. Geoenviron. Eng. 2017, 143, 04016083. [Google Scholar] [CrossRef]
- Soon, N.W.; Lee, L.M.; Khun, T.C.; Ling, H.S. Factors affecting improvement in engineering properties of residual soil through microbial-induced calcite precipitation. J. Geotech. Geoenviron. Eng. 2014, 140, 04014006. [Google Scholar] [CrossRef]
- Umar, M.; Kassim, K.A.; Chiet, K.T.P. Biological process of soil improvement in civil engineering: A review. J. Rock Mech. Geotech. Eng. 2016, 8, 767–774. [Google Scholar] [CrossRef]
- Mahawish, A.; Bouazza, A.; Gates, W.P. Factors affecting the bio-cementing process of coarse sand. Proc. Inst. Civ. Eng. Improv. 2018, 1–45. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, L.; Li, C.; Li, M.; Amini, F.; Zhang, H. Factors affecting improvement of engineering properties of MICP-treated soil catalyzed by bacteria and urease. J. Mater. Civ. Eng. 2014, 26, 04014094. [Google Scholar] [CrossRef]
- Wang, R.; Qian, C.; Wang, J. Study on microbiological precipitation of CaCO3. J. Southeast Univ. 2005, 35, 95–191. [Google Scholar]
- Castanier, S.; Le Métayer-Levrel, G.; Perthuisot, J.P. Ca-carbonates precipitation and limestone genesis—the microbiogeologist point of view. Sediment. Geol. 1999, 126, 9–23. [Google Scholar] [CrossRef]
- Sahrawat, K. Effects of temperature and moisture on urease activity in semi-arid tropical soils. Plant Soil 1984, 78, 401–408. [Google Scholar] [CrossRef]
- Liang, Z.P.; Feng, Y.Q.; Meng, S.X.; Liang, Z.Y. Preparation and properties of urease immobilized onto glutaraldehyde cross-linked chitosan beads. Chin. Chem. Lett. 2005, 16, 135–138. [Google Scholar]
- Nemati, M.; Greene, E.; Voordouw, G. Permeability profile modification using bacterially formed calcium carbonate: comparison with enzymic option. Process Biochem. 2005, 40, 925–933. [Google Scholar] [CrossRef]
- Nemati, M.; Voordouw, G. Modification of porous media permeability, using calcium carbonate produced enzymatically in situ. Enzyme Microb. Technol. 2003, 33, 635–642. [Google Scholar] [CrossRef]
- Rebata-Landa, V. Microbial Activity in Sediments: Effects on Soil Behavior. Ph.D. Thesis, Georgia Institute of Technology, Atlanta, GA, USA, 2007. [Google Scholar]
- Cheng, L.; Shahin, M.; Cord-Ruwisch, R.; Addis, M.; Hartanto, T.; Elms, C. In Soil stabilisation by microbial-induced calcite precipitation (micp): Investigation into some physical and environmental aspects. In Proceedings of the 7th International Congress on Environmental Geotechnics, Melbourne, Australia, 10–14 November 2014; p. 1105. [Google Scholar]
- Jones, T.; Detwiler, R. In Fracture-aperture alteration induced by calcite precipitation. In Proceedings of the 49th US Rock Mechanics/Geomechanics Symposium, San Francisco, CA, USA, 28 June–1 July 2015. [Google Scholar]
- Ferris, F.; Stehmeier, L.; Kantzas, A.; Mourits, F. Bacteriogenic mineral plugging. J. Can. Pet. Technol. 1996, 35. [Google Scholar] [CrossRef]
- Stocks-Fischer, S.; Galinat, J.K.; Bang, S.S. Microbiological precipitation of CaCO3. Soil Biol. Biochem. 1999, 31, 1563–1571. [Google Scholar] [CrossRef]
- Evans, D.; Evans, D.G.; Takemura, T.; Nakano, H.; Lampert, H.C.; Graham, D.Y.; Granger, D.N.; Kvietys, P.R. Characterization of a Helicobacter pylori neutrophil-activating protein. Infect. Immun. 1995, 63, 2213–2220. [Google Scholar] [PubMed]
- Arunachalam, K.D.; Sathyanarayanan, K.; Darshan, B.; Raja, R.B. Studies on the characterisation of Biosealant properties of Bacillus sphaericus. Int. J. Eng. Sci. Technol. 2010, 2, 270–277. [Google Scholar]
- Mobley, H.; Island, M.D.; Hausinger, R.P. Molecular biology of microbial ureases. Microbiol. Rev. 1995, 59, 451–480. [Google Scholar] [PubMed]
- Ciurli, S.; Marzadori, C.; Benini, S.; Deiana, S.; Gessa, C. Urease from the soil bacterium Bacillus pasteurii: immobilization on Ca-polygalacturonate. Soil Biol. Biochem. 1996, 28, 811–817. [Google Scholar] [CrossRef]
- Dupraz, S.; Ménez, B.; Gouze, P.; Leprovost, R.; Bénézeth, P.; Pokrovsky, O.S.; Guyot, F. Experimental approach of CO2 biomineralization in deep saline aquifers. Chem. Geol. 2009, 265, 54–62. [Google Scholar] [CrossRef]
- Fujita, Y.; Redden, G.D.; Ingram, J.C.; Cortez, M.M.; Ferris, F.G.; Smith, R.W. Strontium incorporation into calcite generated by bacterial ureolysis1. Geochim. Cosmochim. Acta 2004, 68, 3261–3270. [Google Scholar] [CrossRef] [Green Version]
- Ferris, F.; Phoenix, V.; Fujita, Y.; Smith, R. Kinetics of calcite precipitation induced by ureolytic bacteria at 10 to 20 °C in artificial groundwater. Geochim. Cosmochim. Acta 2004, 68, 1701–1710. [Google Scholar] [CrossRef]
- Khan, J.A. Biodegradation of azo dye by moderately halotolerant Bacillus megaterium and study of enzyme azoreductase involved in degradation. Adv. Biotech. 2011, 10, 21–27. [Google Scholar]
- DeJong, J.T.; Mortensen, B.M.; Martinez, B.C.; Nelson, D.C. Bio-mediated soil improvement. Ecol. Eng. 2010, 36, 197–210. [Google Scholar] [CrossRef]
- Ehrlich, H.L. Geomicrobiology: its significance for geology. Earth Sci. Rev. 1998, 45, 45–60. [Google Scholar] [CrossRef]
- Akiyama, M.; Kawasaki, S. Microbially mediated sand solidification using calcium phosphate compounds. Eng. Geol. 2012, 137, 29–39. [Google Scholar] [CrossRef] [Green Version]
- Stabnikov, V.; Jian, C.; Ivanov, V.; Li, Y. Halotolerant, alkaliphilic urease-producing bacteria from different climate zones and their application for biocementation of sand. World J. Microbiol. Biotechnol. 2013, 29, 1453–1460. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, B.; DeJong, J. Strength and stiffness of MICP treated sand subjected to various stress paths. In Proceedings of the Geo-Frontiers 2011, Advances in Geotechnical Engineering, Dallas, TX, USA, 13–16 March 2011; pp. 4012–4020. [Google Scholar]
- Kim, G.; Youn, H. Microbially induced calcite precipitation employing environmental isolates. Materials 2016, 9, 468. [Google Scholar] [CrossRef] [PubMed]
- ASTM-D4373. Standard Test Method for Rapid Determination of Carbonate Content of Soils; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- Frankenberger, W.; Dick, W. Relationships between enzyme activities and microbial growth and activity indices in soil1. Soil Sci. Soc. Am. J. 1983, 47, 945–951. [Google Scholar] [CrossRef]
- Mobley, H.; Hausinger, R. Microbial ureases: Significance, regulation, and molecular characterization. Microbiol. Rev. 1989, 53, 85–108. [Google Scholar] [PubMed]
- Lauchnor, E.G.; Topp, D.; Parker, A.; Gerlach, R. Whole cell kinetics of ureolysis by S porosarcina pasteurii. J. Appl. Microbiol. 2015, 118, 1321–1332. [Google Scholar] [CrossRef] [PubMed]
- Bang, S.S.; Galinat, J.K.; Ramakrishnan, V. Calcite precipitation induced by polyurethane-immobilized Bacillus pasteurii. Enzyme Microb. Technol. 2001, 28, 404–409. [Google Scholar] [CrossRef]
- Whiffin, V.S. Microbial CaCO3 Precipitation for the Production of Biocement. Ph.D. Thesis, Murdoch University, Murdoch, Australia, 2004. [Google Scholar]
- Dhami, N.K.; Reddy, M.S.; Mukherjee, A. Synergistic role of bacterial urease and carbonic anhydrase in carbonate mineralization. Appl. Biochem. Biotechnol. 2014, 172, 2552–2561. [Google Scholar] [CrossRef] [PubMed]
- Warth, A. Relationship between the heat resistance of spores and the optimum and maximum growth temperatures of Bacillus species. J. Bacteriol. 1978, 134, 699–705. [Google Scholar] [PubMed]
- Balan, S.S.; Fathima, F.; Jayalakshmi, S. Characterization of urease enzyme from marine bacterium Klebsiella species. Afr. J. Microbiol. Res. 2012, 6, 5914–5923. [Google Scholar]


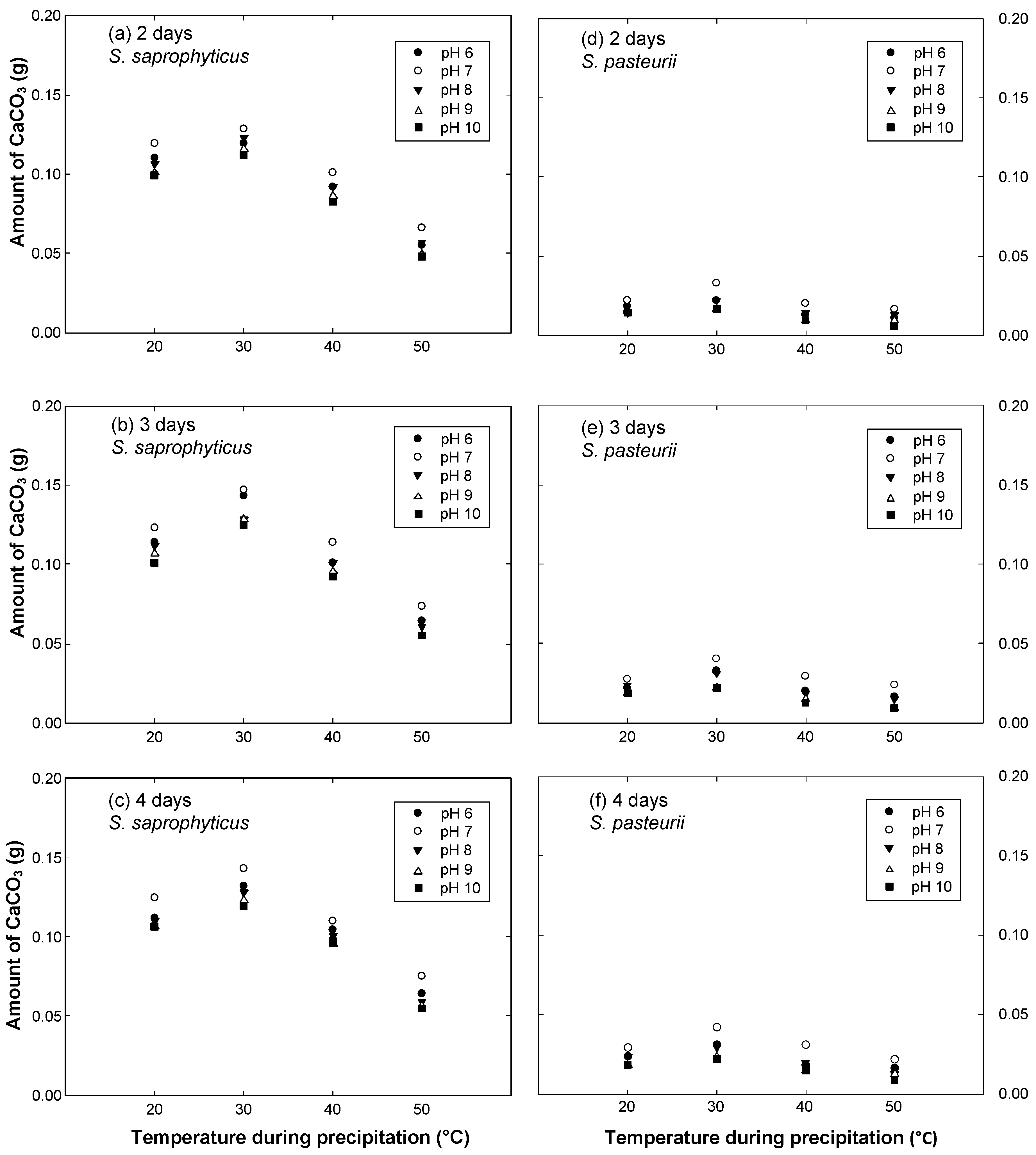
| Reaction Duration (Day) | Amount of Calcite Precipitation (g) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Staphylococcus saprophyticus | Sporosarcina pasteurii | ||||||||||
| pH Temp. | 6 | 7 | 8 | 9 | 10 | 6 | 7 | 8 | 9 | 10 | |
| 2 | 20 | 0.1102 | 0.1193 | 0.1065 | 0.1010 | 0.0991 | 0.0184 | 0.0220 | 0.0147 | 0.0147 | 0.0147 |
| 30 | 0.1193 | 0.1285 | 0.1230 | 0.1157 | 0.1120 | 0.0220 | 0.0330 | 0.0220 | 0.0165 | 0.0165 | |
| 40 | 0.0918 | 0.1010 | 0.0918 | 0.0863 | 0.0826 | 0.0129 | 0.0202 | 0.0147 | 0.0092 | 0.0092 | |
| 50 | 0.0551 | 0.0661 | 0.0569 | 0.0496 | 0.0477 | 0.0110 | 0.0165 | 0.0129 | 0.0092 | 0.0055 | |
| 3 | 20 | 0.1138 | 0.1230 | 0.1120 | 0.1065 | 0.1010 | 0.0220 | 0.0275 | 0.0239 | 0.0184 | 0.0184 |
| 30 | 0.1432 | 0.1469 | 0.1285 | 0.1285 | 0.1249 | 0.0330 | 0.0404 | 0.0312 | 0.0220 | 0.0220 | |
| 40 | 0.1010 | 0.1138 | 0.1010 | 0.0955 | 0.0918 | 0.0202 | 0.0294 | 0.0184 | 0.0147 | 0.0129 | |
| 50 | 0.0643 | 0.0734 | 0.0606 | 0.0551 | 0.0551 | 0.0165 | 0.0239 | 0.0147 | 0.0092 | 0.0092 | |
| 4 | 20 | 0.1120 | 0.1249 | 0.1102 | 0.1065 | 0.1065 | 0.0239 | 0.0294 | 0.0239 | 0.0184 | 0.0184 |
| 30 | 0.1322 | 0.1432 | 0.1285 | 0.1230 | 0.1193 | 0.0312 | 0.0422 | 0.0294 | 0.0239 | 0.0220 | |
| 40 | 0.1047 | 0.1102 | 0.1010 | 0.0955 | 0.0973 | 0.0184 | 0.0312 | 0.0202 | 0.0147 | 0.0147 | |
| 50 | 0.0643 | 0.0753 | 0.0588 | 0.0569 | 0.0551 | 0.0165 | 0.0220 | 0.0129 | 0.0129 | 0.0092 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, G.; Kim, J.; Youn, H. Effect of Temperature, pH, and Reaction Duration on Microbially Induced Calcite Precipitation. Appl. Sci. 2018, 8, 1277. https://doi.org/10.3390/app8081277
Kim G, Kim J, Youn H. Effect of Temperature, pH, and Reaction Duration on Microbially Induced Calcite Precipitation. Applied Sciences. 2018; 8(8):1277. https://doi.org/10.3390/app8081277
Chicago/Turabian StyleKim, Gunjo, Janghwan Kim, and Heejung Youn. 2018. "Effect of Temperature, pH, and Reaction Duration on Microbially Induced Calcite Precipitation" Applied Sciences 8, no. 8: 1277. https://doi.org/10.3390/app8081277
APA StyleKim, G., Kim, J., & Youn, H. (2018). Effect of Temperature, pH, and Reaction Duration on Microbially Induced Calcite Precipitation. Applied Sciences, 8(8), 1277. https://doi.org/10.3390/app8081277





