Abstract
Recent developments in high-density lithium-ion battery technologies have greatly expanded the electric vehicle (EV) market. Due to the fact that the rapid charging of an EV battery pack while maintaining a suitable cell cycle life is necessary for further growth of the EV market, we herein propose an innovative adaptive rapid charging pattern that minimizes cell degradation and reflects the degradation characteristics. This technology is advantageous in that cells can be developed by analyzing the charging characteristics in the latter stages of cell development of the rapid charging pattern, while also considering the complexity and heterogeneity of the manufacturing process. Furthermore, the battery charging pattern is optimized and controlled in real-time by reflecting the characteristics of the battery module and pack degradation as the cycle number is increased. More specifically, we present a preliminary study that simplifies the implementation of the new optimization pattern to improve the cell cycle life by over 45% in comparison to conventional fast charging patterns, and to address the drop in capacity in the latter half of cell life during rapid charging.
1. Introduction
Charging electric vehicle batteries at rates comparable to the refueling of gasoline-powered cars remains an unresolved problem. To address this issue and promote further growth in the electric vehicle market, technology capable of rapidly charging lithium-ion batteries while maintaining excellent cycle lives is essential. As such, the development of materials and manufacturing technologies appropriate for high-speed charging has received attention from both battery manufacturers and academia [1,2,3,4,5]. However, due to a lack of interest in the development of rapid charging patterns, battery companies are providing vehicle original equipment manufacturers (OEMs) with previously analyzed data from typical charging patterns.
In this context, the majority of typical rapid charging patterns consist of conventional single or multiple constant-current constant-voltage (CCCV) step charge processes as shown in Figure 1a [6,7,8,9]. To reduce charging times, conventional methods tend to involve charging the battery with a high constant current in a low state of charge (SOC) region prior to charging the battery through a series of decreasing charge current steps as the SOC increases with the aim of minimizing cell degradation. However, these conventional step charging methods do not reflect the environmental variables of the cell and the state caused by internal degradation, which ultimately results in rapid cell degradation, as shown in Figure 1b [6]. Other recently reported rapid charging methods include pulse charging and boost charging [10,11,12,13], which insert intermittent pauses to prevent lithium plating. Such plating is caused by high lithium ion concentrations around the anode due to the high charge current employed in these processes. However, as the design of a rapid charging pattern is related to the balance between the charging time and the cell cycle life, the use of higher charge currents to compensate for pause times can lead to rapid capacity losses.
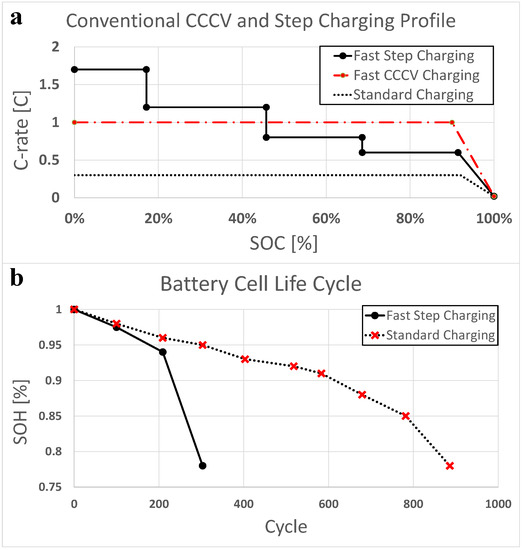
Figure 1.
Current pattern examples. Results from cycle life evaluations for (a) a conventional charging profile according to the charging method; and (b) a battery cell life cycle according the charging method.
As the environments where batteries are being used and their target applications become more diverse, a thorough understanding and accurate prediction of the behaviors of commercialized cells are becoming increasingly important in the context of developing novel cell charging patterns. To meet the requirements of automobile OEMs, batteries with higher energy densities and higher performances have been developed based on the design of batteries with higher packing densities and complex form factors. However, in the context of automobiles, the lithium-ion battery is difficult to understand through the prediction of cell operations by the analysis of laboratory-scale coin cells, or by predicting or analyzing actual cell operations through simulations. Furthermore, the prediction of battery characteristics becomes more challenging as the cycle life increases.
To address this issue, differential voltage analysis (DVA) can aid in understanding commercial cell behaviors in actual applications as changes in voltage with varying capacitance can be plotted as either dQ/dV or dV/dQ (dQ = the capacity increase, dV = the voltage increase) [14,15]. The observed changes in the peak values of the corresponding plots thereby provide information regarding phase changes, active material losses, and chemical changes within the battery [16,17,18,19,20,21,22]. Thus, we herein report our study into cell resistance characteristics based on DVA, and further propose two adaptive rapid charging patterns that reflect the intrinsic characteristics of the cells in a specific SOC, in addition to considering variations in degradation characteristics as the battery cycle life progresses.
Initially, we examined the development of the CV step charging pattern, which is a rapid charging method that allows self-charge control to reflect cell degradation and reduces cycle life degradation. Second, we report on the development of a charging pattern that controls the C-rate of the charging step according to the capacity degradation of the battery cycle to ultimately adjust the charging time to the initially set rapid charging time. In addition, by using the 18650 cylindrical lithium battery cell that is employed in electric vehicles, we examined the advantages of two new rapid charging methods (i.e., the CV step charging pattern and the constant time (CT) adaptive rapid charging pattern) compared to the conventional high-speed step charging method. Furthermore, additional analytical results are presented following the evaluation of the cell cycle life. Finally, we discuss the application of this novel method to battery module and pack applications.
2. Experimental
To design a new rapid charging pattern and test the newly proposed rapid charging method, we selected a 18650 cylindrical lithium ion battery cell. This 3.3 Ah lithium cobalt oxide (LCO)/graphite 18650 type cell has been used in various information technology applications following the reinforcement of its lifespan and safety characteristics [23].
In this study, DVA was employed to analyze the operating characteristics at the commercial cell level and to generate optimized rapid charging patterns. The DVA profile typically varies depending on the type of battery cell and the shape of the DVA plot as the 3.3 Ah cell is mainly determined by the LCO used as the cathode active material, although it can be further influenced by the cell shape and the active material loading. To obtain the DVA profile, basic performance tests were initially conducted for each cell. To test the 3.3 Ah cell, a constant temperature chamber was prepared with a maximum charge and discharge current of 10 A at 25 °C. The charge/discharge conditions of the cell were suitably adjusted for the cell design and the target application.
As shown in Figure 2, 3.3 Ah cells were charged at 0.3 C intervals between a 0.2 and 2.2 C-rate with a 4.25 V CCCV charge and a cutoff value lower than 1/10 C to obtain the cell IV curve data. After charging, the battery was discharged at a discharge rate of 1 C under a 3.0 V cutoff voltage. The data were converted into an DVA plot, which was analyzed to derive a new CV charging pattern and an adaptive rapid step charging pattern. Cycle life evaluation was then performed between 3.0 and 4.25 V using the proposed rapid charging pattern. During cycle life evaluation, a reference performance test (RPT) was performed after each block of 50 cycles to measure the quantitative changes in the battery capacity. Based on the measured capacities, the adaptive rapid charging pattern was updated with a new charging pattern optimized for the degraded capacity. A typical CCCV pattern was then employed to determine the charge C-rate value for the target charge time. The RPT evaluation consisted of capacity evaluation and DC resistance measurement. The capacity measurement was performed only for the nominal currents of 0.3 C for charging and 0.3 C for discharging, which allowed moving to the next step of resistance measurement without an additional pre-conditioning cycle in between. The resistance measurements were performed by the pulse train at 50% SOC.
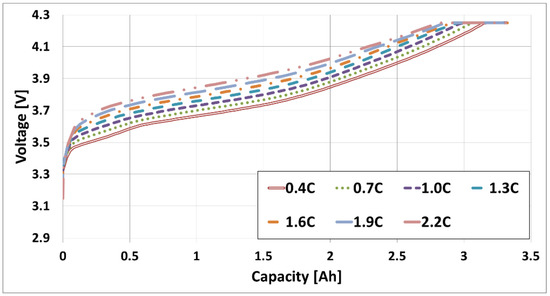
Figure 2.
The current-voltage curve of the 3.3 Ah cell.
Figure 3 shows the detailed experimental parameters for each experimental pattern, where the life cycle test was terminated at 80% of the initial capacity. After measuring the thickness of the cell following life cycle evaluation, the cell was fully discharged to 3.0 V to evaluate its additional characteristics. The headspace gas was extracted and transferred to a gas chromatography mass spectrometer (GC-MS) for analysis. Scanning electron microscopy (SEM), x-ray diffraction (XRD), x-ray photoelectron spectroscopy (XPS), and inductively coupled plasma mass spectrometry (ICP-MS) were also performed after cell degradation. Finally, we investigated the suitability of the new method in solving the cell balance issue by using data analysis, and compared the peak currents applied to each cell.
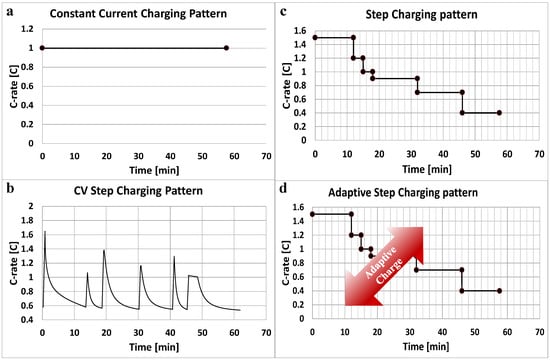
Figure 3.
Rapid charging patterns: (a) A typical step charging pattern; (b) the novel constant current step rapid charging pattern; (c) a constant current charging pattern; and (d) an adaptive rapid charging pattern.
3. Results and Discussion
3.1. Methodology of the New Fast Charge Pattern
Unlike the rapid charging patterns generated by simulations or by laboratory-scale capacity (mAh) cell studies, rapid charging patterns optimized for commercial-level cells (>Ah) were generated, and DVA was directly performed to analyze the charge/discharge behavior of the cells. The resulting DVA plot showed the capacity increase (dQ) per voltage increase (dV) (or vice versa) on the y-axis, and the voltage (V) or capacity (Q) on the x-axis.
Thus, the DVA plot shows in what state (i.e., voltage or capacity) the cell exhibits changes in resistance during charge/discharge due to factors such as phase transition, Li-ion diffusion, electron mobility reduction, and by-product formation. Equation (1) represents the mathematical interpretation above.
In this system, three possible causes of cell resistance can be identified. The first is the Ohmic resistance, which originates from the limitations of electron transport at the interfaces and in the passages of the conductive active materials and the electrical components of the cells such as outer cell-can materials, metal substrates, and metal taps. The second cause is the interfacial resistance that occurs during charge transfer at the interface between the active material and the solid electrolyte interphase (SEI) layer. The third cause is the diffusion resistance due to mechanical and chemical disruption of Li-ion transport across the separator, electrolyte, SEI layer, and the solid active material. Such resistance varies greatly depending on the type of component material used and the manufacturing method employed.
Thus, understanding the DVA plot of a commercial cell allows us to understand the complex effects of cell resistance.
Figure 4 shows the DVA plot of the 3.3 Ah cell used in the experiments reported herein. Specifying the target CV voltage at the peak location is expected to reduce the internal stress generated in relation to the large increase in the rate of resistance. In contrast to applying the CC steps that are inherently related to the external characteristics, the CV step represents the eigenstate of the cell and can determine the optimum current that varies continuously with the changes in cell resistance based on Ohm’s law. To compensate for the rapid charging time required, a number of charge termination steps have been specified.
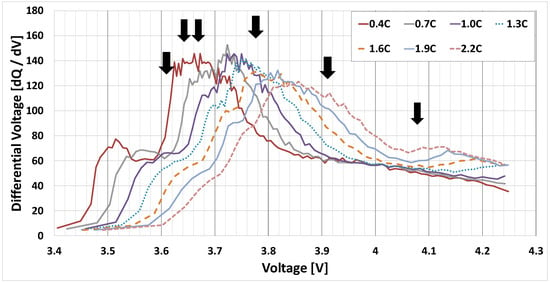
Figure 4.
The differential voltage analysis plot of the ICR18650-33F 3.3 Ah cell employed herein.
Thus, the signals shown in Figure 4 presumably represent the starting point for the phase transition between LCO and graphite.
Typically, the higher the number of phase transitions occurring during graphite charging in non-high voltage applications in the x > 0.5 range for LixCoO2, the lower the number of phase transitions reported for LCO when compared to I–IV [24,25,26,27], where the LCO stays in the O3 phase [28,29,30,31,32,33]. Therefore, the new CV step-fill pattern designed for a specific 3.3 Ah battery is expected to have a positive impact on reducing anode degradation.
Figure 5 shows the variation in charge time and available capacity relative to the cycle number for the 18650 cylindrical battery. As indicated, following repeated charge/discharge cycles, the capacity degrades, and so when the battery is charged at the same charge C-rate as the initial value of the test, the charge time is reduced.
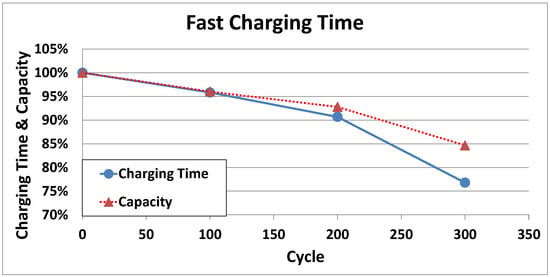
Figure 5.
Variation in the battery charging time and capacity with increasing cycle numbers.
Thus, as the number of cycles is increased, charging is performed at a higher C-rate, and degradation is accelerated. As shown in Figure 6a, the adaptive rapid charging pattern memorizes the battery capacity of the current state of the battery management system, and when the battery is charged, the charging pattern optimized in the current environment is communicated to the charger for further charging. The proposed adaptive rapid charging pattern uses a constant time control method to maintain the same charging time during degradation, as the value corresponding to the capacity degradation rate is applied to the charge C-rate of each stage, as shown in Figure 6b.
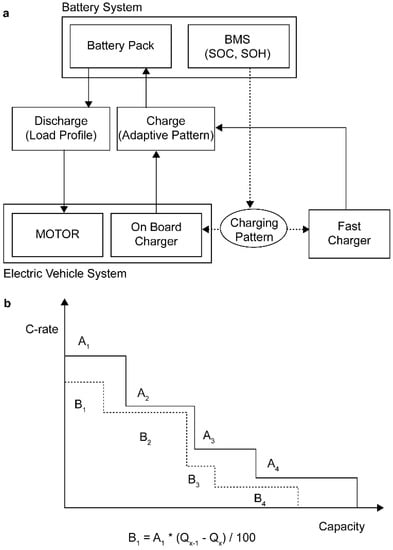
Figure 6.
Adaptive rapid charging: (a) Battery capacity degradation tracking algorithm; and (b) adaptive rapid charge pattern control based on capacity degradation.
3.2. Verification of the New Method
The plot shown in Figure 7a indicates that the number of feasible cycle numbers for the new CV step pattern charging method with a 20% capacity degradation in the 3.2 Ah battery increased by 48.9% when compared with the conventional CCCV step charging method, while those of the adaptive step rapid charging method increased by 61.7%. In addition, the conventional step charging procedure resulted in a sharp drop in the available capacity at the beginning of the cycle, while the proposed novel adaptive charging pattern gave no drop in capacity.
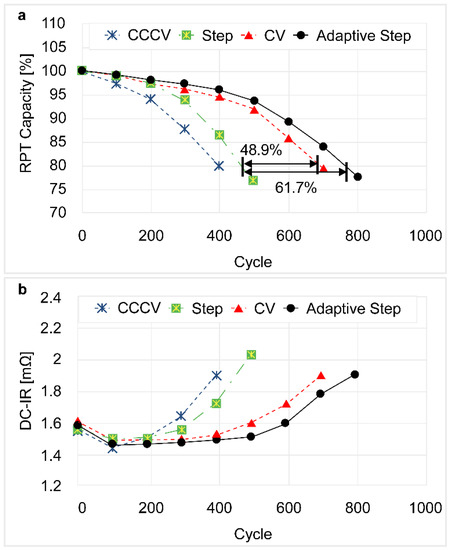
Figure 7.
DVA plots of ICR18650-33F showing the variations in cell cycle lives upon the implementation of different charging methods. (a) The capacity of the battery; and (b) The direct current internal resistance of the battery.
In addition, the plot given in Figure 7b shows the increase in the rate of internal resistance upon standard charging, conventional CCCV step rapid charging, CV step rapid charging, and adaptive step rapid charging. In this context, it must be considered that internal resistance is related to the battery output, and is an important indicator for potential electric vehicle batteries. In all charging patterns indicated, no increase in the internal resistance was observed at the beginning of the cycle, although an increase took place when the capacity began to drop.
3.3. Post-Test Cell Analysis
The results of the post-test diagnostics showed the distinct advantages of the new CV step and the adaptive step rapid charging methods compared to the conventional CCCV step method. In addition, cycle life evaluation was complete at 80% (20% degradation) relative to the initial capacity under all charging conditions. Interestingly, 400 cycles were achieved for the CCCV step charging method, the new CV step charging reached 650 cycles, and over 780 cycles were carried out for the adaptive step rapid charging method, thereby indicating the improved performances of the new CV and adaptive step rapid charging methods when compared to the conventional CCCV step method.
The gas analysis results shown in Figure 8 were consistent with the measurement data regarding the cell thickness. More specifically, the total amount of gas extracted from the expanded cell and the quantities of methane and ethane produced were significantly higher in the cell that employed the CCCV step pattern. Interestingly, methane and ethane are typically formed in the cell due to electrolyte decomposition and SEI layer formation [34,35,36].
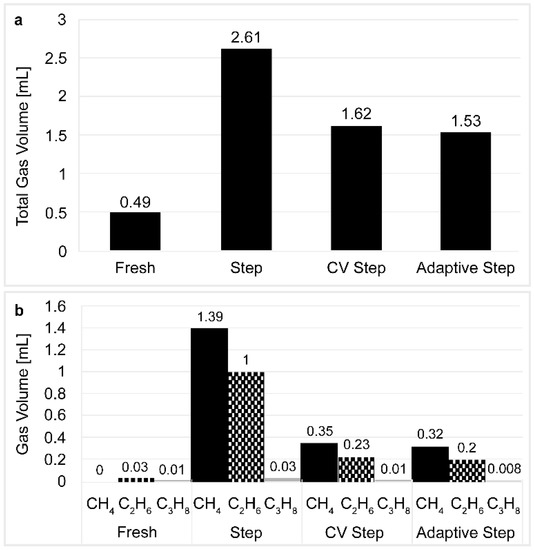
Figure 8.
(a) The total volume of gas extracted; and (b) the composition of the extracted gas after reaching the end of the cell lives. Data provided for the fresh cell, the step charging cell, the CV step charging cell, and the adaptive step charging cell.
The residual electrolyte extracted from the dissembled separator was analyzed by GC-MS, and a higher level of propylene carbonate (PC) decomposition was found to take place for the cell subjected to CCCV step charging (Figure 9). This was consistent with the gas analysis results, and it should be noted that the difference in additive consumption was negligible.
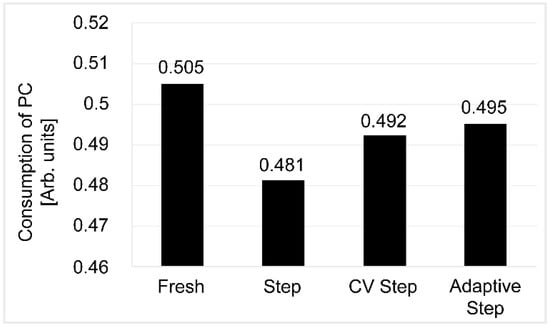
Figure 9.
Standardized residual electrolyte propylene carbonate components of the 3.3 Ah Cell.
Figure 10 shows the thickness of each electrode following disassembly of the cell. Upon comparison with the slight difference in cathode thickness, the CCCV step charging cell exhibited a greater increase in anode thickness than the CV step charging cell. Based on the rapid charging pattern design methodology, it was assumed that the novel CV step pattern allows the cell to self-regulate the charge current and that the adaptive step rapid charging method adjusts the charge current at each RPT, thereby alleviating the accumulation of resistance-increasing factors, and affecting the anode to a greater extent. Minimizing the increase in resistance is thereby expected to minimize cell degradation and both inhibit electrolyte decomposition and unwanted side reactions. We then validated these hypotheses using various analytical techniques and surface characterization methods.
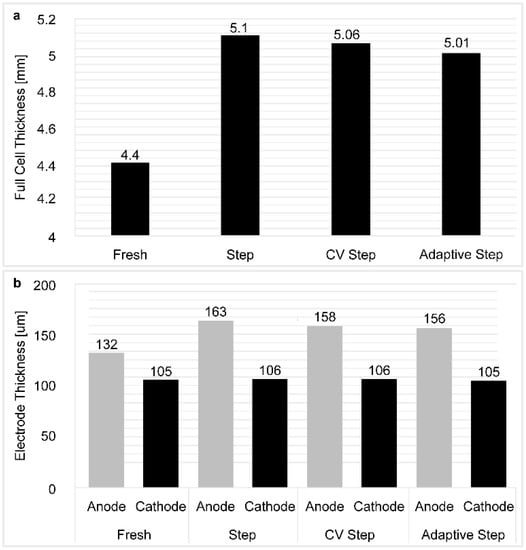
Figure 10.
(a) Electrode thickness following disassembly; and (b) differences in the electrode thicknesses between the positive and negative electrodes.
3.3.1. Bulk Characterization
Bulk characterization of the electrodes was carried out by ICP-MS and XRD, as shown in Figure 11. Considering that the cell was completely discharged prior to its disassembly, a significant degree of degradation occurred in the cell based on the CCCV step charging method. This was determined based on the fact that the quantity of Li found in the anode was large, whereas that found in the cathode was small (Figure 11a).
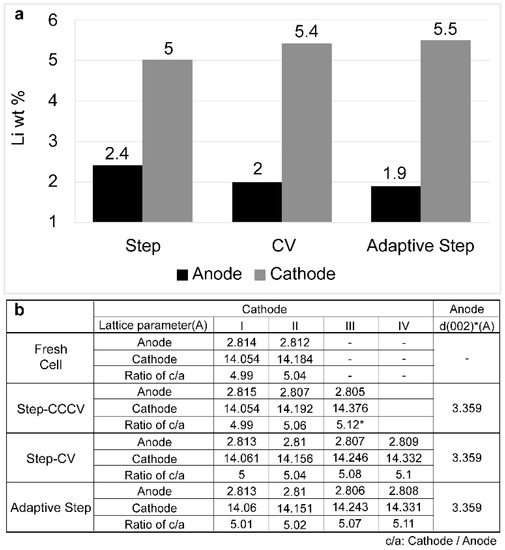
Figure 11.
Analysis of the electrode bulk characteristics: (a) the inductively coupled plasma mass spectrometry results, and (b) the x-ray diffraction results.
The low lithium content of the cathode could be accounted for by the higher amount of irreversible lithium trapped in the graphite lattice or consumed during SEI formation. However, the lattice parameters determined from the XRD data for the anode were comparable for both the CV step and the CCCV charging cells (Figure 11b). We therefore assumed that the majority of irreversible lithium was involved in the formation of by-products at the anode. Indeed, this result as consistent with the electrode thickness data presented in Figure 9. Furthermore, Figure 11b gives the distorted lattice parameters of the LCO as determined by the analysis of the XRD peaks of the cathode. During CCCV step charging, the cathode has the largest distorted c/a lattice ratio, and so the enhanced cathode degradation appears to be related to this process. However, further investigation is required to better understand the origins of these widely distributed lattice structures.
3.3.2. Surface Characterization
Based on the SEM measurements of the disassembled electrode of the 3.3 Ah cell, formation of the SEI layer was confirmed to be relatively uniform with respect to the cathodes, although the cathodes of the battery subjected to cyclic charge/discharge using the CCCV charging pattern were thicker due to the presence of an inorganic SEI layer on the organic SEI layer.
XPS measurements were then performed to identify the composition of the SEI layer and to understand the origin of the increase in anode thickness.
Figure 12 shows the qualitative analysis of the organic and inorganic components on the anode surface. Despite few differences in the compositions of the SEI layers formed on the cathodes, the cell subjected to the novel charging pattern contained an SEI layer composed of more inorganic components than organic components. This was consistent with the SEM observations. Furthermore, the detection of high numbers of Lix–C bonds suggests that the exposure of graphite below the SEI layer was high, which indicates that the formed SEI layer was thinner on the cell subjected to the proposed charging pattern. These results confirmed that the greater increase in anode thickness for the CCCV step charging cell was due to the formation of a thicker SEI with a higher inorganic content. It was therefore concluded that this thicker SEI layer resulted in a rapid capacity loss due to a lack of optimized current level to reduce the resistance polarization.
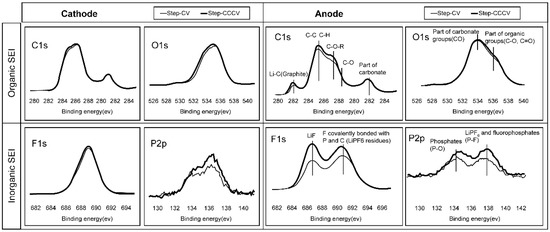
Figure 12.
The x-ray photoelectron spectroscopy results of the electrodes from the disassembled 3.3 Ah cell.
4. Conclusions
We herein proposed an innovative adaptive rapid charging pattern that minimized cell degradation in Li-ion batteries and reflected degradation characteristics.
Based on the obtained results, the proposed constant voltage (CV) step charging and adaptive rapid charging patterns produced an innovative rapid charging pattern, and exhibited several advantages over conventional methods. More specifically, the novel rapid charging pattern allowed self-compensation of the degradation mechanism by constant control of the optimized current level, thereby permitting charging within a minimum time.
Using the lithium cobalt oxide/graphite 18650 cylindrical 3.3 Ah cell, the proposed CV step charging and adaptive rapid charging methods were verified. Following charge/discharge, it was confirmed that the new rapid charging pattern was effective in controlling undesirable side reactions between the anode and the electrolyte, ultimately preventing capacity loss. Furthermore, the proposed rapid charging patterns were generated by accurately analyzing cell operations through the incremental capacity analysis of commercial-level cells (>Ah) rather than through simulations or laboratory-scale capacity (i.e., mAh) cell studies.
Furthermore, a number of cell characteristics and the inherent heterogeneity resulting from complex manufacturing processes were considered. We therefore expect that our method will be a good candidate in the development of rapidly charging electric vehicle battery packs while maintaining suitable cell cycle lives.
Author Contributions
D.-R.K. conceived and designed the experiment; D.-R.K. analyzed the theory; J.-W.K. wrote the manuscript; J.-W.K., T.-H.E., J.-M.K. and J.L., C.-Y.W. participated in the research plan development and revised the manuscript. All authors have contributed to the manuscript.
Funding
This work was supported by the Korea Institute of Energy Technology Evaluation and Planning (KETEP) and the Ministry of Trade, Industry & Energy (MOTIE) of the Republic of Korea. (No. 20172410104900).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zaghib, K.; Dontigny, M.; Guerfi, A.; Charest, P.; Rodrigues, I.; Mauger, A.; Julien, C.M. Safe and fast-charging Li-ion battery with long shelf life for power applications. J. Power Sources 2010, 196, 3943–3954. [Google Scholar] [CrossRef]
- Byoungwoo, K.; Gerbrand, C. Battery materials for ultrarapid charging and discharging. Nature 2009, 458, 190–193. [Google Scholar]
- Lin, M.; Gong, M.; Lu, B.; Wu, Y.; Wang, D.Y.; Guan, M.; Angell, M.; Chen, C.; Yang, J.; Hwang, B.; et al. An ultrafast rechargeable aluminium-ion battery. Nature 2015, 520, 324–328. [Google Scholar] [CrossRef] [PubMed]
- John, B.; Youngsik, K. Challenges for Rechargeable Li Batteries. Chem. Mater. 2009, 22, 587–603. [Google Scholar]
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.S. The effect of the charging protocol on the cycle life of a Li-ion battery. J. Power Sources 2006, 161, 1385–1391. [Google Scholar] [CrossRef]
- Mohammed, F.H.; Chen, C.F.; Christian, E.S.; Partha, P.M. Analysis of the implications of rapid charging on lithium-ion battery performance. J. Electrochem. Soc. 2015, 162, A1382–A1395. [Google Scholar]
- Anseán, D.; González, M.; Viera, J.C.; García, V.M.; Blanco, C.; Valledor, M. Fast charging technique for high power lithium iron phosphate batteries. J. Power Sources 2013, 239, 9–15. [Google Scholar] [CrossRef]
- Wang, C.Y.; Xu, T.; Ge, S.; Zhang, G.; Yang, X.G.; Ji, Y. A fast rechargeable lithium-ion battery at subfreezing temperatures. J. Electrochem. Soc. 2016, 163, A1944–A1950. [Google Scholar] [CrossRef]
- Ramachandrana, Z.S.; Khandelwala, A.; Hariharana, K.S.; Bong Chul, K.; Young, K. Rapid analysis charging profiles of lithium ion batteries using a hybrid simplified electrochemical model. J. Electrochem. Soc. 2016, 163, A1101–A1111. [Google Scholar] [CrossRef]
- Purushothaman, B.K.; Landau, U. Rapid charging of lithium-ion batteries using pulsed currents a theoretical analysis. J. Electrochem. Soc. 2006, 153, A533–A542. [Google Scholar] [CrossRef]
- Lin, C.Y.; Ye, C. The applications of pulse charge for secondary lithium battery. ECS Trans. 2008, 11, 55–62. [Google Scholar]
- Notten, P.H.L.; Op Het Veld, J.H.G.; Van Beek, J.R.G. Boostcharging Li-ion batteries: A challenging new charging concept. J. Power Sources 2005, 145, 89–94. [Google Scholar] [CrossRef]
- Dubarry, M.; Truchot, C.; Liaw, B.Y. Synthesize battery degradation modes via a diagnostic and prognostic model. J. Power Sources 2012, 219, 204–216. [Google Scholar] [CrossRef]
- Han, X.; Ouyang, M.; Lu, L.; Li, J.; Zheng, Y.; Li, Z. A comparative study of commercial lithium ion battery cycle life in electrical vehicle. J. Power Sources 2014, 251, 38–54. [Google Scholar] [CrossRef]
- Keil, P.; Jossen, A. Charging protocols for lithium-ion batteries and their impact on cycle life—An experimental study with different 18,650 high-power cells. J. Energy Storage 2016, 6, 125–141. [Google Scholar] [CrossRef]
- Anseán, D. Fast charging technique for high power LiFePO4 batteries: A mechanistic analysis of aging. J. Power Sources 2016, 321, 201–209. [Google Scholar] [CrossRef]
- Abdel-Monem, M.; Trad, K.; Omar, N.; Hegazy, O.; Van den Bossche, P.; Van Mierlo, J. Influence analysis of static and dynamic fast-charging current profiles on ageing performance of commercial lithium-ion batteries. Energy 2017, 120, 179–191. [Google Scholar] [CrossRef]
- Smith, A.J.; Dahn, J.R. Delta differential capacity analysis. J. Electrochem. Soc. 2012, 159, A290–A293. [Google Scholar] [CrossRef]
- Fathi, R.; Burns, J.C.; Stevens, D.A.; Ye, H.; Hu, C.; Jain, G.; Scott, E.; Schmidt, C.; Dahn, J.R. Ultra High-Precision Studies of Degradation Mechanisms in Aged LiCoO2/Graphite Li-Ion Cells. J. Electrochem. Soc. 2014, 161, A1572–A1579. [Google Scholar] [CrossRef]
- Dubarry, M.; Liaw, B.Y. Identify capacity fading mechanism in a commercial LiFePO4 cell. J. Power Sources 2009, 194, 541–549. [Google Scholar] [CrossRef]
- Deschpande, R.; Verbrudgge, M.; Cheng, Y.T.; Wang, J.; Liu, P. Battery cycle life prediction with coupled chemical degradation and fatigue mechanics. J. Electrochem. Soc. 2012, 159, A1730–A1738. [Google Scholar] [CrossRef]
- Blomgren, G.E. The Development and Future of Lithium Ion Batteries. J. Electrochem. Soc. 2017, 164, A5019–A5025. [Google Scholar] [CrossRef]
- Persson, K.; Hinuma, Y.; Meng, Y.S.; Ven, A.V.D.; Ceder, G. Thermodynamic and kinetic properties of the Li-graphite system from first-principles calculations. Phys. Rev. B 2010, 82, 12–15. [Google Scholar] [CrossRef]
- Wang, X.; Cai, L. Visualizing the chemistry and structure dynamics in lithium-ion batteries by in-situ neutron diffraction. Sci. Rep. 2012, 2, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Striebel, K.A.; Sierra, A.; Shim, J.; Wang, C.W.; Sastry, A.M. The effect of compression on natural graphite anode performance and matrix conductivity. J. Power Sources 2004, 134, 241–251. [Google Scholar] [CrossRef]
- Wang, C.; Kakwan, I.; Appleby, A.; Little, F.E. In situ investigation of electrochemical lithium intercalation into graphite powder. J. Electroanal. Chem. 2000, 489, 55–67. [Google Scholar] [CrossRef]
- Xia, H.; Lu, L.; Meng, Y.S.; Ceder, G. Phase transitions and high-voltage electrochemical behavior of LiCoO2; thin films grown by pulsed laser deposition. J. Electrochem. Soc. 2007, 154, A337–A342. [Google Scholar] [CrossRef]
- Sun, Y.K.; Yoon, C.S.; Myung, S.T.; Belharouak, L.; Amine, K. Role of AlF3 coating on LiCoO₂ particles during cycling to cutoff voltage above 4.5 V. J. Electrochem. Soc. 2009, 156, A1005–A1010. [Google Scholar] [CrossRef]
- Ohzuku, T.; Ueda, A. Solid-state redox reactions of LiCoO (R3m) for 4 volt secondary lithium cells. J. Electrochem. Soc. 1994, 141, 2972–2977. [Google Scholar] [CrossRef]
- Abe, T.; Koyama, T. Thermodynamic modeling of the LiCoO2-CoO2 pseudo-binary system. Calphad 2011, 35, 209–218. [Google Scholar] [CrossRef]
- Marianetti, C.A.; Kotliar, G.; Ceder, G. A first-order Mott transition in LixCoO2. Nat. Mater. 2004, 3, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Lu, Z.; Dahn, J.R. Staging phase transitions in LixCoO2. J. Electrochem. Soc. 2002, 149, A1604–A1609. [Google Scholar] [CrossRef]
- Gauthier, M.; Carney, T.J.; Grimaud, A.; Giordano, L.; Pour, N.; Chang, H.H.; Fenning, D.P.; Lux, S.F.; Paschos, O.; Bauer, C.; et al. Electrode-electrolyte interface in Li-ion batteries: Current understanding and new insights. J. Phys. Chem. Lett. 2015, 6, 4653–4672. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.S.; Han, C.H.H.; Lee, S.I.; Kim, H.J.; Kim, K. Effect of Li2CO3 additive on gas generation in lithium-ion batteries. J. Power Sources 2002, 109, 47–52. [Google Scholar] [CrossRef]
- Teng, X.; Zhan, C.; Bai, Y.; Ma, L.; Liu, Q.; Wu, C.; Wu, F.; Yang, Y.; Lu, J.; Amine, K. In situ analysis of gas generation in lithium-ion batteries with different carbonate-based electrolytes. ACS Appl. Mater. Interfaces 2015, 7, 22751–22755. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).