Femtosecond Laser Processing of Biodegradable Polymers
Abstract
:1. Introduction
2. Femtosecond Laser Processing of Biodegradable Polymers
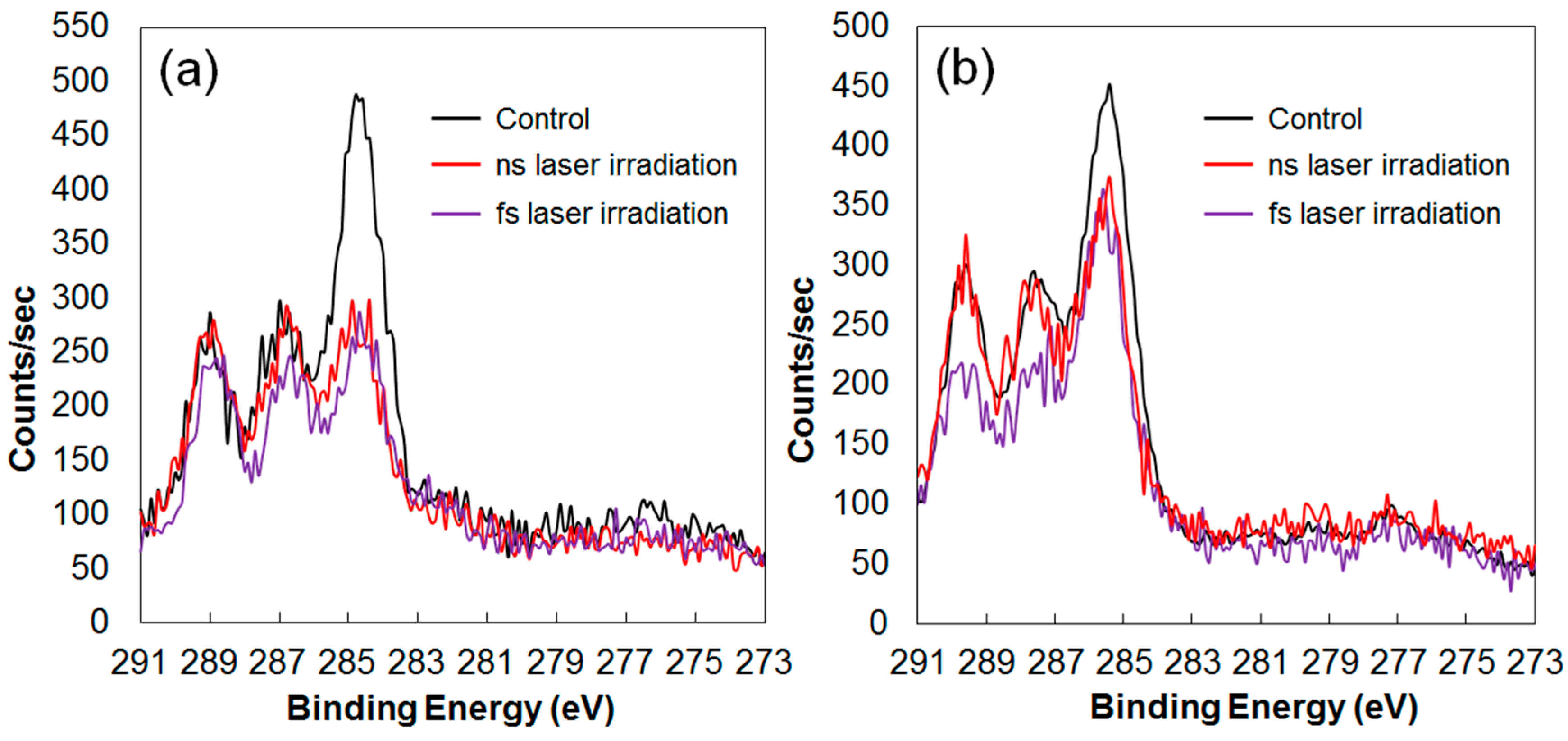
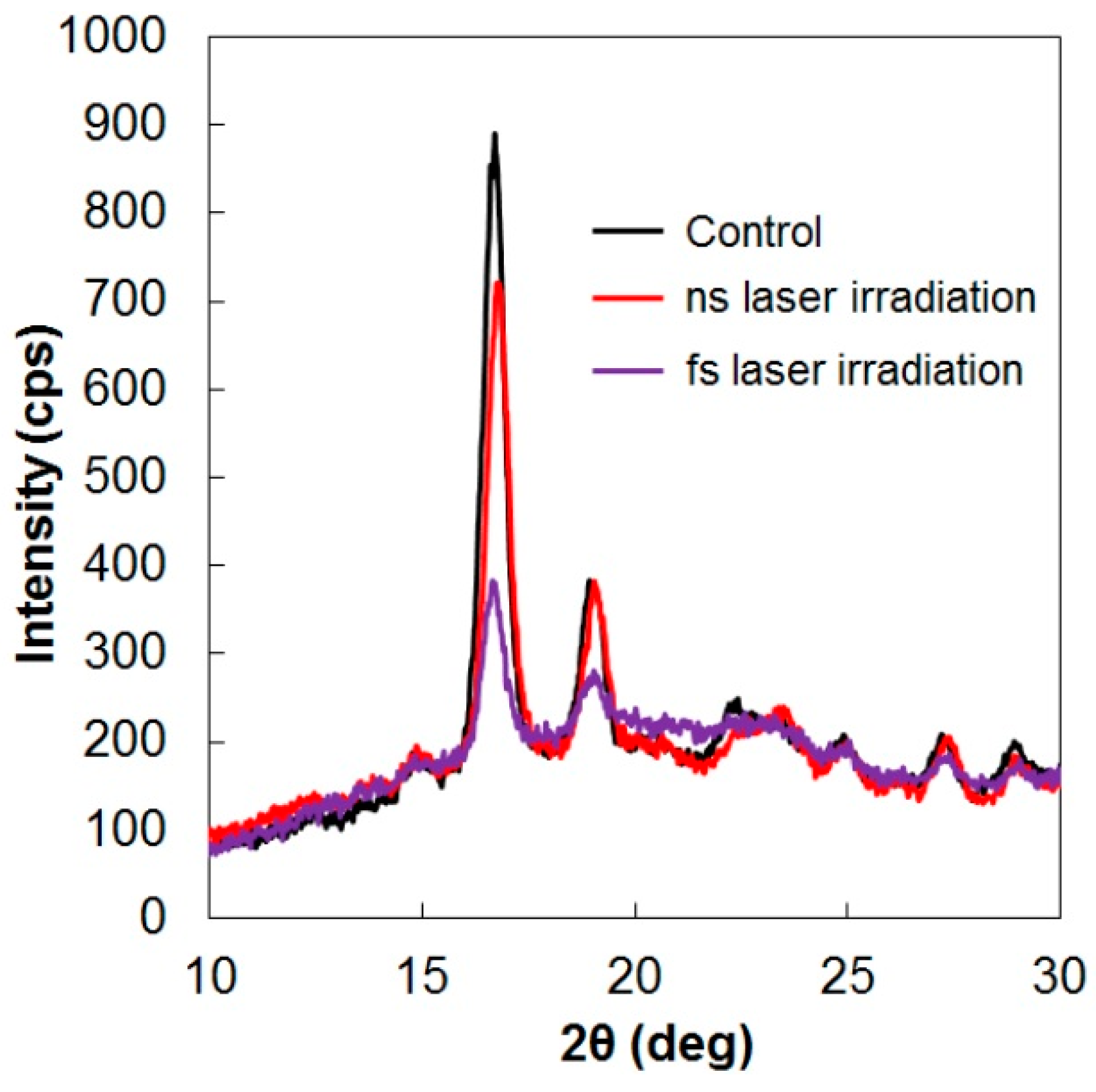
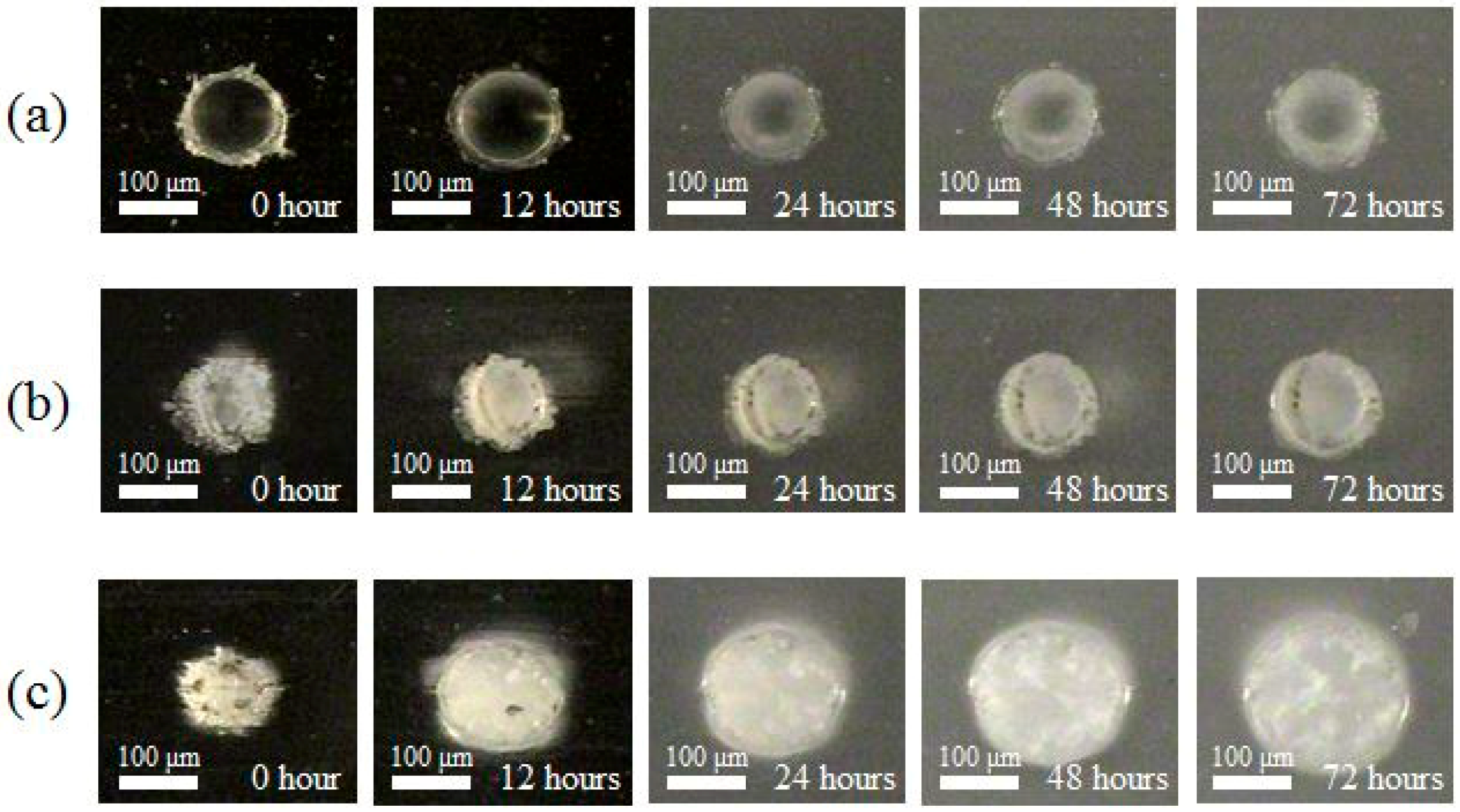
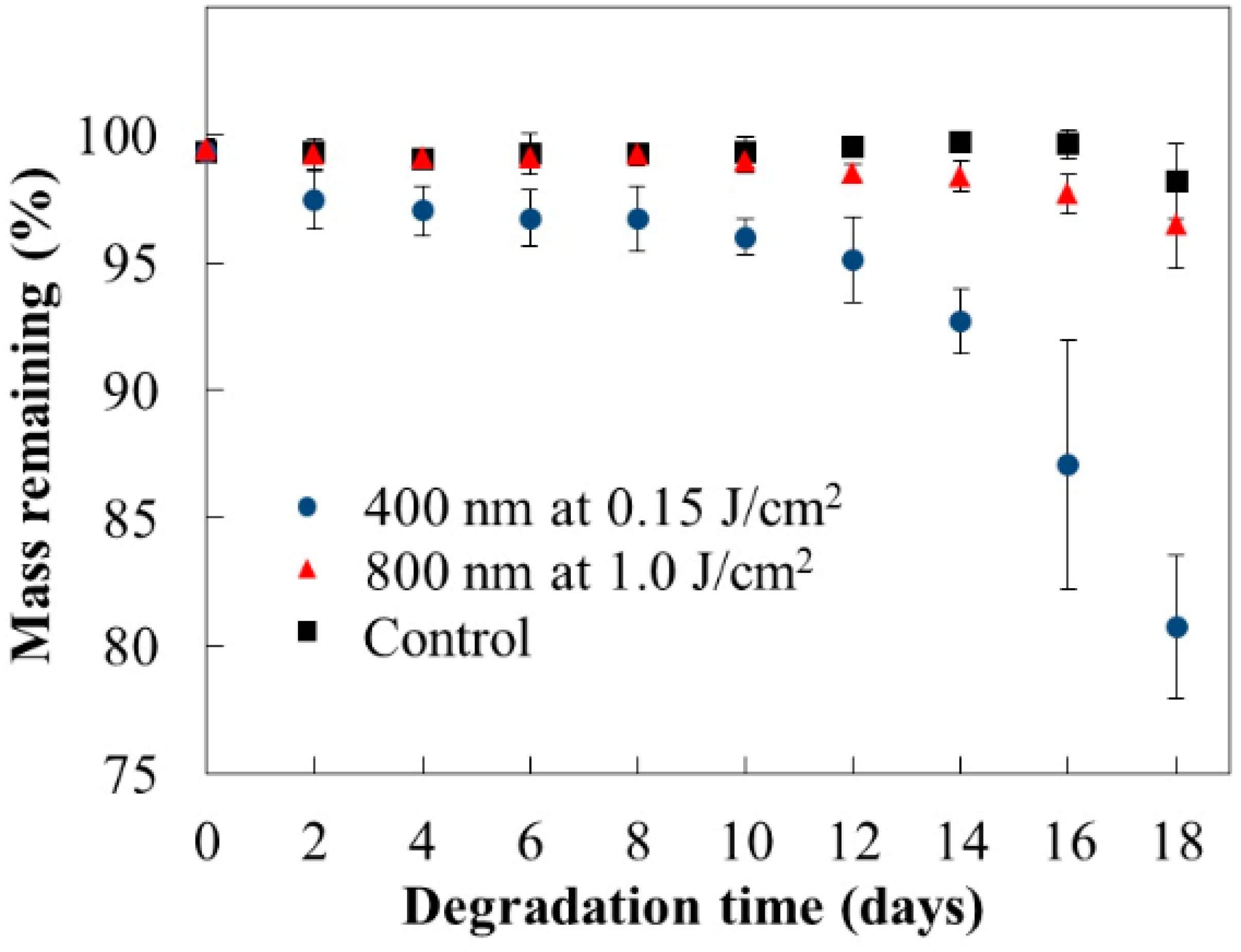
3. Degradable Property after Laser Irradiation
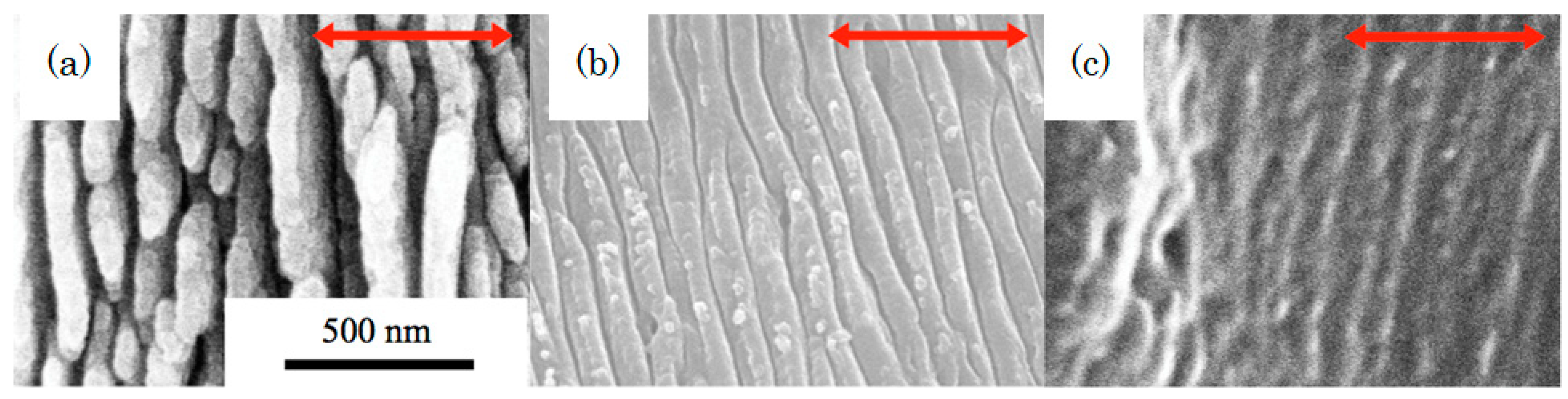
4. Formation of Laser-Induced Periodic Surface Structure (LIPSS) on the Surface of Biodegradable Polymers
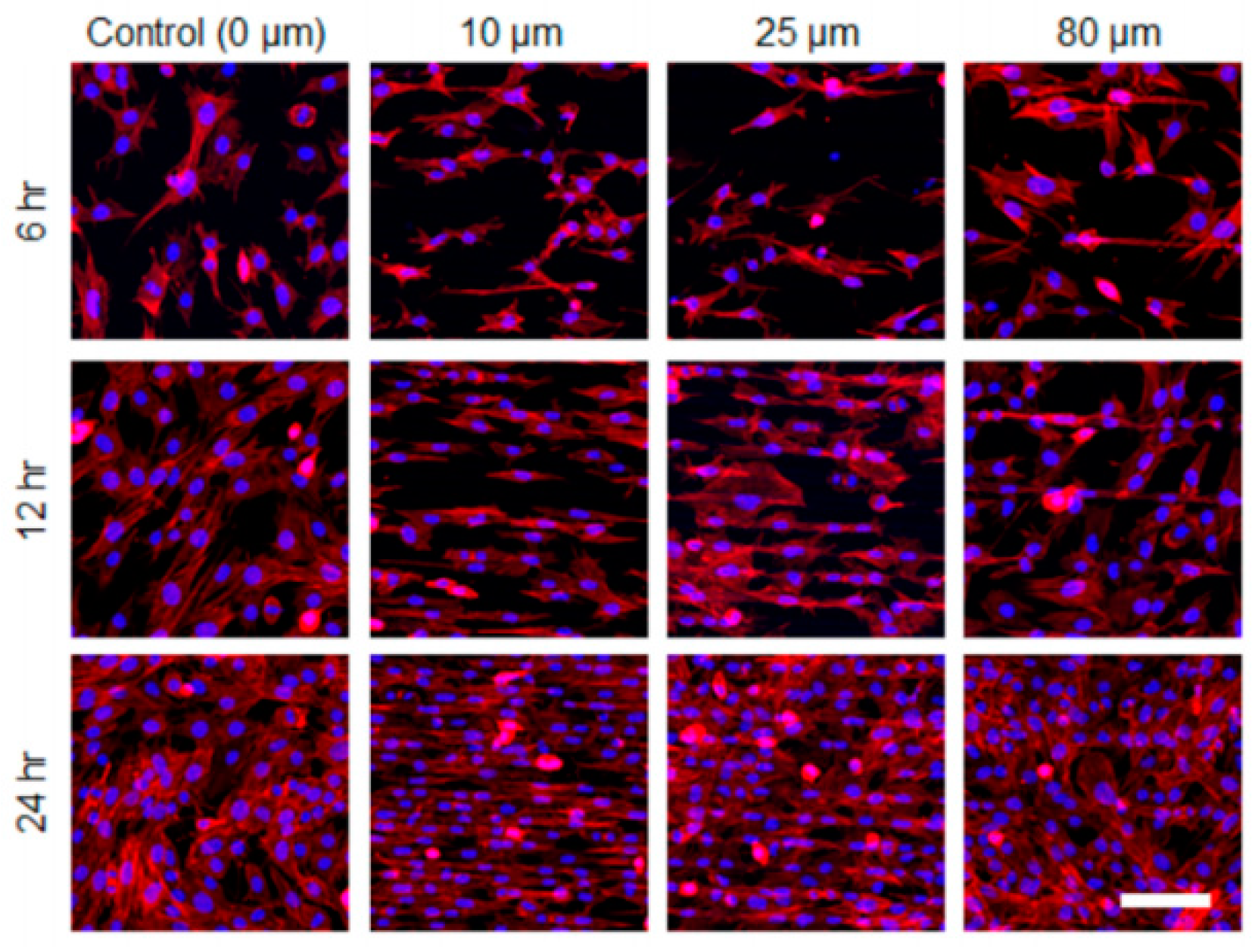
5. Cell Adhesion and Behavior on the Laser-Irradiated Surface of Biodegradable Polymers
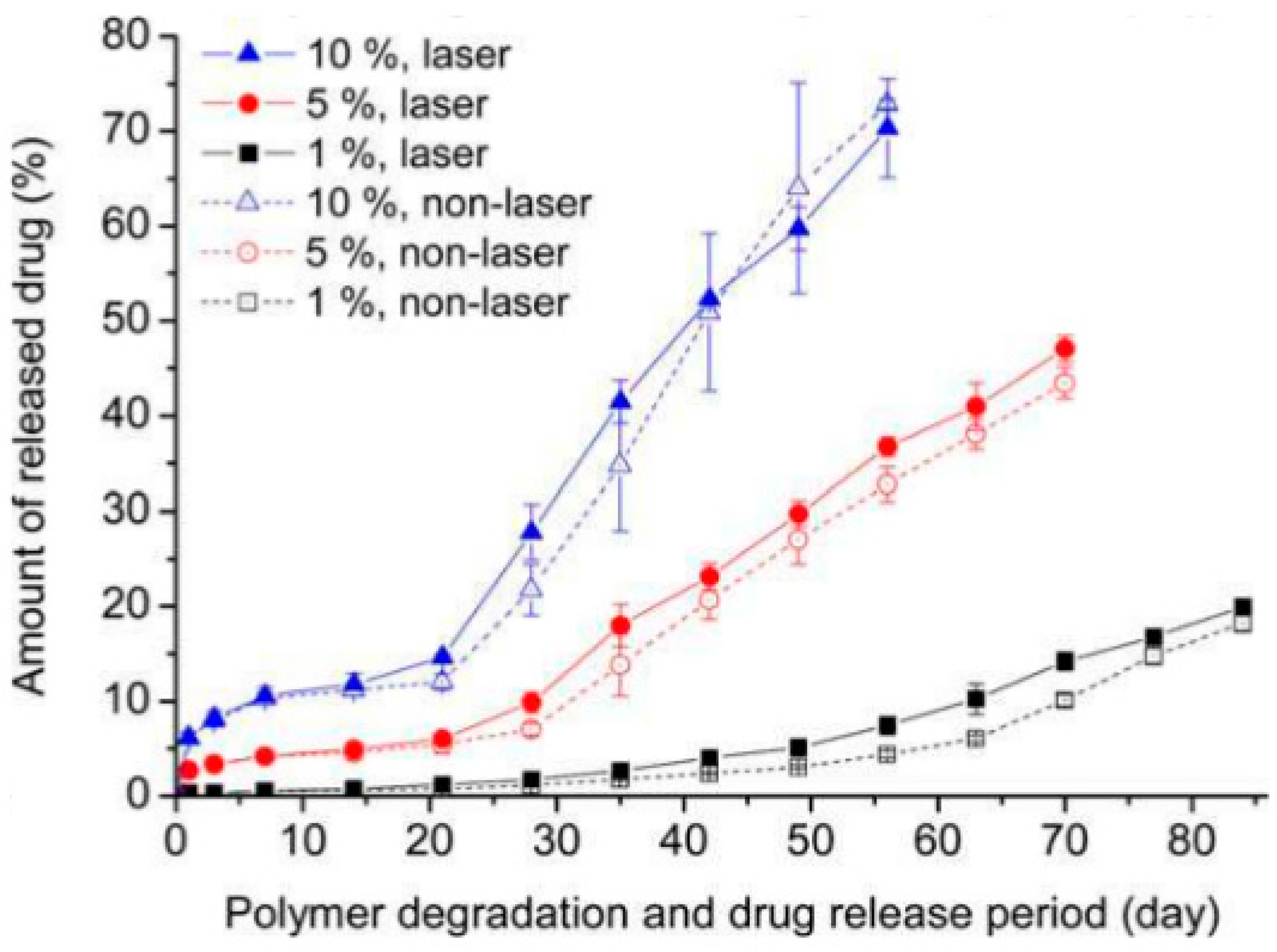
6. Laser-Triggered Molecular Release from Biodegradable Polymers
7. Conclusions and Perspectives
Acknowledgments
Conflicts of Interest
Abbreviations
| CPA | chirped pulse amplification |
| FHG | fourth harmonics generation |
| HAZ | heat affected zone |
| LIPSS | laser induced periodic surface structure |
| PCL | polycaprolactone |
| PGA | polyglycolic acid |
| PLLA | poly-l-lactic acid |
| PLGA | polylactic-co-glycolic acid |
| PPP | polylactide-polyethylene glycol-polylactide |
| PVP | polyvinylpyrrolidone |
| SEM | scanning electron microscopy |
| SHG | second harmonics generation |
| THG | third harmonics generation |
| UV | ultraviolet |
| WAXD | wide-angle X-ray diffraction |
| XPS | X-ray photoelectron spectroscopy |
| XRD | X-ray diffraction |
| 3D | three-dimensional |
References
- Kancharla, V.V.; Chen, S. Laser micromachining of a biodegradable polymer. Trans. NAMRI/SME 2001, 29, 407–412. [Google Scholar]
- Chen, S.; Kancharla, V.V.; Lu, Y. Laser-based microscale patterning of biodegradable polymers for biomedical applications. Int. J. Mater. Prod. Technol. 2003, 18, 457–468. [Google Scholar] [CrossRef]
- Rytlewski, P.; Mróz, W.; Żenkiewicz, M.; Czwartos, J.; Budner, B.J. Laser induced surface modification of polylactide. Mater. Process. Technol. 2012, 212, 1700–1704. [Google Scholar] [CrossRef]
- Lazare, S.; Tokarev, V.; Sionkowska, A.; Wiśniewski, M. Surface foaming of collagen, chitosan and other biopolymer films by KrF excimer laser ablation in the photomechanical regime. Appl. Phys. A 2005, 81, 465–470. [Google Scholar] [CrossRef]
- Küper, S.; Stuke, M. Femtosecond UV excimer laser ablation. Appl. Phys. B 1987, 44, 199–204. [Google Scholar] [CrossRef]
- Srinivasan, R.; Sutcliffe, E.; Braren, B. Ablation and etching of polymethylmethacrylate by very short (160 fs) ultraviolet (308 nm) laser pulses. Appl. Phys. Lett. 1987, 51, 1285–1287. [Google Scholar] [CrossRef]
- Krüger, J.; Kautek, W. Ultrashort pulse laser interaction with dielectrics and polymers. Adv. Polym. Sci. 2004, 168, 247–289. [Google Scholar] [CrossRef]
- Sima, F.; Sugioka, K.; Vazquez, R.M.; Osellame, R.; Kelemen, L.; Ormos, P. Three-dimensional femtosecond laser processing for lab-on-a-chip applications. Nanophotonics 2018, 7, 613–647. [Google Scholar] [CrossRef]
- Toenshoff, H.K.; Ostendorf, A.; Nolte, S.; Korte, F.; Bauer, T. Micromachining using femtosecond lasers. Proc. SPIE 2000, 4088, 136–139. [Google Scholar] [CrossRef]
- Heya, M.; Fukami, Y.; Nagats, H.; Nishida, Y.; Awazu, K. Gelatin ablation wavelength dependency in the range of 5.6–6.7 m using a mid-infrared free electron laser. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrom. Detect. Assoc. Equip. 2003, 507, 564–568. [Google Scholar] [CrossRef]
- Tiaw, K.S.; Goh, S.W.; Hong, M.; Wang, Z.; Lan, B.; Teoh, S.H. Laser surface modification of poly(epsilon-caprolactone) (PCL) membrane for tissue engineering applications. Biomaterials 2005, 26, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, C.A.; Lu, Y.; Mao, S.; Chen, S. Direct micro-patterning of biodegradable polymers using ultraviolet and femtosecond lasers. Biomaterials 2005, 26, 7642–7649. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, S.; Singha, S.; Cho, M.R.; Gordon, R.J. 3D femtosecond laser patterning of collagen for directed cell attachment. Biomaterials 2005, 26, 4597–4605. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.W.; Johnson, J.K.; Nam, J.; Farson, D.F.; Lannutti, J. Structuring electrospun polycaprolactone nanofiber tissue scaffolds by femtosecond laser ablation. J. Laser Appl. 2007, 19, 225–231. [Google Scholar] [CrossRef]
- Gaspard, S.; Oujja, M.; de Nalda, R.; Abrusci, C.; Catalina, F.; Bañares, L.; Lazare, S.; Castillejo, M. Submicron foaming in gelatine by nanosecond and femtosecond pulsed laser irradiation. Appl. Surf. Sci. 2007, 253, 6420–6424. [Google Scholar] [CrossRef]
- Gaspard, S.; Oujja, M.; de Nalda, R.; Abrusci, C.; Catalina, F.; Bañares, L.; Lazare, S.; Castillejo, M. Nanofoaming in the surface of biopolymers by femtosecond pulsed laser irradiation. Appl. Surf. Sci. 2007, 254, 117–1184. [Google Scholar] [CrossRef]
- Gaspard, S.; Forster, M.; Huber, C.; Zafiu, C.; Trettenhahn, G.; Kautek, W.; Castillejo, M. Femtosecond laser processing of biopolymers at high repetition rate. Phys. Chem. Chem. Phys. 2008, 10, 6174–6181. [Google Scholar] [CrossRef] [PubMed]
- Gaspard, S.; Oujja, M.; de Nalda, R.; Castillejo, M.; Bañares, L.; Lazare, S.; Bonneau, R. Nanofoaming dynamics in biopolymers by femtosecond laser irradiation. Appl. Phys. A 2008, 93, 209–213. [Google Scholar] [CrossRef] [Green Version]
- Oujja, M.; Pérez, S.; Fadeeva, E.; Koch, J.; Chichkov, B.N.; Castillejo, M. Three dimensional microstructuring of biopolymers by femtosecond laser irradiation. Appl. Phys. Lett. 2010, 95, 263703. [Google Scholar] [CrossRef] [Green Version]
- Yeong, W.Y.; Lim, K.P.; Ng, G.K.L.; Tan, L.P.; Boey, F.Y.C.; Venkatraman, S.S. Annealing of biodegradable polymer induced by femtosecond laser micromachining. Adv. Eng. Mater. 2010, 12, B89–B93. [Google Scholar] [CrossRef]
- Yeong, W.Y.; Yu, H.; Lim, K.P.; Ng, K.L.; Boey, Y.C.; Subbu, V.S.; Tan, L.P. Multiscale topological guidance for cell alignment via direct laser writing on biodegradable polymer. Tissue Eng. Part C Methods 2010, 16, 1011–1021. [Google Scholar] [CrossRef] [PubMed]
- Melissinaki, V.; Gill, A.A.; Ortega, I.; Vamvakaki, M.; Ranella, A.; Haycock, J.W.; Fotakis, C.; Farsari, M.; Claeyssens, F. Direct laser writing of 3D scaffolds for neural tissue engineering applications. Biofabrication 2011, 3, 045005. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.C.; Johnson, J.; Fei, Z.; Wu, Y.; Farson, D.F.; Lannutti, J.J.; Choi, H.W.; Lee, L.J. Micropatterning and characterization of electrospun poly(ε-caprolactone)/gelatin nanofiber tissue scaffolds by femtosecond laser ablation for tissue engineering applications. Biotechnol. Bioeng. 2011, 108, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Lim, Y.C.; Farson, D.F.; Powell, H.M.; Lannutti, J.J. Vascular wall engineering via femtosecond laser ablation: Scaffolds with self-containing smooth muscle cell populations. Ann. Biomed. Eng. 2011, 39, 3031–3041. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, R.; Quintana, I.; Etxarri, J.; Lejardi, A.; Sarasua, J.-R. Picosecond laser ablation of poly-l-lactide: Effect of crystallinity on the material response. J. Appl. Phys. 2011, 110, 094902. [Google Scholar] [CrossRef]
- Li, H.; Wen, F.; Wong, Y.S.; Boey, F.Y.; Subbu, V.S.; Leong, D.T.; Ng, K.W.; Ng, G.K.; Tan, L.P. Direct laser machining-induced topographic pattern promotes up-regulation of myogenic markers in human mesenchymal stem cells. Acta Biomater. 2012, 8, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.L.; Jeon, H.; Wang, A.; Yan, Z.; Yu, J.; Grigoropoulos, C.; Li, S. Femtosecond laser ablation enhances cell infiltration into three-dimensional electrospun scaffolds. Acta Biomater. 2012, 8, 2648–2658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.W.; Cheng, C.W.; Li, C.W.; Chang, H.W.; Wu, P.H.; Wang, G.J. Fabrication of pillared PLGA microvessel scaffold using femtosecond laser ablation. Int. J. Nanomed. 2012, 7, 1865–1873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castillejo, M.; Rebollar, E.; Oujja, M.; Sanz, M.; Selimis, A.; Sigletou, M.; Psycharakis, S.; Ranella, A.; Fotakis, C. Fabrication of porous biopolymer substrates for cell growth by UV laser: The role of pulse duration. Appl. Surf. Sci. 2012, 258, 8919–8927. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Wong, Y.S.; Wen, F.; Ng, K.W.; Ng, G.K.; Venkatraman, S.S.; Boey, F.Y.; Tan, L.P. Human mesenchymal stem-cell behaviour on direct laser micropatterned electrospun scaffolds with hierarchical structures. Macromol. Biosci. 2013, 13, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, R.; Moreno-Flores, S.; Quintana, I.; Vivanco, M.; Sarasua, J.R.; Toca-Herrera, J.L. Ultra-fast laser microprocessing of medical polymers for cell engineering applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 1, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Daskalova, A.; Nathala, C.S.R.; Bliznakova, I.; Stoyanova, E.; Zhelyazkova, A.; Ganz, T.; Lueftenegger, S.; Husinsky, W. Controlling the porosity of collagen, gelatin and elastin biomaterials by ultrashort laser pulses. Appl. Surf. Sci. 2014, 292, 367–377. [Google Scholar] [CrossRef]
- Stolberg, K.; Friedel, S.; Kremser, B.; Roehner, M. IR and green femtosecond laser machining of heat sensitive materials for medical devices at micrometer scale. In Proceedings of the Proceeding SPIE, San Francisco, CA, USA, 6 March 2014; Volume 8968, p. 89680E. [Google Scholar] [CrossRef]
- Laser Micromachining of Bio-Absorbable Polymers: Impact of the Laser Process Parameters on the Machining Throughput and Quality. Available online: http://www.spectra-physics.cn/assets/client_files/files/documents/M405%20paper%20manuscript_final.pdf (accessed on 1 June 2018).
- Yada, S.; Terakawa, M. Femtosecond laser induced periodic surface structure on poly-l-lactic acid. Opt. Express 2015, 23, 5694–5703. [Google Scholar] [CrossRef] [PubMed]
- Paun, I.A.; Zamfirescu, M.; Mihailescu, M.; Luculescu, C.R.; Mustaciosu, C.C.; Dorobantu, I.; Calenic, B.; Dinescu, M. Laser micro-patterning of biodegradable polymer blends for tissue engineering. J. Mater. Sci. 2015, 50, 923–936. [Google Scholar] [CrossRef]
- Jia, W.; Luo, Y.; Yu, J.; Liu, B.; Hu, M.; Chai, L.; Wang, C. Effects of high-repetition-rate femtosecond laser micromachining on the physical and chemical properties of polylactide (PLA). Opt. Express 2015, 23, 26932–26939. [Google Scholar] [CrossRef] [PubMed]
- Jun, I.; Chung, Y.; Heo, Y.; Han, H.; Park, J.; Jeong, H.; Lee, H.; Lee, Y.B.; Kim, Y.; Seok, H.; et al. Creating hierarchical topographies on fibrous platforms using femtosecond laser ablation for directing myoblasts behavior. ACS Appl. Mater. Interfaces 2016, 8, 3407–3417. [Google Scholar] [CrossRef] [PubMed]
- Daskalova, A.; Nathala, C.S.R.; Kavatzikidou, P.; Ranella, A.; Szoszkiewicz, R.; Husinsky, W.; Fotakis, C. FS laser processing of bio-polymer thin films for studying cell-to-substrate specific response. Appl. Surf. Sci. 2016, 382, 178–191. [Google Scholar] [CrossRef]
- Shibata, A.; Yada, S.; Terakawa, M. Biodegradability of poly(lactic-co-glycolic acid) after femtosecond laser irradiation. Sci. Rep. 2016, 6, 27884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stępak, B.; Antończak, A.J.; Abramski, K.M. Optimization of femtosecond laser cutting of a biodegradable polymer for medical devices manufacturing. Photonics Lett. Pol. 2016, 8, 116–118. [Google Scholar] [CrossRef]
- Umemoto, T.; Shibata, A.; Terakawa, M. Femtosecond laser irradiation of the fluorescent molecules-loaded poly(lactic-co-glycolic acid). Appl. Surf. Sci. 2017, 417, 165–169. [Google Scholar] [CrossRef]
- Shibata, A.; Machida, M.; Kondo, N.; Terakawa, M. Biodegradability of poly(lactic-co-glycolic acid) and poly(l-lactic acid) after deep-ultraviolet femtosecond and nanosecond laser irradiation. Appl. Phys. A 2017, 123, 438. [Google Scholar] [CrossRef]
- Viertel, T.; Pabst, L.; Olbrich, M.; Ebert, R.; Horn, A.; Exner, H. Generation of nano-voids inside polylactide using femtosecond laser radiation. Appl. Phys. A 2017, 123, 789. [Google Scholar] [CrossRef]
- Assaf, Y.; Kietzig, A.-M. Optical and chemical effects governing femtosecond laser-induced structure formation on polymer surfaces. Mater. Today Commun. 2018, 14, 169–179. [Google Scholar] [CrossRef]
- Daskalova, A.; Trifonov, A.; Bliznakova, I.; Nathala, C.; Ajami, A.; Husinsky, W.; Declercq, H.; Buchvarov, I. Selective cell response on natural polymer bio-interfaces textured by femtosecond laser. Appl. Phys. A 2018, 124, 207. [Google Scholar] [CrossRef]
- Daskalova, A.; Bliznakova, I.; Zhelyazkova, A.; Ostrowska, B.; Trifonov, A.; Buchvarov, I.; Avramov, L.; Husinsky, W. Femtosecond laser surface texturing of 3D poly-ε-caprolactone matrices for bone tissue engineering applications. J. Phys. Conf. Ser. 2018, 992, 012048. [Google Scholar] [CrossRef] [Green Version]
- Stępak, B.D.; Szustakiewicz, K.; Antończak, A.J. Optimization of femtosecond laser micromachining of polylactide and PLLA/HAp composite. Proc. SPIE 2018, 10520, 105201V. [Google Scholar] [CrossRef]
- Sekosan, G.; Vasanthan, N. Morphological changes of annealed poly-ε-caprolactone by enzymatic degradation with lipase. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 202–211. [Google Scholar] [CrossRef]
- Vey, E.; Rodger, C.; Meehan, L.; Booth, J.; Claybourn, M.; Miller, A.F.; Saiani, A. The impact of chemical composition on the degradation kinetics of poly(lactic-co-glycolic) acid copolymers cast films in phosphate buffer solution. Polym. Degrad. Stab. 2012, 97, 358–365. [Google Scholar] [CrossRef]
- Wu, L.; Ding, J. Effects of porosity and pore size on in vitro degradation of three-dimensional porous poly(d,l-lactide-coglycolide) scaffolds for tissue engineering. J. Biomed. Mater. Res. A 2005, 75, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Ikada, E. Photo- and bio-degradable polyesters photodegradation behaviors of aliphatic polyesters. J. Photopolym. Sci. Technol. 1997, 10, 265–270. [Google Scholar] [CrossRef]
- Tsuji, H.; Echizen, Y.; Nishimura, Y. Enzymatic degradation of poly(l-lactic acid): Effects of UV irradiation. J. Polym. Environ. 2006, 14, 239–248. [Google Scholar] [CrossRef]
- Hsu, S.-T.; Tan, H.; Yao, Y.L. Effect of laser-induced crystallinity modification on biodegradation profile of poly(l-lactic acid). J. Manuf. Sci. Eng. 2013, 136, 011005. [Google Scholar] [CrossRef]
- Farkas, B.; Romano, I.; Ceseracciu, L.; Diaspro, A.; Brandi, F.; Beke, S. Four-order stiffness variation of laser-fabricated photopolymer biodegradable scaffolds by laser parameter modulation. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 55, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Slepička, P.; Michaljaničová, I.; Sajdl, P.; Fitl, P.; Švorčík, V. Surface ablation of PLLA induced by KrF eximer laser. Appl. Surf. Sci. 2013, 283, 438–444. [Google Scholar] [CrossRef]
- Oya, K.; Aoki, S.; Shimomura, K.; Sugita, N.; Suzuki, K.; Nakamura, N.; Fujie, H. Morphological observations of mesenchymal stem cell adhesion to a nanoperiodic-structured titanium surface patterned using femtosecond laser processing. Jpn. J. Appl. Phys. 2012, 51, 125203. [Google Scholar] [CrossRef]
- Shinonaga, T.; Tsukamoto, M.; Nagai, A.; Yamashita, K.; Hanawa, T.; Matsushita, N.; Xie, G.; Abe, N. Cell spreading on titanium dioxide film formed and modified with aerosol beam and femtosecond laser. Appl. Surf. Sci. 2014, 288, 649–653. [Google Scholar] [CrossRef]
- Srinivasan, R.; Lazare, S. Modification of polymer surfaces by far-ultraviolet radiation of low and high (laser) intensities. Polymer 1985, 26, 1297–1300. [Google Scholar] [CrossRef]
- Pérez, S.; Rebollar, E.; Oujja, M.; Martín, M.; Castillejo, M. Laser-induced periodic surface structuring of biopolymers. Appl. Phys. A 2013, 110, 683–690. [Google Scholar] [CrossRef]
- Toosi, S.; Maradi, S.; Hatzikiriakos, S.G. Fabrication of micro/nano patterns on polymeric substrates using laser ablation methods to control wettability behavior: A critical review. Rev. Adhes. Adhes. 2017, 5, 55–78. [Google Scholar] [CrossRef]
- Chu, C.; Wang, Y.; Tai, L.; Wu, L.; Yang, C. Surface deformation of gold nanorod-loaded poly(d,l-lactide-co-glycolide) nanoparticles after near infrared irradiation: An active and controllable drug release system. J. Mater. Chem. 2010, 20, 3260–3264. [Google Scholar] [CrossRef]
- Hsu, S.-T.; Yao, Y.L. Effect of drug loading and laser surface melting on drug release profile from biodegradable polymer. J. Appl. Polym. Sci. 2013, 130, 4147–4156. [Google Scholar] [CrossRef]
- Ariyasu, K.; Ishii, A.; Umemoto, T.; Terakawa, M. Laser-triggered release of encapsulated molecules from polylactic-co-glycolic acid microcapsules. J. Biomed. Opt. 2016, 21, 085003. [Google Scholar] [CrossRef] [PubMed]
- Kerse, C.; Kalaycıoğlu, H.; Elahi, P.; Çetin, B.; Kesim, D.K.; Akçaalan, Ö.; Yavaş, S.; Aşık, M.D.; Öktem, B.; Hoogland, H.; et al. Ablation-cooled material removal with ultrafast bursts of pulses. Nature 2016, 537, 84–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, F.; Yu, J.; Tan, Y.; Chu, W.; Zhou, C.; Cheng, Y.; Sugioka, K. Tailoring femtosecond 1.5-μm Bessel beams for manufacturing high-aspect-ratio through-silicon vias. Sci. Rep. 2017, 7, 40785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Year | Wavelength | Pulse Width | Material | Note | Ref. |
|---|---|---|---|---|---|
| 2000 | n/a | n/a | n/a | medical stents fabrication | [9] |
| 2003 | mid IR | 15 us, 5 ps | Gelatin | surface ablation | [10] |
| 2005 | 800, 248 nm | 110 fs, 23 ns | PCL | enhancement of wettability and hydrophobic nature | [11] |
| 2005 | 800 nm, 193 nm | 220 fs, 35 ns | PCL, PGA | analysis of chemical structure and biodegradability | [12] |
| 2005 | 800 nm | 45 fs | collagen | cell attachment on patterned surface | [13] |
| 2007 | 775 nm, n/a | 150 fs, 120 ns | PCL, PET | laser ablation on electrospun scaffolds | [14] |
| 2007 | 800, 400, 266 nm, 1064, 532, 355, 266 nm | 90 fs, 6 ns | gelatin | submicron structures foaming | [15] |
| 2007 | 800, 400, 266 nm | 90 fs | gelatin | nano-foaming | [16] |
| 2008 | 790 nm | 60 fs | gelatin, curcumin-doped gelatin, collagen | femtosecond laser ablation | [17] |
| 2008 | 800, 400, 266 nm, 248 nm | 90 fs, 25 ns | gelatin, collagen | Discussion on the mechanism of nano-foaming | [18] |
| 2010 | 800 nm | 30 fs | gelatin, PVP, PVP/chitosan | 3D internal modification of biopolymers | [19] |
| 2010 | 800 nm | 150 fs | PCL, PLA | evaluation of HAZ | [20] |
| 2010 | 800 nm | 150 fs | PLLA-PCL copolymer | evaluation of cell alignment on direct laser written surface | [21] |
| 2011 | 800 nm | 20 fs | PLA | 3D scaffold fabrication | [22] |
| 2011 | 775 nm | 150 fs | PCL/gelatin | micro-patterned electrospun scaffold and cell behavior | [23] |
| 2011 | 775 nm | 150 fs | PCL | effect of micro-channel on cell behavior | [24] |
| 2011 | 1064 nm | 10 ps | PLLA | effect of crystallinity on micro-grating fabrication | [25] |
| 2011 | 800 nm | 150 fs | PLA-PCL | effect of laser patterned surface on up regulation of myogenic markers | [26] |
| 2012 | 800 nm | 100 fs | PLLA | effect of laser processed electrospun scaffolds on cell behavior | [27] |
| 2012 | 800 nm | 120 fs | PLGA | micro-vessel scaffold fabrication | [28] |
| 2012 | 248 nm, 213 nm, 248 nm | 20 ns, 150 ps, 500 fs | chitosan, starch, chitosan/starch | effect of pulse duration on porous surface fabrication | [29] |
| 2013 | 800 nm | 150 fs | PLA-PCL | nano/micro-grating and cell behavior | [30] |
| 2014 | 1064 nm | 10 ps | PLLA, PS | micro-patterned surface for cancer cell confinement | [31] |
| 2014 | 800 nm | 30–100 fs | Gelatin, collagen, collagen-elastin | porous structure in ablation area, cell proliferation. | [32] |
| 2014 | 1030, 515 nm | 400–800 fs | PLLA, Poly(d,l-lactid) | high repetition rate up to 500 kHz | [33] |
| 2015 | 520 nm, 532 nm | 350 fs, 10 ps | PLLA, PLGA | parameter dependence | [34] |
| 2015 | 800 nm, 400 nm | 100 fs | PLLA | LIPSS formation | [35] |
| 2015 | 775 nm | 200 fs | PLGA, PPP | femtosecond laser ablation and MAPLE for cell adhesion | [36] |
| 2015 | 1040 nm | 115 fs | PLA | processing in air and in water, high-rep rate of 57 MHz | [37] |
| 2016 | 515 nm | 400 fs | PLLA electrospun fibers | fibrous platforms for cell adhesion and direction | [38] |
| 2016 | 800 nm | 30 fs | Collagen/elastin blends, gelatin | laser ablation for changing cell adhesion and wettability | [39] |
| 2016 | 800 nm, 400 nm | 100 fs | PLGA | biodegradability depending on the laser wavelength | [40] |
| 2016 | 515 nm | 450 fs | PLLA | experimental optimization of laser parameters | [41] |
| 2017 | 800 nm, 400 nm | 100 fs | PLGA | release of loaded molecules after laser ablation | [42] |
| 2017 | 266 nm | 100 fs, 5 ns | PLGA, PLLA | comparison of fs and ns lasers | [43] |
| 2017 | 1030 nm | 180 fs, | PLA | nano-void formation | [44] |
| 2018 | 800, 275 nm | 85 fs | PLA | comparison of six polymers | [45] |
| 2018 | 800 nm | 30 fs | chitosan | texturing for bio-interfaces | [46] |
| 2018 | 800 nm | 30 fs | PCL | osteoblast cell adhesion | [47] |
| 2018 | 515 nm | 450 fs | PLLA | detailed study on physicochemical properties | [48] |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terakawa, M. Femtosecond Laser Processing of Biodegradable Polymers. Appl. Sci. 2018, 8, 1123. https://doi.org/10.3390/app8071123
Terakawa M. Femtosecond Laser Processing of Biodegradable Polymers. Applied Sciences. 2018; 8(7):1123. https://doi.org/10.3390/app8071123
Chicago/Turabian StyleTerakawa, Mitsuhiro. 2018. "Femtosecond Laser Processing of Biodegradable Polymers" Applied Sciences 8, no. 7: 1123. https://doi.org/10.3390/app8071123
APA StyleTerakawa, M. (2018). Femtosecond Laser Processing of Biodegradable Polymers. Applied Sciences, 8(7), 1123. https://doi.org/10.3390/app8071123




