Abstract
Air pollutants such as volatile organic compounds (VOCs), nitrogen oxides (NOx), and sulfur dioxide (SO2), as well as water pollutants (e.g., heavy metals phosphorous, fluoride, boron, phenolic compounds, and dyes), are harmful to humans and the environment. Effective control and reduction of their pollution is therefore an important topic for today’s scientists. Fly ash (FA) is a type of industrial waste that can cause multiple environmental problems if discharged into the air. On the other hand, because of its high porosity, large specific surface area, and other unique characteristics, FA can also be used as a low-cost and high efficient adsorbent for treatment of environment pollutants. This paper reviews the effects of FA on treatment of the air and water pollution, including to the current status of global FA utilization, physicochemical properties, principle of adsorption, and the application direction of FA in the future. Since most researchers only studied the adsorption capacity of pure FA or zeolite (synthesized from FA), the research on the fabrication of nanofiber membranes using FA is still lacking, especially the adsorption of VOCs from air and heavy metals from wastewater using FA nanofiber membranes. Therefore, in this paper, we focus on reviewing and summarizing that FA can be spun into a fiber membrane via electrospinning with the ability to adsorb VOCs and heavy metals from air and wastewater. Moreover, we also evaluate the future application value of FA nanofiber membranes in the field of environmental pollution control. Utilization of nanofiber technology to fabricate multi-functional FA emerging composite materials to mitigate air and water pollution has great potential in the future, especially the use of pollutant materials to control other pollutants.
1. Introduction
Along with unceasing economic development, people’s living standard enhances, and humans currently live in a time of rapid development, with daily life constantly becoming more high-tech and intelligent. However, the continuous improvement of human life will inevitably increase the demand for energy and other substances, and it will cause some environmental pollution problems, such as air and water pollutants including nitrogen oxides (NOx) [1], volatile organic compounds (VOCs) [2], sulfur dioxide (SO2) [3], and heavy metal pollution [4]. Fly ash (FA), a type of industrial waste, is a coal combustion product composed of particulates [5]. With rapid industrial development, large demand for electricity has led to hundreds of millions of tons of FA (around 80% of which is fly ash) being discharged every year worldwide. The annual coal consumption of China in 2015 was about 50% of the global coal consumption [6]. Power generation using coal provides energy for daily life and industry, but it also produces atmospheric and water pollution, which disrupts ecological cycles. FA pollutes the atmosphere and threatens human health if it is discharged directly from power plant chimneys into the atmosphere without treatment [7,8]. This is a significant contributor to recent air quality and haze problems in China [9,10].
However, with developing science and technology, FA from power plants is changing from industrial waste into an industrial raw material [11,12,13]. FA is a large porous structure exhibiting an irregular shape, with large specific surface areas and high adsorption activities. The characteristics of FA mainly depend on the type of coal and the combustion conditions [14,15]. Yao et al. pointed out that the average diameter of FA in the form of fine particles is less than 20 μm, and their bulk density and specific surface area are in the range of 0.54–0.86 g/cm3 and 300–500 m2/kg, respectively [15]. In addition, FA contains valuable oxide components, such as SiO2, Al2O3, CaO, MgO, Na2O, and TiO2, and essential elements, including P, K, Mg, Zn, Fe, Mn, and others [5,7]. Therefore, FA has attracted the attention of many researchers. FA is a pozzolanic mixture formed by high temperature combustion of pulverized coal. Its chemical composition is similar to clay, with a good lime binding capacity. Thus, it is primarily used for production of cement [16], clay bricks [17], hollow blocks [18], aerated concrete [19], and other building materials [20,21,22]. It can also be used as an agricultural fertilizer and soil conditioner due to its low bulk density, high water retention capacity, favorable pH, and other properties [15,23,24]. Ram et al. [25] studied the effects of FA on rice crop production from 1996 to 2000 and found that FA improved texture, fertility, and crop productivity of mine spoil. Figure 1 shows the various applications of FA in different fields.
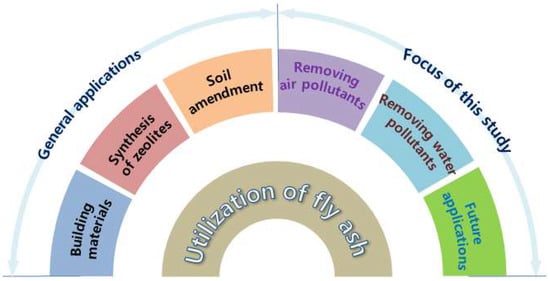
Figure 1.
Various applications of fly ash in different fields.
FA can also be used as a low-cost adsorbent because of its excellent specific surface area, porosity, particle size [26], and other natural characteristics [27]. Bhattacharyya et al. [28] found that FA could be used as an adsorbent in a cascade with a plasma reactor to NOx emission from biodiesel engines. Hower et al. [29] showed that FA carbon captures Hg efficiently. Other researchers have found that, after simple modification, FA is a good adsorbent of SO2 [30] and VOCs [31]. Furthermore, one-step fabrication of FA into a fibrous membrane by electrospun technology to adsorb VOCs from air has been successfully reported by our research group [32,33]. On the other hand, FA can also be combined with TiO2 [34] or AgNO3 [35] to produce a multifunctional fibrous membrane under electrospinning for purifying water, such as adsorption of heavy metals (Hg and Pb), removal of dyes (methylene blue), antibacterial activity, carcinogenic arsenic (As) and toxic organic dyes. To sum up, FA can be fabricated into a variety of composite materials including zeolites and fiber membranes. They have excellent adsorption capacity in controlling air and water pollutants, such as VOCs, SOx, NOx, heavy metals and toxic organic dyes. In addition, the perfect combination of nanotechnology and FA can reduce environmental pollution while producing low-cost and high-performance nanocomposites.
2. Global Overview of Fly Ash
2.1. China
China is a populous country with high electricity consumption per capita. Coal is one of the main energy sources in China, accounting for 70% of the total energy consumption from 1978 to 2009 [36]. China is the largest coal mining and consuming country in the world, and it has surpassed Japan to become the world’s largest coal-importing country in 2008 [36]. China consumed more than 3 billion tons of coals in 2010 [37], and most cities in northern China use coal-fired heating in the winter. Figure 2 shows the coal consumption of China compared with other countries and its relationship with Gross Domestic Product (GDP). China clearly is dependent on energy from coal. However, burning coal produces FA in the air that causes environmental problems such as acid rain, fog and haze, and heavy metal pollution [38,39,40]. Solving the problem of FA pollutant from burning coal would be a great contribution to environmental and human health.
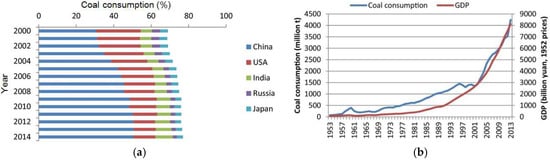
Figure 2.
Comparison of coal consumption in China with other important coal consuming countries (a); and relationship between coal consumption and GDP in China (b) (reproduced with permission from [36], Elsevier, 2018).
Figure 3 shows the production and utilization rate of FA from 2001 to 2015 and the degree of utilization of FA in different cities of China in 2010. The annual output of FA is huge in China. At the end of 2015, about 580 million tons of FA were produced, accounting for about 77% of the global FA production [41]. FA utilization in China increased after 2004 and reached about 70% in 2015 (Figure 3a), which is closely related to the rapid development of China’s economy and technology. However, different statistical offices have different estimates of the utilization rate of fly ash in China. For example, Greenpeace [41] estimated the utilization rate to be only 30%. Regardless of the value, the utilization rate of FA in China is very high. According to statistics, the global average utilization rate of FA is about 25% [42]. Other researchers reported that the utilization rate of FA is about 67% [43]. The utilization rate of FA in different cities in China is shown in Figure 3b. The utilization rate of fly ash in eastern China is clearly higher than in western China, due to the large market for FA in the east [37]. The specific utilization of FA is shown in Table 1. Although there are many ways to utilize FA in China, few technologically advanced products use FA. If processes involving FA are not strictly controlled, they can cause secondary pollution [37].
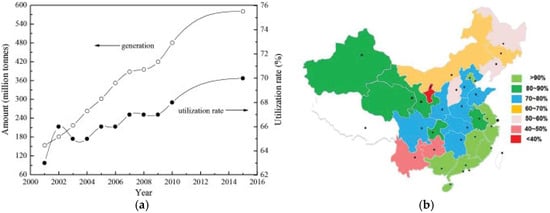
Figure 3.
Production and utilization of fly ash from 2001 to 2015 (reproduced with permission from [41], Elsevier, 2018) (a); and comprehensive utilization rate of fly ash in Chinese cities in 2010 (reproduced with permission from [37], Elsevier, 2018) (b).

Table 1.
Specific utilization of fly ash in China.
2.2. India
With the development of economy and the increase of the population, the energy consumption in India is also increasing year by year. U.S. Energy Information Administration Department reported that the energy consumption growth in India will far exceed that of China, the United States, and Russia until the end of 2035 [44]. In terms of generating capacity, Indian power plants rely mainly on fossil fuels. In general, 76.4% of the power generation is coal-based power plants, while renewable energy sources only generate 6.62% [44]. Annual production of FA in India is therefore also increasing rapidly. Production of FA during 2010 and 2011 increased to about 130 million tons, which was about 85.7% higher than during 1996 to 1997. The FA utilization rate also increased from 9.63% in 1996 to 1997 to 54.53% in 2010 to 2011, an increase of 466% over a 15 year period [45]. This shows that people are aware of the value of FA utilization with technological advances and increased incomes. Figure 4 shows uses of FA in India: 44.76% for cement, 16.72% for reclamation, 9.1% for mine filling, 6.89% for ash dyke raising, 6.86% for bricks and tiles, 6.51% for road and embankments, 0.74% for concrete, and 7.38% for other uses [15]. The Ministry of Environment, Forests and Climate Change (MoEFCC) expanded the standard of using FA to make it more used in agriculture. Moreover, to improve the utilization of FA and reduce the cost, the Ministry of Railways has also made relevant regulation policies, such as providing FA free of charge to the surrounding users, and establishing the cement industry near coal power plant [46]. Parab et al. [47] and other researchers [48,49,50,51,52] found that FA of India can improve soil structure and be used to reclaim dumps and degraded soils by making soil more fertile and increasing the yield of agricultural products. Given the advantages of FA and the policies in place in India, it is expected that the utilization rate of FA in India will continue to increase in the near future.
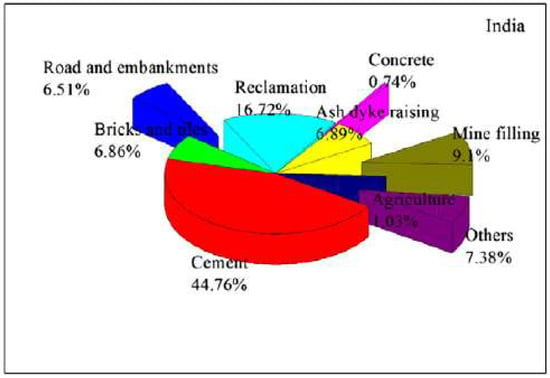
Figure 4.
Utilization of fly ash in India (reproduced with permission from [15], Elsevier, 2018).
2.3. USA
The United States has multiple energy sources and high energy efficiency due to their advanced technology and equipment. Americans use many types of energy, including petroleum, natural gas, coal, renewable energy, and nuclear electric power. The energy consumption of the United States in 2016 is displayed in Figure 5a. Coal consumption accounted for only 15% of total United States primary energy consumption. Natural gas and renewable energy accounted for 39% of total energy. Although renewable energy only accounted for 10%, it includes many sources such as biomass waste, biofuel, and hydroelectric. It can be inferred that the United States has a strong sense of environmental protection, which makes it particularly favor use of clean energy based on advanced technology. Because of these factors, coal-fired power generation in the United States is not substantial. The energy consumption for power stations in 2013 is shown in Figure 5b. It can be clearly seen that the United States relies mainly on natural gas to generate electricity, which accounts for about 50.8% of energy production. Coal accounts for only 21.9% of all power generation. The growth rate of coal consumption in the United States from 2010 to 2017 is almost zero, as shown in Figure 6. Basu et al. [24] presented that utilization of FA in the United States is as high as 65%, including many fields, such as mine fill, cement, wallboard, snow and ice control, agriculture, cosmetics, bowling balls and carpeting. In 2006, the use of FA in cement kilns was about four times more than that in 2001, about 4 million tons. In addition, housing projects and rising demand in the ready-mixed concrete market are expected to be major drivers for future FA utilization in the United States [53].
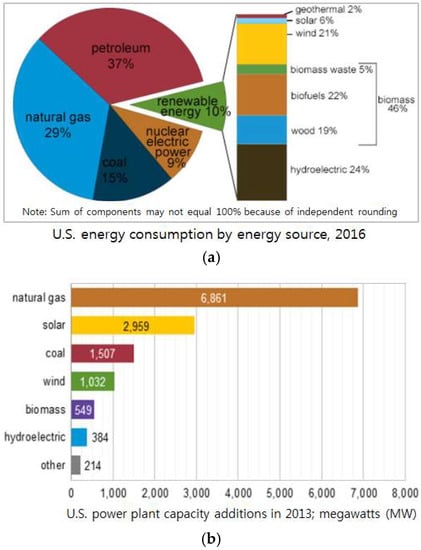
Figure 5.
Energy consumption in the United States in 2016 (sources: EIA; U.S. energy consumption by energy source, 2016) (a); and energy consumption of power stations in the United States in 2013 (sources: EIA; U.S. power plant capacity additions, 2013) (b).
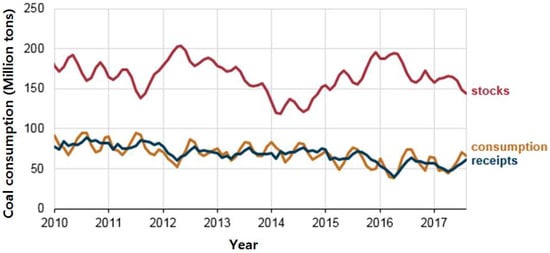
Figure 6.
Coal consumption in the United States from 2010 to 2017 (sources: EIA; U.S. Energy Information Administration, Electric Power Monthly).
2.4. Other Countries
Other countries produce a small amount of FA from coal-fired power. For example, Germany produces 40 million tons per year, the UK produces 15 million tons, and Australia produces 10 million tons. The annual production of FA has not reached 10 million tons per year in Canada, France, Denmark, Italy, or the Netherlands. These countries produce very little FA every year compared to China and India, but their FA utilization is high. The average utilization rate of Germany, the UK, Australia, Canada, and France is 76%. The average of Denmark, Italy, and the Netherlands is up to 100%. FA is used in the same ways in these countries as in China, India, and USA [24,47,54].
3. Physical and Chemical Properties of Fly Ash
The physical and chemical properties of FA play an important role in its study, including its morphology, its chemical properties and its mineralogical properties. FA properties may vary depending on combustion conditions and collector setup. The average size of FA fine particles is less than 20 microns, with a bulk density of 0.54–0.86 g/cm3, a surface area of 300–500 m2/kg, and a pH value of 1.2–12.5, with most ashes tending toward alkalinity [55]. FA can be classified into three main groups: acidic ash (pH ~4.5), mildly alkaline ash (pH 8–9), and strongly alkaline ash (pH 11–13) [15,56].
3.1. Morphological Characteristics
Figure 7 and Figure 8 show scanning electron microscopy (SEM) images of FA. Figure 7a,b shows the morphological characteristics of non-carbonated FA, including different sized spherical particles, cenospheres, and plerospheres. Most of the small particles are well-rounded and solid spheres. Some large particles are vesicular cenospheres due to the presence of gas bubbles or vapor [57]. Figure 7c,d shows morphologies after FA carbonization, which has the “cubic-like” structures of calcite (Figure 7c) and “needle-like” structures of aragonite (Figure 7d). In addition, FA particles are reported to be globular and irregular with high porosity [58], glassy cenospheres [59,60], spherical-shaped and flake-like particles [61,62], spherical particles of varying sizes and particles of unburned coal [59,63,64], and predominantly spherical in shape and consisting of solid spheres, cenospheres, irregular-shaped debris and porous unburnt carbon [15]. In Figure 8, the FA particles are mainly spherical, consisting of solid spheres, cenospheres, irregular fragments and porous unburned carbon. The morphological characteristics of FA depend on particle size, but cenospheres are an exception. FA precipitators are sized 20–53 μm and 75–106 μm, while cenospheres are 20–45 μm and 106–150 μm [59]. FA particles smaller than 20 μm consist of mostly smooth spherical particles, while many irregularly shaped grains are between 75 and 106 μm, and fewer irregularly shaped vesicular grains are more than 150 μm [65].
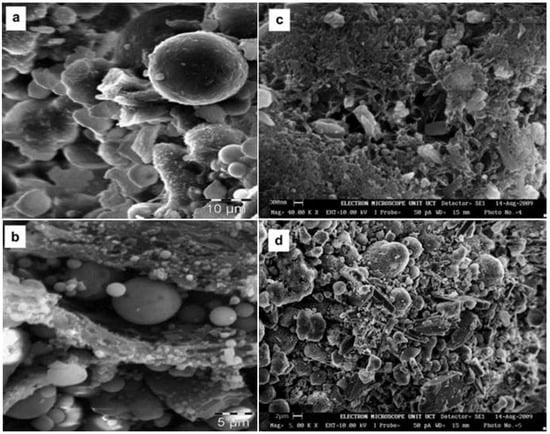
Figure 7.
SEM images of: non-carbonated fly ash (a,b) and carbonated fly ash (c,d) (reproduced with permission from [57], Elsevier, 2018). Note: The fly ash was collected from coal burning power plants in the Mpumalanga province of South Africa.

Figure 8.
SEM images of fly ash fractions. FBC ash, fluidized bed combustion ash (reproduced with permission from [15], Elsevier, 2017).
3.2. Chemical and Mineralogical Composition
The chemical and mineralogical composition of FA plays an important role in the analysis of the application and disposal of FA, including its use as a cement replacement material. The chemical composition of FA can be determined by X-ray fluorescence (XRF) and spectrometry techniques. Researchers [66,67,68,69,70] have reported that FA is composed of SiO2, Al2O3, Fe2O3, CaO, MgO, Na2O, K2O, TiO2, P2O5, and small amounts of MnO, BaO, and SO3. The detailed chemical composition of fly ash is given in Table 2. The chemical composition of FA may differ based on geographical origin and combustion conditions. Some heavy metals are also found in FA, such as Fe, Ca, Zn, Cd, Co, Ni, Cu, Pb and Mn [71], as shown in Table 3. FA can also be divided into high calcium ash (≥10% CaO) and low calcium ash (<10% CaO), according to the content of CaO. High calcium ash can remove acidic gases and fix hazardous heavy metals, such as Cd and Cu. CaO is present in FA as free lime and embedded in glassy spheres. Usually, the CaO content increases with decreasing particle size [57]. Loss on ignition (LOI) has long served as a standard method to measure unburned carbon in FA [72,73]. All types of FA have a certain LOI value indicating their unburnt carbon content (Table 2). Unburnt carbon in FA powder has properties similar to activated carbon, such as good adsorption capacity.

Table 2.
Primary chemical compositions for fly ash produced from different coal types (adapted with permission from [5], Elsevier, 2017).

Table 3.
The concentration of heavy metals in Yenikoy Toxicity Characteristic Leaching Procedure (TCLP) leachates (adapted with permission from [71], Elsevier, 2018).
Some minerals in FA can be measured by X-ray diffraction (XRD) spectroscopy techniques. Nyambura et al. [57] analyzed the XRD spectra of non-carbonated and carbonated FA, as shown in Figure 9. Minerals in non-carbonated FA were found to mainly consist of amorphous, CaO, hematite, mullite, and quartz (see Figure 9a). The minerals in carbonated FA are mainly composed of amorphous CaCO3, magnetite, mullite, plagioclase, quartz, bassanite, and anhydrite (see Figure 9a). The major crystalline phases are mullite and quartz for most ash [74,75,76,77]. The CaO in non-carbonated FA is converted into calcite (main component: CaCO3) when it is carbonized. These results have been described in SEM spectrum analysis, and the “cubic-like” structures in Figure 7c are calcites [57]. Many other researchers have also confirmed these results [78]. Among the many oxides in FA, recovery of alumina has attracted interest in recent decades. Therefore, as science and technology progresses, the recovery and utilization of industrial waste to benefit humans and the environment will help reduce environmental impacts.
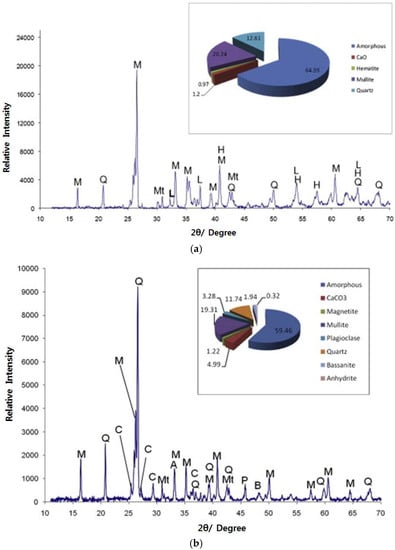
Figure 9.
XRD patterns of: non-carbonated FA (a) and carbonated FA (b). M, mullite; Q, quartz; C, calcite; A, anhydrite; P, plagioclase; B, bassanite; Mt, magnetite; L, lime; and H, hematite (reproduced with permission from [57], Elsevier, 2018).
4. Application of Fly Ash for Treatment of Pollutants
4.1. Removing Air Pollutants
As a cheap adsorbent, FA can effectively reduce environmental pollution through simple processing due to its high porosity, high surface area, appropriate pore size, high porosity, and other characteristics (e.g., unburned carbon remaining in the fly ash particles and CaO percentage) [79]. Higher surface area and carbon content having high micropore volume results in a higher adsorption capacity and larger breakthrough time. In addition, the adsorption effect is not only related to the physicochemical properties of the adsorbent itself but also to the physicochemical properties of the adsorbate, such as pore structure, surface functional groups [80]. The air pollutants that can be adsorbed by FA include volatile organic compounds (VOCs), nitrogen oxides (NOx) and sulfur dioxide (SO2).
4.1.1. Removal of Volatile Organic Compounds
Volatile organic compounds (VOCs) are one of air pollutants mainly produced by fossil fuel combustion [81,82], painting [83], refining [84], building [85], and other industries [86,87]. In recent decades, rapid development of industry has led to a dramatic increase in anthropogenic VOCs. There are more than 300 chemical structures of VOCs, which can be classified as alkanes, aromatics, esters, and aldehydes, among others [88,89]. Many VOCs are harmful to humans in even small amounts, such as benzenes and formaldehyde, which are suspected carcinogens [90]. Xylene and aliphatic hydrocarbons are mainly produced by the paint industry and are harmful to the environment and humans [91,92]. In sunlight, photochemical reactions of VOCs and NOx will generate ozone, peroxide, nitro-aldehydes, and other photochemical smog compounds that cause secondary pollution and affect people’s eyes and respiratory systems, endangering human health [93,94,95,96]. Table 4 shows some VOCs harmful to human health [97]. Thus, the reduction and control of VOCs from air are important research topics for air purification.

Table 4.
Sources and health effects of major volatile organic compounds (adapted with permission from [97], Elsevier, 2018).
Seo et al. [98] evaluated adsorption capacity of seven building materials (activated carbon, gypsum board mixed with a given quantity of activated carbon, board made out of activated carbon, humidity-controlling porous ceramic material, humidity-controlling silicate calcium, ceramic tile and ordinary gypsum board) for adsorption of four kinds of VOCs in a small test chamber. They showed that activated carbon has the highest VOCs adsorption capacity compared with other building materials. Chmielewski et al. [99] studied the reduction of VOCs from coal combustion using electron beam treatment and found that the concentration of polycyclic aromatic hydrocarbons (VOCs) was greatly reduced from 40% to 98%. Other researchers have also found that other materials effectively reduce VOCs, including cashew nut shell liquid [100,101], sol-gel [102], CuO-CeO2 mixed oxides [103], Mn-Cu mixed oxide [104], manganese oxides [105], and other metal oxide catalysts and noble metals [106,107]. Among them, activated carbon is one of the most common adsorbents because of its low-cost, high availability, excellent specific surface area, pore size, porosity, chemical functional group, and other properties [108,109,110]. Nevertheless, activated carbon adsorption of macromolecules and hydrophilic VOCs is not good because it has a multi-microporous structure and is a nonpolar adsorbent [97]. On the other hand, activated carbon fibers adsorb VOCs better than activated carbon because of their short and straight thin-fiber shaped micropores that increase intraparticle adsorption kinetics compared to activated carbon [111,112]. In addition, activated carbon fibers also play an important role during adsorption or desorption, have large adsorption capacity and have high mass transfer rates [113,114].
Similar to adsorption on activated carbon fibers, FA can be used to fabricate a functional adsorption fibrous membrane through simple electrospinning [32]. Electrospinning is an efficient method for fabricating fibers from nanometer to micrometer, with advantages of high efficiency, low cost, and easy operation [115]. Figure 10 shows a schematic of an electrospun fibrous membrane and a VOCs adsorption experiment. The spinnable functional material (e.g., fly ash) and polymer (e.g., polyacrylonitrile (PAN), polyurethane (PU), and Polylactic acid (PLA)) are mixed with the chemical solvent (e.g., N,N dimethylformamide (DMF) and methyl ethyl ketone (MEK)) at a certain ratio, then as-prepared spinning solution is further processed using magnetic stirring and ultrasonication to obtain a homogenous spinning solution, and finally the composite membrane is collected on the roller under the action of the electrostatic field. The size and arrangement of electrospun fibers are affected by viscosity, conductivity and feed rate of spinning solution, distance between nozzle and collector, supply voltage, etc. [88,116]. Kim et al. [33] successfully synthesized polyurethane fibers containing different amounts of FA and analyzed their adsorption of chloroform, benzene, toluene, xylene, and styrene (Figure 11). They found that polyurethane fibers containing 30 wt % FA had the smallest fiber diameter and the maximum specific surface area, it results in the highest VOCs adsorption capacity compared to other fibrous membranes. Furthermore, the adsorption of VOCs by fibrous membranes was in the following order: styrene > xylene > toluene > benzene > chloroform. This is because adsorption of VOCs not only depends on the surface area and fiber diameter, but also on other characteristics, including molecular polarity, molecular structure, molecular weight, pore size, porosity, chemical functional groups electronic and steric effects, π-complexes, adsorption kinetics, ionization potential, dipole moment, boiling point, temperature and humidity [97,117,118]. In general, lower ionization potential aromatic compounds are easier to be absorbed by FA fibrous membranes due to their instability [32]. The adsorbent with high C/O ratio has the high adsorption capacity for adsorption of nonpolar VOCs [119]. Use of FA fibrous membrane to adsorb VOCs from air is an innovative research, which provides a new idea for using FA fibrous membrane to filter air pollutants. As an emerging material, FA fibrous membrane can be modified into a variety of air filters with great development potential in the field of environmental pollution management [32,33].
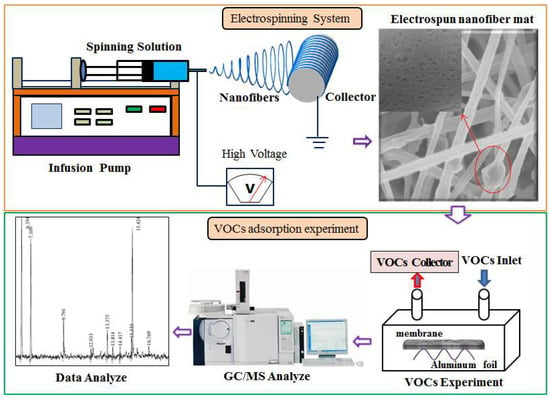
Figure 10.
Schematic diagram of electrospun fibrous membrane and VOCs adsorption experiment.
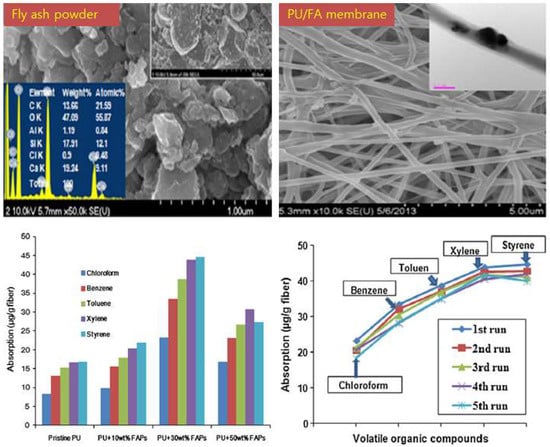
Figure 11.
SEM images and VOCs adsorption capacity of FA membranes (adapted with permission from [33], Elsevier, 2018).
4.1.2. Removal of Nitrogen Oxides
A small amount of NOx can be adsorbed by FA because of the unburned carbon in FA and the physical characteristics of FA such as high porosity and specific surface area [120]. In fact, this carbon is a precursor to activated carbon, but it has gone through devolatilization during combustion in the furnace of a power station [121]. Therefore, the internal carbon can be activated by some simple modifications to the FA to increase its adsorption capacity. The general method used is steam activation and physical separation, but, because the ash content of carbon-enriched FA is too high, using chemical demineralization to activate carbon is the best method [122]. Rubio et al. [122] studied adsorption of NOx by unburnt carbon from FA and found that the conversion curves of NO on different FA samples reached steady state after 10 h. In addition, the enriched-carbon FA showed high NO abatement capacity due to increased carbon content, surface area, and porosity. Izquierdo et al. [30] synthesized Cu and Fe exchange type Y zeolites from FA for removal of NOx from flue gas. Rubel et al. [123] also reported that carbon-rich products from combustion ash could decrease NOx emissions and Hg due to ion-pair interactions between NO+ and O2− at the surface of carbon, with subsequent condensation of NO2 in micropores. In addition, the pore size on the surface of the adsorbent can be changed after adsorption of a certain amount of Hg, producing the optimum pore size (micropores, <2 nm) for adsorbed NOx [124]. Rubel et al. [123] also showed that the surface area of adsorbents increased due to adsorption of NOx, as shown in Figure 12. From the above literature, it can be concluded that unburned carbon concentration in FA plays a decisive role for reducing NOx from air, and the high surface area of FA will further improve the NOx adsorption capacity. In addition, the unburned carbon remaining in the FA particles contribute the main surface area to FA, and the carbon can be activated to further improve the adsorption performance of the FA [5].
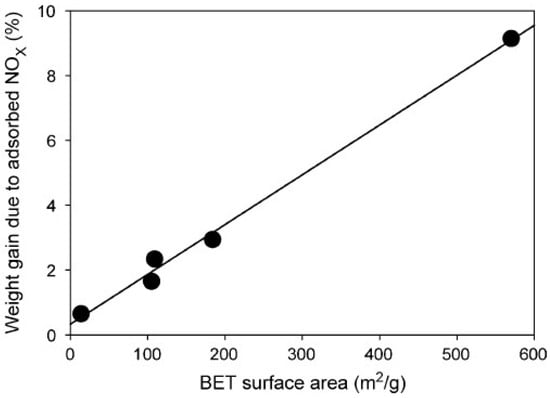
Figure 12.
Relationship between BET surface area of adsorbent and adsorbed NOx (reproduced with permission from [123], Elsevier, 2018).
4.1.3. Removal of Sulfur Dioxide
Coal power generation plays an important role in electricity generation worldwide, accounting for about 41% of the world’s power generation in 2006. In some countries, this percentage is even higher, such as Poland (92%), China (79%), and India (69%) [125,126]. The large amount of coal used causes an increase in SO2 and a small amount of SO3, which pollute the air. SO2 is the main chemical responsible for formation of acid rain [127,128,129]. In addition, SO2 and SO3 are harmful gases that contribute to formation of submicron acid particles that can penetrate human lungs and be absorbed into the bloodstream [130]. Many researchers have reported that blending FA with Ca(OH)2 or CaO can produce an adsorbent via a hydration process with a better SO2 reduction than that of hydrated lime [131,132,133]. Lee et al. [134] found similar results, as shown in Figure 13. An adsorbent composed of a mixture of FA/CaO/CaSO4 was successfully synthesized with a higher SO2 adsorption capacity than that of pure FA, CaSO4, and CaO, due to its larger specific surface area (64.5 m2/g). In addition, no adsorbents exhibited significant desulfurization activity. SEM images of adsorbent before and after SO2 adsorption are shown in Figure 14. The high density of pore structures on the surface of the adsorbent before adsorbing SO2 can be clearly seen. However, the pores disappear after adsorption of SO2, due to SO2 being converted into sulfate salts (CaSO4) and covering the surface of the adsorbent. From the above results, it can be concluded that the ability of FA to capture SO2 is mainly related to its surface area, pore structures and calcium ion content. Specific surface area is increased after the FA is hydrated, leading to more calcium ions that can react with SO2 to produce CaSO4 [135,136].
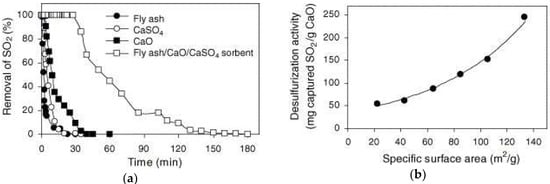
Figure 13.
(a) Desulfurization activity of different adsorbents; and (b) relationship between desulfurization capacity and specific surface area (reproduced with permission from [134], Elsevier, 2018).
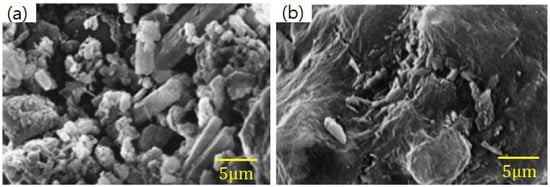
Figure 14.
Comparison of SEM images before (a) and after (b) SO2 adsorption (adapted with permission from [134], Elsevier, 2018).
4.2. Removing Water Pollutants
4.2.1. Removal of Heavy Metals
Industrial development is likely to cause water pollution, especially heavy metal pollution, because of its inherent toxicity, large sources, persistence, non-degradability, and other factors and can cause serious harm to humans, animals, plants, and the environment [137,138]. Some of the common sources of anthropogenic heavy metals are displayed in Table 5. The treatment of waste water containing heavy metal can be divided into three categories: physical methods, chemical methods, and biological methods [5,139]. Physical methods include adsorption (activated carbon, carbon nanotubes, kaolinite and montmorillonite, FA, and other low-cost adsorbents), membrane filtration (ultrafiltration, reverse osmosis, nanofiltration, and electrodialysis), coagulation and flocculation, flotation, and other methods [5,140]. Chemical methods include chemical precipitation (hydroxide precipitation, sulfide precipitation, chelating precipitation, and chemical precipitation combined with other methods), ion-exchange, electrochemical treatment technologies, and others [5,139]. Biological methods include bio-adsorbents and use of microorganism, as well as others [141,142]. Among them, ion-exchange, adsorption and membrane filtration are the most commonly used method, and use of modified FA is an economical and efficient method for treatment of heavy metal wastewater because FA is derived from industrial waste.

Table 5.
Primary sources of common heavy metals and their effects on human organs (reproduced with permission from [143], Elsevier, 2017).
FA can be used directly or indirectly (synthesized as zeolites) to adsorb heavy metal from polluted water [144,145,146]. It is a potential adsorbent that can remove Cu, Pb, Zn, Mn, Cd, Cr, and Ni from wastewater [147,148]. On the other hand, the pH of the aqueous solution directly affects adsorption of metal ions due to changes in surface charge of the adsorbent and the degree of ionization [26,144]. FA has a high adsorption capacity for Cu2+ and Zn2+ ions at pH 8. In addition, using FA to adsorb Cu2+ and Zn2+ ions was faster than that of natural zeolite and peanut husk [149]. The SEM images of the FA before and after the adsorption of heavy metals are shown in Figure 15. As shown in Figure 15b, the porosity between the FA particles is significantly reduced. This could be attributed to that the surface of FA is covered by complexes formed by heavy metal ions [26]. Moreover, FA has a stronger adsorption capacity for Pb2+ and Cd2+ than other heavy metal ions. Adsorption of Pb2+ and Cd2+ can be attributed to chemical sorption or chemisorption involving valence forces through sharing or exchange of electrons between the sorbent and sorbate, because their adsorption kinetics followed second-order reaction kinetics [26,150]. With the development of nanotechnology, FA can be fabricated into a functional fibrous membrane with a high capacity for adsorbing heavy metals [34]. FA particles can be perfectly combined with polyurethane fibers via electrospinning, and the FA fibrous membrane has higher adsorption capacity and faster adsorption rate for adsorption of Pb than that of Hg. This is attributed to the affinity of formation of PbOH+ being higher than to HgOH+ [34]. The results are encouraging for the removal of heavy metals using FA fibrous membrane from industrial wastewater. FA fibrous membrane can be used as a variety of filters with low-cost and high performance. These are a great help to improve the utilization of FA and reduce the pressure of environmental pollution. The mechanism of modified FA for treatment of heavy metal wastewater can be summarized as follows: most FA is alkaline with a high adsorption capacity and high surface area, and negative charge accumulates at the FA surface in alkaline solutions. FA can be expected to remove some metal ions from wastewater by electrostatic interactions or precipitation–adsorption. The ion exchange capacity, high surface area, and unique pore characteristics of modified FA play an important role for treatment of heavy metal wastewater [15,139].
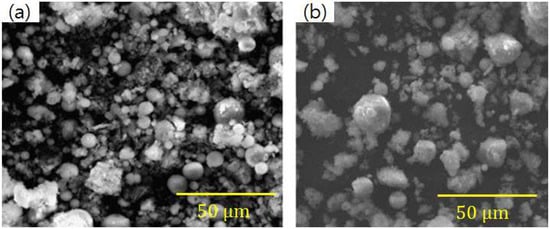
Figure 15.
Comparison of SEM images before (a) and after (b) heavy metal adsorption (adapted with permission from [26], Elsevier, 2018).
4.2.2. Removal of Other Organic/Inorganic Components
On the other hand, FA not only has the capacity to adsorb heavy metals from wastewater, but also has the ability to adsorb other organic/inorganic pollutants from wastewater, including to phosphorous, fluoride, boron, phenolic compounds, pesticides, dyes, etc. [5,151]. Phosphorus is an indispensable element in living organisms. Phosphorus is required for the metabolism of organisms. Therefore, when the phosphorus chemical industry discharges phosphorus compounds into rivers, it will lead to the “eutrophication” of water, which will lead to the rapid growth of algae and other plants, and absorb a large amount of free oxygen in the water, posing a great threat to the ecological environment. Many researchers [152,153] have studied the use of FA to remove phosphorus from wastewater. Kandel et al. reported that mixture of sand with 5% of FA by weight can effectively reduce phosphorus concentration in water [151]. They showed that the FA has a good effect of adsorb or precipitate phosphate because of the FA contains a certain amount of calcium contents. The removal efficiency of phosphate is affected by pH conditions and calcium content in FA. In general, alkaline conditions for high-calcium FA, neutral pH levels for medium-calcium FA have a high ability to remove phosphate, and the low-calcium FA immobilized little phosphate at all pH values [154]. In addition, the iron contained in FA can also react with phosphate to cause phosphate to be precipitated. Using FA to remove phosphorus from wastewater is primarily due to the precipitation of phosphate with Ca2+ ions in solution, and the weak physical interactions between the surface of FA and the phosphate [155]. Xu et al. [156] found that a magnesia-loaded fly ash cenospheres (MLC) is an efficient adsorbent for adsorption of fluoride. The influence of temperature, initial fluoride concentration, pH of the solution, contact time, coexisting ions, and fly ash dosage on fluoride removal was respectively investigated. They indicated that when the pH of the solution is 3, MLC has the maximum adsorption of fluorine, which is mainly due to highly protonated MLC surface and the gradual increase in the attractive forces between the positively charged surface and the negatively charged fluoride ions. Polat et al. [70] investigated the removal of boron from seawater and desalinated seawater on FA. They carried out several series of column and batch experiments and determined that there is a significant reduction of boron concentration in seawater after interaction with FA. Under suitable conditions (e.g., pH = 9, L/S = l/10, reaction time > 6 h), more than 95% of boron was removed by FA. Phenolic compounds are one of the major pollutants in polluted water. Phenol will volatilize into the atmosphere or infiltrate underground, polluting the atmosphere, groundwater and crops when untreated phenolic wastewater is used to irrigate crops. Phenol, cresol and other volatile phenols have attracted wide attention. FA as a waste material can be used as adsorbents for the removal of phenolic compounds. Ahmaruzzaman et al. [157] and Kao et al. [158] reported that the high adsorption capacity of FA was related to its high-surface-area, pore volume, porosity and higher unburned carbon content. Moreover, phenols have a strong hydroxyl functional group which interacts with the adsorbent surfaces, resulting in vertical alignment of the molecule on the surface. Therefore, phenol can be more adsorbed by FA because of the interaction between adsorbed molecules. Other researchers [159,160] have demonstrated that FA also has the ability to remove pesticides, various dyes and pigments from wastewater. FA is low cost and more effective for adsorption of multiple harmful substances to be used widely in wastewater treatment.
5. Conclusions
Fly ash (FA) can be used as the base material of some adsorbents to treat air and water pollution due to its high porosity, high surface area, appropriate pore size, CaO percentage and surface functional groups. It can be fabricated into a variety of composite materials including zeolites and fiber membranes. They have excellent adsorption capacity in controlling air and water pollutants, such as VOCs, SOx, NOx, heavy metals and toxic organic dyes. In this paper, the mechanism of FA for adsorption of air pollutants and water pollutants has been studied, in addition to a comparison of FA production of different countries, as well as the physical and chemical properties of FA. Detailed results are as follows:
- Fly ash can be used in building materials, building works, roads construction, agriculture, and other fields.
- To improve the adsorption capacity and efficiency of fly ash on environmental pollutants and make full use of fly ash, new technologies for the efficient utilization of fly ash should be developed, such as nanofiber technology.
- Fly ash can be converted to inexpensive and high performance adsorbents by simple modifications due to its unique porous properties. For example, is can be made into various kinds of zeolites.
- Modified fly ash can adsorb VOCs, NOx and SO2 emissions in the air and can also remove some heavy metals and other organic/inorganic pollutants (e.g., phosphorous, fluoride, boron, phenolic compounds, pesticides and dyes) from wastewater, due to its high porosity, high surface area, appropriate pore size, high porosity, alkalinity, negative charge, unburned carbon remaining in the fly ash particles and other unique characteristics.
- In addition, the adsorption effect is not only related to the physicochemical properties of the adsorbent itself but also to the physicochemical properties of the adsorbate, e.g., polar/non-polar, and functional groups.
- Pristine fly ash is a powder, which limits its scope of use. Using nanotechnology, it can be synthesized into low-cost, multi-functional and multi-purpose porous hybrid composites for adsorption of air pollutants and water pollutants. Fly ash porous hybrid composites as an emerging material, which has great potential in the future.
Author Contributions
J.C.G. made suggestions, analyzed the physicochemical properties of fly ash, summarized the adsorption mechanism of the fly ash composite materials for treatment of environmental pollutants, and drafted this manuscript. S.K.Y. summarized and analyzed the production and consumption of fly ash from several representative countries, summarized the synthetic methods of the fly ash composite materials and analyzed their development trend in the future. N.J.C. guided the idea of this manuscript, analyzed the application of the fly ash composite materials in the control of environmental pollutants, and supervised the work and the manuscript. All authors participated in the evaluation of the data, and reading and approving the final manuscript.
Funding
This research was funded by [the Ministry of Education] grant number [2016R1D1A1B03931616].
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (Project No. 2016R1D1A1B03931616).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Deng, F.; Pan, Y.; Sun, W.; Yang, F.; Zhang, Y.; Huang, Z. An ignition delay time and chemical kinetic study of ethane sensitized by nitrogen dioxide. Fuel 2017, 207, 389–401. [Google Scholar] [CrossRef]
- Kampa, M.; Castanas, E. Human health effects of air pollution. Environ. Pollut. 2008, 151, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, J.A.; Alexis, N.; Barnes, C.; Bernstein, I.L.; Nel, A.; Peden, D.; Diaz-Sanchez, D.; Tarlo, S.M.; Williams, P.B. Health effects of air pollution. J. Allergy Clin. Immunol. 2004, 114, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.; Liu, J.; Wang, Q.; Liang, Z. Health risk assessment of heavy metal exposure to street dust in the zinc smelting district, northeast of China. Sci. Total Environ. 2010, 408, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Ahmaruzzaman, M. A review on the utilization of fly ash. Prog. Energy Combust. Sci. 2010, 36, 327–363. [Google Scholar] [CrossRef]
- Zhao, C.; Luo, K. Household consumption of coal and related sulfur, arsenic, fluorine and mercury emissions in China. Energy Policy 2018, 112, 221–232. [Google Scholar] [CrossRef]
- Bartoňová, L. Unburned carbon from coal combustion ash: An overview. Fuel Process. Technol. 2015, 134, 136–158. [Google Scholar] [CrossRef]
- Ding, M.; Han, W.; Li, J.; Ma, E.; Shan, Z. In situ study of the mechanical properties of airborne haze particles. Sci. Chin. Technol. Sci. 2015, 58, 2046–2051. [Google Scholar] [CrossRef]
- Chen, S.; Xu, L.; Zhang, Y.; Chen, B.; Wang, X.; Zhang, X.; Zheng, M.; Chen, J.; Wang, W.; Sun, Y. Direct observations of organic aerosols in common wintertime hazes in north China: Insights into direct emissions from chinese residential stoves. Atmos. Chem. Phys. 2017, 17, 1259–1270. [Google Scholar] [CrossRef]
- Ge, J.C.; Yoon, S.K.; Choi, N.J. Using canola oil biodiesel as an alternative fuel in diesel engines: A review. Appl. Sci. 2017, 7, 881. [Google Scholar] [CrossRef]
- Iyer, R.S.; Scott, J.A. Power station fly ash—A review of value-added utilization outside of the construction industry. Resour. Conserv. Recycl. 2001, 31, 217–228. [Google Scholar] [CrossRef]
- Zhuang, X.Y.; Chen, L.; Komarneni, S.; Zhou, C.H.; Tong, D.S.; Yang, H.M.; Yu, W.H.; Wang, H. Fly ash-based geopolymer: Clean production, properties and applications. J. Cleaner Prod. 2016, 125, 253–267. [Google Scholar] [CrossRef]
- Park, J.H.; Edraki, M.; Mulligan, D.; Jang, H.S. The application of coal combustion by-products in mine site rehabilitation. J. Clean. Prod. 2014, 84, 761–772. [Google Scholar] [CrossRef]
- Lanzerstorfer, C. Fly ash from coal combustion: Dependence of the concentration of various elements on the particle size. Fuel 2018, 228, 263–271. [Google Scholar] [CrossRef]
- Yao, Z.; Ji, X.; Sarker, P.; Tang, J.; Ge, L.; Xia, M.; Xi, Y. A comprehensive review on the applications of coal fly ash. Earth Sci. Rev. 2015, 141, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Bouzoubaa, N.; Lachemi, M. Self-compacting concrete incorporating high volumes of class F fly ash: Preliminary results. Cem. Concr. Res. 2001, 31, 413–420. [Google Scholar] [CrossRef]
- Abbas, S.; Saleem, M.A.; Kazmi, S.M.; Munir, M.J. Production of sustainable clay bricks using waste fly ash: Mechanical and durability properties. J. Build. Eng. 2017, 14, 7–14. [Google Scholar] [CrossRef]
- Kumar, S. Fly ash–lime–phosphogypsum hollow blocks for walls and partitions. Build. Environ. 2003, 38, 291–295. [Google Scholar] [CrossRef]
- Dilmore, R.M.; Neufeld, R.D. Autoclaved aerated concrete produced with low NOx burner/selective catalytic reduction fly ash. J. Energy Eng. 2001, 127, 37–50. [Google Scholar] [CrossRef]
- Kumar, S. A perspective study on fly ash–lime–gypsum bricks and hollow blocks for low cost housing development. Constr. Build. Mater. 2002, 16, 519–525. [Google Scholar] [CrossRef]
- Andini, S.; Cioffi, R.; Colangelo, F.; Grieco, T.; Montagnaro, F.; Santoro, L. Coal fly ash as raw material for the manufacture of geopolymer-based products. Waste Manag. 2008, 28, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Koukouzas, N.; Ketikidis, C.; Itskos, G.; Spiliotis, X.; Karayannis, V.; Papapolymerou, G. Synthesis of CFB-coal fly ash clay bricks and their characterisation. Waste Biomass Valoriz. 2011, 2, 87–94. [Google Scholar] [CrossRef]
- Jala, S.; Goyal, D. Fly ash as a soil ameliorant for improving crop production—A review. Bioresour. Technol. 2006, 97, 1136–1147. [Google Scholar] [CrossRef] [PubMed]
- Basu, M.; Pande, M.; Bhadoria, P.; Mahapatra, S. Potential fly-ash utilization in agriculture: A global review. Prog. Nat. Sci. 2009, 19, 1173–1186. [Google Scholar] [CrossRef]
- Ram, L.; Srivastava, N.; Tripathi, R.; Jha, S.; Sinha, A.K.; Singh, G.; Manoharan, V. Management of mine spoil for crop productivity with lignite fly ash and biological amendments. J. Environ. Manag. 2006, 79, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.; Gandhimathi, R. Removal of heavy metal ions from municipal solid waste leachate using coal fly ash as an adsorbent. J. Hazard. Mater. 2009, 169, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Bayat, B. Comparative study of adsorption properties of turkish fly ashes: I. The case of nickel (II), copper (II) and zinc (II). J. Hazard. Mater. 2002, 95, 251–273. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Kerketta, S.; Kumar, M.S.; Rajanikanth, B. Discharge Plasma Cascaded with Fly Ash for Removal of NOx in Biodiesel Exhaust: A Feasibility Study. Int. J. Plasma Environ. Sci. Technol. 2014, 8, 98–102. [Google Scholar]
- Hower, J.C.; Maroto-Valer, M.M.; Taulbee, D.N.; Sakulpitakphon, T. Mercury capture by distinct fly ash carbon forms. Energy Fuels 2000, 14, 224–226. [Google Scholar] [CrossRef]
- Izquierdo, M.; Rubio, B. Carbon-enriched coal fly ash as a precursor of activated carbons for SO2 removal. J. Hazard. Mater. 2008, 155, 199–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.; Chen, Y.-L.; Zhang, X.-H.; Tian, F.-M.; Zu, Z.-N. Zeolites developed from mixed alkali modified coal fly ash for adsorption of volatile organic compounds. Mater. Lett. 2014, 119, 140–142. [Google Scholar] [CrossRef]
- Kim, H.J.; Pant, H.R.; Choi, N.J.; Kim, C.S. Fly ash/polyurethane thin film for the adsorption of volatile organic compounds (VOCs) from air. Fibers Polym. 2014, 15, 1393–1398. [Google Scholar] [CrossRef]
- Kim, H.J.; Pant, H.R.; Choi, N.J.; Kim, C.S. Composite electrospun fly ash/polyurethane fibers for absorption of volatile organic compounds from air. Chem. Eng. J. 2013, 230, 244–250. [Google Scholar] [CrossRef]
- Joo Kim, H.; Raj Pant, H.; Hee Kim, J.; Jung Choi, N.; Sang Kim, C. Fabrication of multifunctional TiO2–fly ash/polyurethane nanocomposite membrane via electrospinning. Ceram. Int. 2014, 40, 3023–3029. [Google Scholar] [CrossRef]
- Pant, H.R.; Kim, H.J.; Joshi, M.K.; Pant, B.; Park, C.H.; Kim, J.I.; Hui, K.; Kim, C.S. One-step fabrication of multifunctional composite polyurethane spider-web-like nanofibrous membrane for water purification. J. Hazard. Mater. 2014, 264, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhou, M.; Li, H. The drag effect of coal consumption on economic growth in China during 1953–2013. Resour. Conserv. Recycl. 2018, 129, 326–332. [Google Scholar] [CrossRef]
- He, Y.; Luo, Q.; Hu, H. Situation analysis and countermeasures of China’s fly ash pollution prevention and control. Procedia Environ. Sci. 2012, 16, 690–696. [Google Scholar] [CrossRef]
- Dong, P.; Wang, S.Y. Risks and countermeasures of the shale gas development in China. Adv. Mater. Res. 2013, 734, 1253–1256. [Google Scholar] [CrossRef]
- Hu, D.; Xu, S. Opportunity, challenges and policy choices for China on the development of shale gas. Energy Policy 2013, 60, 21–26. [Google Scholar] [CrossRef]
- Pi, G.; Dong, X.; Dong, C.; Guo, J.; Ma, Z. The status, obstacles and policy recommendations of shale gas development in China. Sustainability 2015, 7, 2353–2372. [Google Scholar] [CrossRef]
- Yao, Z.; Xia, M.; Sarker, P.K.; Chen, T. A review of the alumina recovery from coal fly ash, with a focus in China. Fuel 2014, 120, 74–85. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Wang, L.; Sun, X. Study on adsorption of Cr(VI) using singlephase zeolites synthesized from fly ash. Chin. J. Environ. Eng. 2008, 2, 1121–1126. [Google Scholar]
- Ram, L.; Masto, R. Fly ash for soil amelioration: A review on the influence of ash blending with inorganic and organic amendments. Earth Sci. Rev. 2014, 128, 52–74. [Google Scholar] [CrossRef]
- Singh, J.; Mantha, S.S.; Phalle, V.M. Characterizing domestic electricity consumption in the indian urban household sector. Energy Build. 2018, 170, 74–82. [Google Scholar] [CrossRef]
- Singh, R.; Gupta, N. Value added utilization of fly ash-prospective and sustainable solutions. Int. J. Appl. Sci. Eng. Res. 2014, 3, 1–16. [Google Scholar]
- Surabhi. Fly ash in India: Generation vis-à-vis Utilization and Global perspective. Int. J. Appl. Chem. 2017, 13, 29–52. [Google Scholar]
- Parab, N.; Mishra, S.; Bhonde, S. Prospects of bulk utilization of fly ash in agriculture for integrated nutrient management. Bull. Nat. Inst. Ecol. 2012, 23, 31–46. [Google Scholar]
- Kalra, N.; Harit, R.; Sharma, S. Effect of flyash incorporation on soil properties of texturally variant soils. Bioresour. Technol. 2000, 75, 91–93. [Google Scholar] [CrossRef]
- Yeledhalli, N.; Prakash, S.; Gurumurthy, S.; Ravi, M. Coal fly ash as modifier of physico-chemical and biological properties of soil. Karnataka J. Agric. Sci. 2010, 20, 531–534. [Google Scholar]
- Garg, R.N.; Pathak, H.; Das, D.; Tomar, R. Use of flyash and biogas slurry for improving wheat yield and physical properties of soil. Environ. Monit. Assess. 2005, 107, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Dhadse, S.; Kumari, P.; Bhagia, L. Fly ash characterization, utilization and government initiatives in India —A review. J. Sci. Ind. Res. 2008, 67, 11–18. [Google Scholar]
- Dermatas, D.; Meng, X. Utilization of fly ash for stabilization/solidification of heavy metal contaminated soils. Eng. Geol. 2003, 70, 377–394. [Google Scholar] [CrossRef]
- Production and Use of Coal Combustion Products in the U.S. Market Forecast through 2033. Prepared by: American road & Transportation builders association. Prepared for: American coal ash association. June 2015. Available online: https://www.acaa-usa.org/Portals/9/Files/PDFs/ReferenceLibrary/ARTBA-final-historical.compressed.pdf (accessed on 3 June 2015).
- Kishor, P.; Ghosh, A.; Kumar, D. Use of flyash in agriculture: A way to improve soil fertility and its productivity. Asian J. Agric. Res. 2010, 4, 1–14. [Google Scholar] [CrossRef]
- Kolbe, J.L.; Lee, L.S.; Jafvert, C.T.; Murarka, I.P. Proceedings of the Use of Alkaline Coal Ash for Reclamation of a Former Strip Mine, World of Coal Ash (WOCA) Conference, Denver, CO, USA, 9–12 May 2011; pp. 1–15.
- Grubb, D.G.; Guimaraes, M.A.S.; Valencia, R. Phosphate immobilization using an acidic type F fly ash. J. Hazard. Mater. 2000, 76, 217–236. [Google Scholar] [CrossRef]
- Nyambura, M.G.; Mugera, G.W.; Felicia, P.L.; Gathura, N.P. Carbonation of brine impacted fractionated coal fly ash: Implications for CO2 sequestration. J. Environ. Manag. 2011, 92, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Kalaw, M.E.; Culaba, A.; Hinode, H.; Kurniawan, W.; Gallardo, S.; Promentilla, M.A. Optimizing and characterizing geopolymers from ternary blend of philippine coal fly ash, coal bottom ash and rice hull ash. Materials 2016, 9, 580. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, T.; Kim, J.; Hardcastle, S.; Rohatgi, P. Crystallinity and selected properties of fly ash particles. Mater. Sci. Eng. A 2002, 325, 333–343. [Google Scholar] [CrossRef]
- Safiuddin, M.; Jumaat, M.Z.; Salam, M.; Islam, M.; Hashim, R. Utilization of solid wastes in construction materials. Int. J. Phys. Sci. 2010, 5, 1952–1963. [Google Scholar]
- Cao, J.; Dong, X.; Li, L.; Dong, Y.; Hampshire, S. Recycling of waste fly ash for production of porous mullite ceramic membrane supports with increased porosity. J. Eur. Ceram. Soc. 2014, 34, 3181–3194. [Google Scholar] [CrossRef]
- Yang, T.; Ji, H.; Yoon, S.; Kim, B.; Park, H. Porous mullite composite with controlled pore structure processed using a freeze casting of TBA-based coal fly ash slurries. Resour. Conserv. Recycl. 2010, 54, 816–820. [Google Scholar] [CrossRef]
- Kutchko, B.G.; Kim, A.G. Fly ash characterization by SEM–EDS. Fuel 2006, 85, 2537–2544. [Google Scholar] [CrossRef]
- Külaots, I.; Hurt, R.H.; Suuberg, E.M. Size distribution of unburned carbon in coal fly ash and its implications. Fuel 2004, 83, 223–230. [Google Scholar] [CrossRef]
- Zhu, Z.; Wang, X.; Dai, S.; Huang, B.; He, Q. Fractional characteristics of coal fly ash for beneficial use. J. Mater. Civ. Eng. 2012, 25, 63–69. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Menendez, R.; Alvarez, D.; Diaz-Somoano, M.; Martinez-Tarazona, M.R. Phase-mineral and chemical composition of coal fly ashes as a basis for their multicomponent utilization. 1. Characterization of feed coals and fly ashes☆. Fuel 2003, 82, 1793–1811. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Menendez, R. Phase-mineral and chemical composition of coal fly ashes as a basis for their multicomponent utilization. 4. Characterization of heavy concentrates and improved fly ash residues. Fuel 2005, 84, 973–991. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G. Methods for characterization of composition of fly ashes from coal-fired power stations: A critical overview. Energy Fuels 2005, 19, 1084–1098. [Google Scholar] [CrossRef]
- Sarode, D.B.; Jadhav, R.N.; Khatik, V.A.; Ingle, S.T.; Attarde, S.B. Extraction and leaching of heavy metals from thermal power plant fly ash and its admixtures. Polish J. Environ. Stud. 2010, 6, 1325–1330. [Google Scholar]
- Polat, H.; Vengosh, A.; Pankratov, I.; Polat, M. A new methodology for removal of boron from water by coal and fly ash. Desalination 2004, 164, 173–188. [Google Scholar] [CrossRef] [Green Version]
- Akar, G.; Polat, M.; Galecki, G.; Ipekoglu, U. Leaching behavior of selected trace elements in coal fly ash samples from Yenikoy coal-fired power plants. Fuel Process. Technol. 2012, 104, 50–56. [Google Scholar] [CrossRef] [Green Version]
- Sushil, S.; Batra, V.S. Analysis of fly ash heavy metal content and disposal in three thermal power plants in india. Fuel 2006, 85, 2676–2679. [Google Scholar] [CrossRef]
- Fan, M.; Brown, R.C. Comparison of the loss-on-ignition and thermogravimetric analysis techniques in measuring unburned carbon in coal fly ash. Energy Fuels 2001, 15, 1414–1417. [Google Scholar] [CrossRef]
- Querol, X.; Umaña, J.C.; Plana, F.; Alastuey, A.; Lopez-Soler, A.; Medinaceli, A.; Valero, A.; Domingo, M.J.; Garcia-Rojo, E. Synthesis of zeolites from fly ash at pilot plant scale. Examples of potential applications. Fuel 2001, 80, 857–865. [Google Scholar] [CrossRef]
- Inada, M.; Tsujimoto, H.; Eguchi, Y.; Enomoto, N.; Hojo, J. Microwave-assisted zeolite synthesis from coal fly ash in hydrothermal process. Fuel 2005, 84, 1482–1486. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A. Mid-infrared spectroscopic studies of alkali-activated fly ash structure. Microporous Mesoporous Mater. 2005, 86, 207–214. [Google Scholar] [CrossRef]
- Wang, S.; Boyjoo, Y.; Choueib, A.; Zhu, Z. Removal of dyes from aqueous solution using fly ash and red mud. Water Res. 2005, 39, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Montes-Hernandez, G.; Perez-Lopez, R.; Renard, F.; Nieto, J.; Charlet, L. Mineral sequestration of CO2 by aqueous carbonation of coal combustion fly-ash. J. Hazard. Mater. 2009, 161, 1347–1354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belviso, C. State-of-the-art applications of fly ash from coal and biomass: A focus on zeolite synthesis processes and issues. Prog. Energy Combust. Sci. 2018, 65, 109–135. [Google Scholar] [CrossRef]
- Wang, S.; Ma, Q.; Zhu, Z.H. Characteristics of coal fly ash and adsorption application. Fuel 2008, 87, 3469–3473. [Google Scholar] [CrossRef]
- Ge, J.C.; Kim, H.Y.; Yoon, S.K.; Choi, N.J. Reducing volatile organic compound emissions from diesel engines using canola oil biodiesel fuel and blends. Fuel 2018, 218, 266–274. [Google Scholar] [CrossRef]
- Yilmaz, N.; Davis, S.M. Polycyclic aromatic hydrocarbon (PAH) formation in a diesel engine fueled with diesel, biodiesel and biodiesel/n-butanol blends. Fuel 2016, 181, 729–740. [Google Scholar] [CrossRef]
- Li, G.; Wei, W.; Shao, X.; Nie, L.; Wang, H.; Yan, X.; Zhang, R. A comprehensive classification method for VOC emission sources to tackle air pollution based on VOC species reactivity and emission amounts. J. Environ. Sci. 2018, 67, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Malakar, S.; Saha, P.D.; Baskaran, D.; Rajamanickam, R. Comparative study of biofiltration process for treatment of VOCs emission from petroleum refinery wastewater—A review. Environ. Technol. Innov. 2017, 8, 441–461. [Google Scholar] [CrossRef]
- Lee, S.; Lam, S.; Fai, H.K. Characterization of VOCs, ozone, and PM10 emissions from office equipment in an environmental chamber. Build. Environ. 2001, 36, 837–842. [Google Scholar] [CrossRef]
- Atkinson, R. Atmospheric chemistry of VOCs and NOx. Atmos. Environ. 2000, 34, 2063–2101. [Google Scholar] [CrossRef]
- Sakai, N.; Yamamoto, S.; Matsui, Y.; Khan, M.F.; Latif, M.T.; Mohd, M.A.; Yoneda, M. Characterization and source profiling of volatile organic compounds in indoor air of private residences in Selangor state, Malaysia. Sci. Total Environ. 2017, 586, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.C.; Choi, N.J. Fabrication of functional polyurethane/rare earth nanocomposite membranes by electrospinning and its VOCs absorption capacity from air. Nanomaterials 2017, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Mendell, M.J. Indoor residential chemical emissions as risk factors for respiratory and allergic effects in children: A review. Indoor Air 2007, 17, 259–277. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Yoon, J.-W.; Choi, K.-I.; Jang, H.W.; Umar, A.; Lee, J.-H. Ultraselective and sensitive detection of xylene and toluene for monitoring indoor air pollution using Cr-doped NiO hierarchical nanostructures. Nanoscale 2013, 5, 7066–7073. [Google Scholar] [CrossRef] [PubMed]
- Rayalu, S.; Meshram, S.; Biniwale, R.B.; Srivasatava, A.; Jadhav, P.; Devotta, S. Volatile organic carbon monitoring in indoor environment using a versatile hydrophobic flyash-based zeolite as adsorbent. Curr. Sci. 2006, 497–503. [Google Scholar]
- Guo, H.; Lee, S.; Li, W.; Cao, J. Source characterization of btex in indoor microenvironments in Hong Kong. Atmos. Environ. 2003, 37, 73–82. [Google Scholar] [CrossRef]
- Barna, M.; Lamb, B.; Westberg, H. Modeling the effects of VOC/NOx emissions on ozone synthesis in the cascadia airshed of the pacific northwest. J. Air Waste Manag. Assoc. 2001, 51, 1021–1034. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Park, R.J.; Kim, J.-J. Urban air quality modeling with full O3–NOx–VOC chemistry: Implications for O3 and PM air quality in a street canyon. Atmos. Environ. 2012, 47, 330–340. [Google Scholar] [CrossRef]
- Shao, M.; Zhang, Y.; Zeng, L.; Tang, X.; Zhang, J.; Zhong, L.; Wang, B. Ground-level ozone in the pearl river delta and the roles of VOC and NOx in its production. J. Environ. Manag. 2009, 90, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Cheng, S.; Li, G.; Wang, G.; Wang, H. Characteristics of ozone and ozone precursors (VOCs and NOx) around a petroleum refinery in Beijing, China. J. Environ. Sci. 2014, 26, 332–342. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, B.; Creamer, A.E.; Cao, C.; Li, Y. Adsorption of VOCs onto engineered carbon materials: A review. J. Hazard. Mater. 2017, 338, 102–123. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.; Kato, S.; Ataka, Y.; Chino, S. Performance test for evaluating the reduction of VOCs in rooms and evaluating the lifetime of sorptive building materials. Build. Environ. 2009, 44, 207–215. [Google Scholar] [CrossRef]
- Chmielewski, A.; Ostapczuk, A.; Zimek, Z.; Licki, J.; Kubica, K. Reduction of VOCs in flue gas from coal combustion by electron beam treatment. Radiat. Phys. Chem. 2002, 63, 653–655. [Google Scholar] [CrossRef]
- Kim, S. The reduction of formaldehyde and VOCs emission from wood-based flooring by green adhesive using cashew nut shell liquid (CNSL). J. Hazard. Mater. 2010, 182, 919–922. [Google Scholar] [CrossRef] [PubMed]
- Suresh, S.; Vijayalakshmi, G.; Rajmohan, B.; Subbaramaiah, V. Adsorption of benzene vapor onto activated biomass from cashew nut shell: Batch and column study. Recent Patents Chem. Eng. 2012, 5, 116–133. [Google Scholar] [CrossRef]
- Tseng, T.K.; Lin, Y.S.; Chen, Y.J.; Chu, H. A review of photocatalysts prepared by sol-gel method for VOCs removal. Int. J. Mol. Sci. 2010, 11, 2336–2361. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Zhu, Q.; Jiang, Z.; Zhang, Y.; Wang, Y. Preparation and formation mechanism of mesoporous CuO–CeO2 mixed oxides with excellent catalytic performance for removal of VOCs. Microporous Mesoporous Mater. 2008, 113, 427–434. [Google Scholar] [CrossRef]
- Belkouch, J.; Ould-Dris, A.; Taouk, B. Removal of hazardous chlorinated VOCs over Mn–Cu mixed oxide based catalyst. J. Hazard. Mater. 2009, 169, 758–765. [Google Scholar]
- Kim, S.C.; Shim, W.G. Catalytic combustion of VOCs over a series of manganese oxide catalysts. Appl. Catal. B 2010, 98, 180–185. [Google Scholar] [CrossRef]
- Scire, S.; Minico, S.; Crisafulli, C.; Satriano, C.; Pistone, A. Catalytic combustion of volatile organic compounds on gold/cerium oxide catalysts. Appl. Catal. B 2003, 40, 43–49. [Google Scholar] [CrossRef]
- Liotta, L. Catalytic oxidation of volatile organic compounds on supported noble metals. Appl. Catal. B 2010, 100, 403–412. [Google Scholar] [CrossRef]
- Chuang, C.; Chiang, P.; Chang, E. Modeling VOCs adsorption onto activated carbon. Chemosphere 2003, 53, 17–27. [Google Scholar] [CrossRef]
- Chiang, Y.-C.; Chiang, P.-C.; Huang, C.-P. Effects of pore structure and temperature on VOC adsorption on activated carbon. Carbon 2001, 39, 523–534. [Google Scholar] [CrossRef]
- Li, L.; Liu, S.; Liu, J. Surface modification of coconut shell based activated carbon for the improvement of hydrophobic VOC removal. J. Hazard. Mater. 2011, 192, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Gaur, V.; Verma, N. Removal of volatile organic compound by activated carbon fiber. Carbon 2004, 42, 2949–2962. [Google Scholar] [CrossRef]
- Tsai, J.-H.; Chiang, H.-M.; Huang, G.-Y.; Chiang, H.-L. Adsorption characteristics of acetone, chloroform and acetonitrile on sludge-derived adsorbent, commercial granular activated carbon and activated carbon fibers. J. Hazard. Mater. 2008, 154, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Oh, G.-Y.; Ju, Y.-W.; Jung, H.-R.; Lee, W.-J. Preparation of the novel manganese-embedded pan-based activated carbon nanofibers by electrospinning and their toluene adsorption. J. Anal. Appl. Pyrolysis 2008, 81, 211–217. [Google Scholar] [CrossRef]
- Bai, Y.; Huang, Z.-H.; Kang, F. Synthesis of reduced graphene oxide/phenolic resin-based carbon composite ultrafine fibers and their adsorption performance for volatile organic compounds and water. J. Mater. Chem. A 2013, 1, 9536–9543. [Google Scholar] [CrossRef]
- Liu, Y.; Hou, C.; Jiao, T.; Song, J.; Zhang, X.; Xing, R.; Zhou, J.; Zhang, L.; Peng, Q. Self-assembled AgNP-containing nanocomposites constructed by electrospinning as efficient dye photocatalyst materials for wastewater treatment. Nanomaterials 2018, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-M.; Zhang, Y.-Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M.; Gupta, V. Application of coal fly ash in air quality management. Ind. Eng. Chem. Res. 2012, 51, 15299–15314. [Google Scholar] [CrossRef]
- Qian, Q.; Gong, C.; Zhang, Z.; Yuan, G. Removal of VOCs by activated carbon microspheres derived from polymer: A comparative study. Adsorption 2015, 21, 333–341. [Google Scholar] [CrossRef]
- Huang, Z.-H.; Kang, F.; Zheng, Y.-P.; Yang, J.-B.; Liang, K.-M. Adsorption of trace polar methy-ethyl-ketone and non-polar benzene vapors on viscose rayon-based activated carbon fibers. Carbon 2002, 40, 1363–1367. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, Z.; Liu, F.; Wang, S. Study of NO/NOx removal from flue gas contained fly ash and water vapor by pulsed corona discharge. J. Electrostat. 2005, 63, 155–164. [Google Scholar] [CrossRef]
- Rokni, E.; Panahi, A.; Ren, X.; Levendis, Y.A. Curtailing the generation of sulfur dioxide and nitrogen oxide emissions by blending and oxy-combustion of coals. Fuel 2016, 181, 772–784. [Google Scholar] [CrossRef]
- Rubio, B.; Izquierdo, M.T.; Mayoral, M.C.; Bona, M.T.; Andres, J.M. Unburnt carbon from coal fly ashes as a precursor of activated carbon for nitric oxide removal. J. Hazard. Mater. 2007, 143, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Rubel, A.; Andrews, R.; Gonzalez, R.; Groppo, J.; Robl, T. Adsorption of Hg and NOx on coal by-products. Fuel 2005, 84, 911–916. [Google Scholar] [CrossRef]
- Hwang, J.; Sun, X.; Li, Z. Unburned carbon from fly ash for mercury adsorption: I. Separation and characterization of unburned carbon. J. Miner. Mater. Charact. Eng. 2002, 1, 39. [Google Scholar] [CrossRef]
- Mehmood, S.; Reddy, B.V.; Rosen, M.A. Energy analysis of a biomass co-firing based pulverized coal power generation system. Sustainability 2012, 4, 462–490. [Google Scholar] [CrossRef]
- Bukhari, S.S.; Behin, J.; Kazemian, H.; Rohani, S. Conversion of coal fly ash to zeolite utilizing microwave and ultrasound energies: A review. Fuel 2015, 140, 250–266. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, B.; Bi, J. More efforts, more benefits: Air pollutant control of coal-fired power plants in China. Energy 2015, 80, 1–9. [Google Scholar] [CrossRef]
- Long, X.-L.; Xin, Z.-L.; Wang, H.-X.; Xiao, W.-D.; Yuan, W.-K. Simultaneous removal of no and SO2 with hexamminecobalt (II) solution coupled with the hexamminecobalt (II) regeneration catalyzed by activated carbon. Appl. Catal. B 2004, 54, 25–32. [Google Scholar] [CrossRef]
- Liu, Y.x.; Zhang, J. Photochemical oxidation removal of NO and SO2 from simulated flue gas of coal-fired power plants by wet scrubbing using UV/H2O2 advanced oxidation process. Ind. Eng. Chem. Res. 2011, 50, 3836–3841. [Google Scholar] [CrossRef]
- Srivastava, R.; Miller, C.; Erickson, C.; Jambhekar, R. Emissions of sulfur trioxide from coal-fired power plants. J. Air Waste Manag. Assoc. 2004, 54, 750–762. [Google Scholar] [CrossRef]
- Ishizuka, T.; Tsuchiai, H.; Murayama, T.; Tanaka, T.; Hattori, H. Preparation of active absorbent for dry-type flue gas desulfurization from calcium oxide, coal fly ash, and gypsum. Ind. Eng. Chem. Res. 2000, 39, 1390–1396. [Google Scholar] [CrossRef]
- Renedo, M.; Fernandez, J. Preparation, characterization, and calcium utilization of fly ash/Ca(OH)2 sorbents for dry desulfurization at low temperature. Ind. Eng. Chem. Res. 2002, 41, 2412–2417. [Google Scholar] [CrossRef]
- Lin, R.-B.; Shih, S.-M.; Liu, C.-F. Characteristics and reactivities of Ca(OH)2/silica fume sorbents for low-temperature flue gas desulfurization. Chem. Eng. Sci. 2003, 58, 3659–3668. [Google Scholar] [CrossRef]
- Lee, K.; Mohamed, A.; Bhatia, S.; Chu, K. Removal of sulfur dioxide by fly ash/CaO/CaSO4 sorbents. Chem. Eng. J. 2005, 114, 171–177. [Google Scholar] [CrossRef]
- Lee, K.T.; Bhatia, S.; Mohamed, A.R. Preparation and characterization of sorbents prepared from ash (waste material) for sulfur dioxide (SO2) removal. J. Mater. Cycles Waste Manag. 2005, 7, 16–23. [Google Scholar] [CrossRef]
- Lin, R.-B.; Shih, S.-M.; Liu, C.-F. Structural properties and reactivities of Ca(OH)2/fly ash sorbents for flue gas desulfurization. Ind. Eng. Chem. Res. 2003, 42, 1350–1356. [Google Scholar] [CrossRef]
- Duruibe, J.; Ogwuegbu, M.; Egwurugwu, J. Heavy metal pollution and human biotoxic effects. Int. J. Phys. Sci. 2007, 2, 112–118. [Google Scholar]
- Wang, S.-L.; Xu, X.-R.; Sun, Y.-X.; Liu, J.-L.; Li, H.-B. Heavy metal pollution in coastal areas of south China: A review. Mar. Pollut. Bull. 2013, 76, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Rios, C.A.; Williams, C.D.; Roberts, C.L. Removal of heavy metals from acid mine drainage (AMD) using coal fly ash, natural clinker and synthetic zeolites. J. Hazard. Mater. 2008, 156, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Huisman, J.L.; Schouten, G.; Schultz, C. Biologically produced sulphide for purification of process streams, effluent treatment and recovery of metals in the metal and mining industry. Hydrometallurgy 2006, 83, 106–113. [Google Scholar] [CrossRef]
- Amuda, O.; Giwa, A.; Bello, I. Removal of heavy metal from industrial wastewater using modified activated coconut shell carbon. BioChem. Eng. J. 2007, 36, 174–181. [Google Scholar] [CrossRef]
- Edelstein, M.; Ben-Hur, M. Heavy metals and metalloids: Sources, risks and strategies to reduce their accumulation in horticultural crops. Sci. Hortic. 2018, 234, 431–444. [Google Scholar] [CrossRef]
- Cho, H.; Oh, D.; Kim, K. A study on removal characteristics of heavy metals from aqueous solution by fly ash. J. Hazard. Mater. 2005, 127, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Hui, K.; Chao, C.Y.H.; Kot, S. Removal of mixed heavy metal ions in wastewater by zeolite 4A and residual products from recycled coal fly ash. J. Hazard. Mater. 2005, 127, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Itskos, G.; Koukouzas, N.; Vasilatos, C.; Megremi, I.; Moutsatsou, A. Comparative uptake study of toxic elements from aqueous media by the different particle-size-fractions of fly ash. J. Hazard. Mater. 2010, 183, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Babel, S.; Kurniawan, T.A. Low-cost adsorbents for heavy metals uptake from contaminated water: A review. J. Hazard. Mater. 2003, 97, 219–243. [Google Scholar] [CrossRef]
- Weng, C.-H.; Huang, C. Adsorption characteristics of Zn(II) from dilute aqueous solution by fly ash. Colloids Surf. A 2004, 247, 137–143. [Google Scholar] [CrossRef]
- Salam, O.E.A.; Reiad, N.A.; ElShafei, M.M. A study of the removal characteristics of heavy metals from wastewater by low-cost adsorbents. J. Adv. Res. 2011, 2, 297–303. [Google Scholar] [CrossRef]
- Nascimento, M.; Soares, P.S.M.; de Souza, V.P. Adsorption of heavy metal cations using coal fly ash modified by hydrothermal method. Fuel 2009, 88, 1714–1719. [Google Scholar] [CrossRef]
- Kandel, S.; Vogel, J.; Penn, C.; Brown, G. Phosphorus retention by fly ash amended filter media in aged bioretention cells. Water 2017, 9, 746. [Google Scholar] [CrossRef]
- Yildiz, E. Phosphate removal from water by fly ash using crossflow microfiltration. Sep. Purif. Technol. 2004, 35, 241–252. [Google Scholar] [CrossRef]
- Agyei, N.M.; Strydom, C.; Potgieter, J. The removal of phosphate ions from aqueous solution by fly ash, slag, ordinary portland cement and related blends. Cem. Concr. Res. 2002, 32, 1889–1897. [Google Scholar] [CrossRef]
- Chen, J.; Kong, H.; Wu, D.; Chen, X.; Zhang, D.; Sun, Z. Phosphate immobilization from aqueous solution by fly ashes in relation to their composition. J. Hazard. Mater. 2007, 139, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Oguz, E. Sorption of phosphate from solid/liquid interface by fly ash. Colloids Surf. A 2005, 262, 113–117. [Google Scholar] [CrossRef]
- Xu, X.; Li, Q.; Cui, H.; Pang, J.; Sun, L.; An, H.; Zhai, J. Adsorption of fluoride from aqueous solution on magnesia-loaded fly ash cenospheres. Desalination 2011, 272, 233–239. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M. Adsorption of phenolic compounds on low-cost adsorbents: A review. Adv. Colloid Interface Sci. 2008, 143, 48–67. [Google Scholar] [CrossRef] [PubMed]
- Kao, P.-C.; Tzeng, J.-H.; Huang, T.-L. Removal of chlorophenols from aqueous solution by fly ash. J. Hazard. Mater. 2000, 76, 237–249. [Google Scholar] [CrossRef]
- Matheswaran, M.; Karunanithi, T. Adsorption of chrysoidine R by using fly ash in batch process. J. Hazard. Mater. 2007, 145, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Singh, K.P.; Singh, G.; Kumar, K. Removal of dyes from wastewater using flyash, a low-cost adsorbent. Ind. Eng. Chem. Res. 2002, 41, 3688–3695. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).