Stability of Halloysite, Imogolite, and Boron Nitride Nanotubes in Solvent Media
Abstract
Featured Application
Abstract
1. Introduction
2. Halloysite Nanotubes
2.1. Colloidal Stability in Water
2.2. Colloidal Stability in Organic Media
3. Imogolite
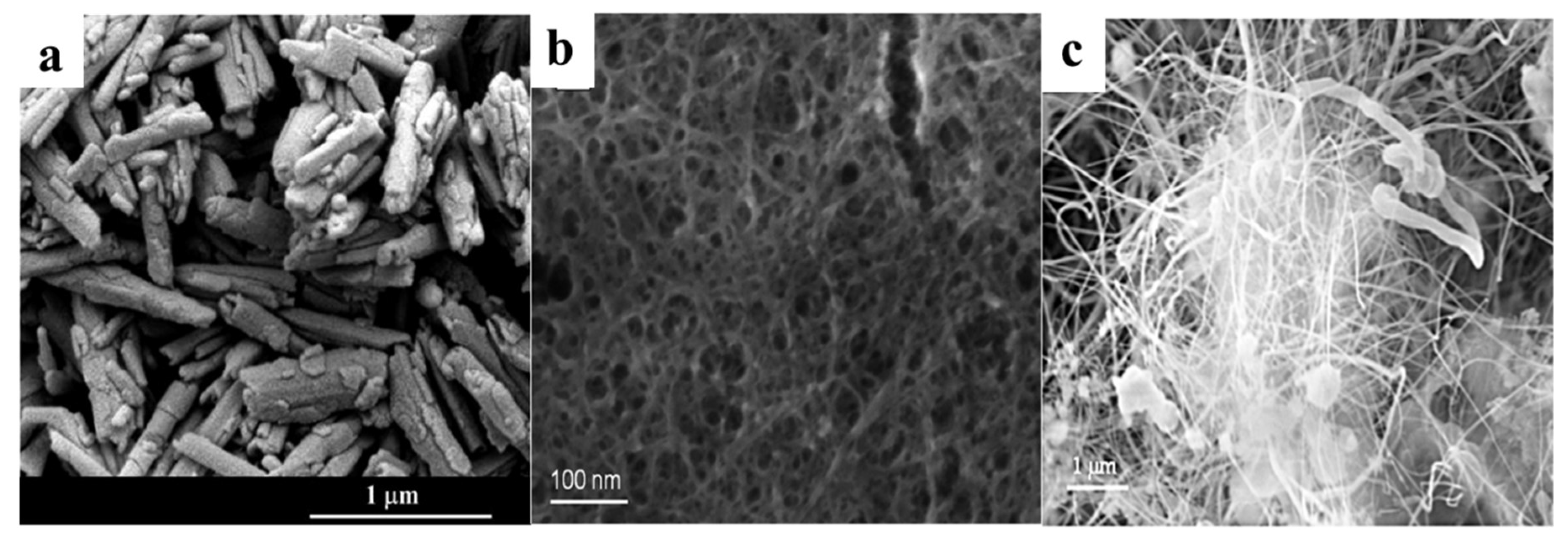
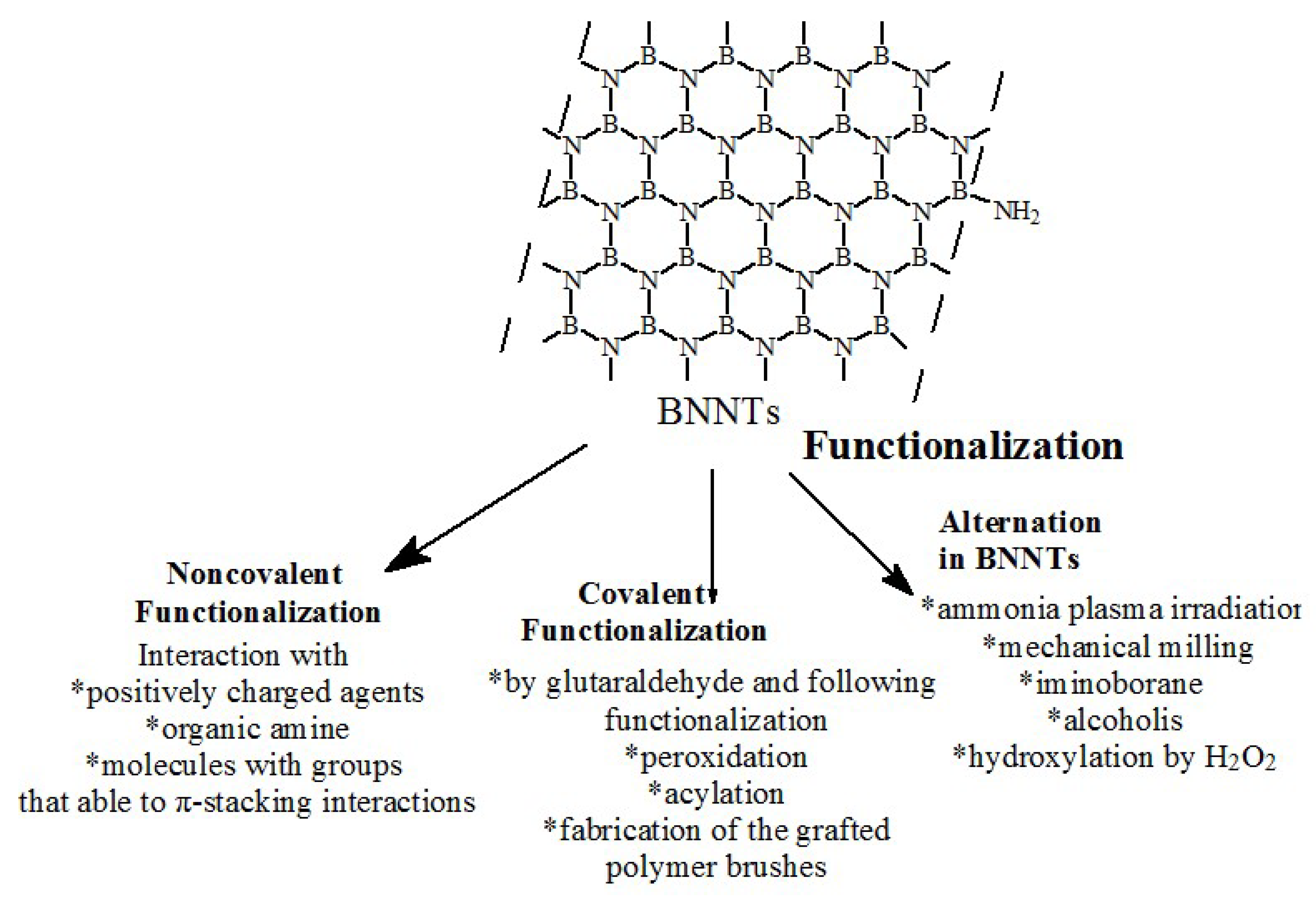
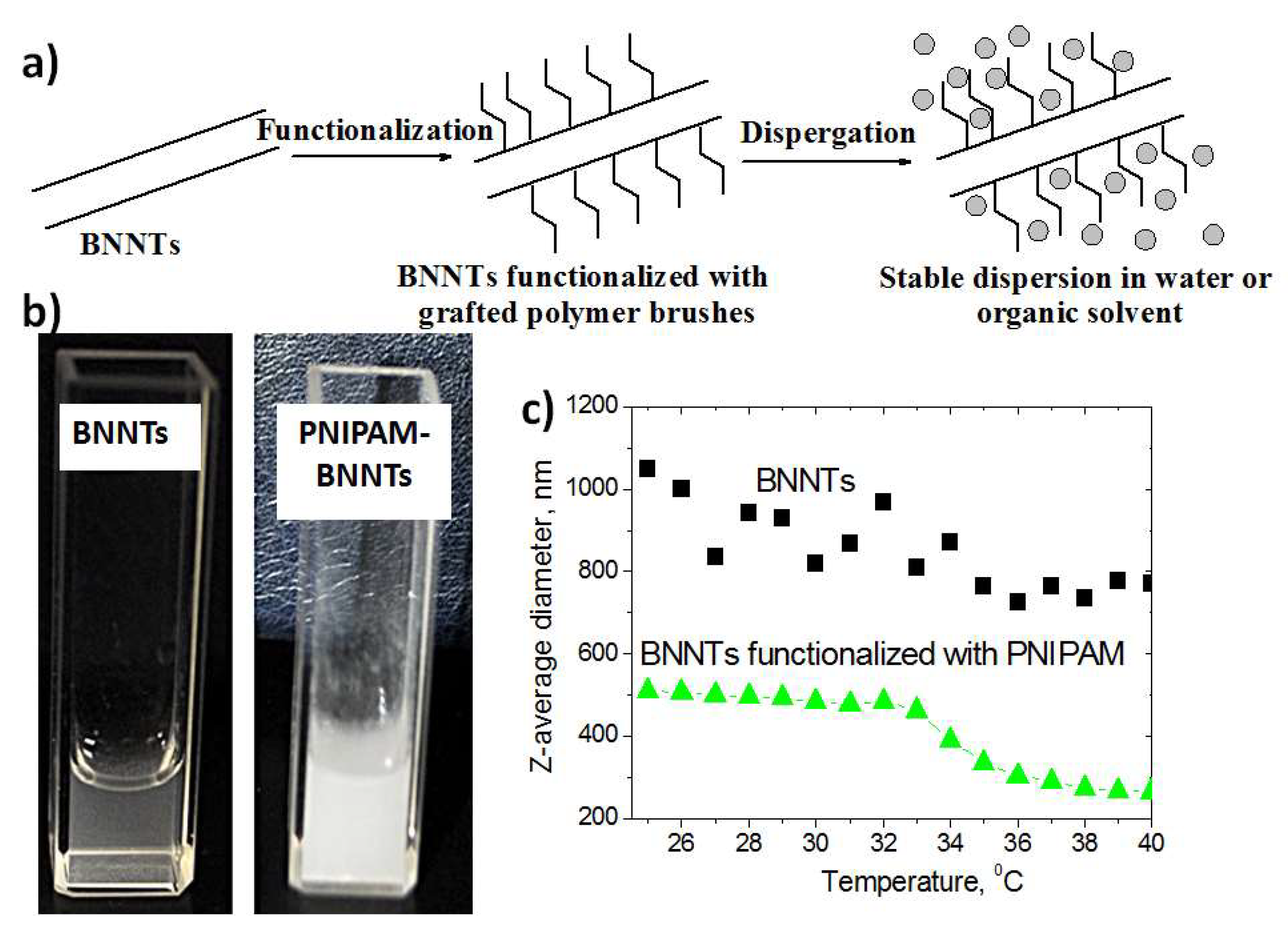
4. Boron Nitride Nanotubes
5. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Sun, J.; Wang, J.; Li, Z.; Yang, Z.; Yang, S. Controllable synthesis of 3D hierarchical bismuth compounds with good electrochemical performance for advanced energy storage devices. RSC Adv. 2015, 5, 51773–51778. [Google Scholar] [CrossRef]
- Yang, G.; Yang, X.; Yang, C.; Yang, Y. A reagentless amperometric immunosensor for human chorionic gonadotrophin based on a gold nanotube arrays electrode. Colloids Surf. Physicochem. Eng. Asp. 2011, 389, 195–200. [Google Scholar] [CrossRef]
- Lai, Y.; Huang, Y.; Wang, H.; Huang, J.; Chen, Z.; Lin, C. Selective formation of ordered arrays of octacalcium phosphate ribbons on TiO2 nanotube surface by template-assisted electrodeposition. Colloids Surf. B Biointerfaces 2010, 76, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Ng, J.; Zhang, X.; Bai, H.; Sun, D.D. Adsorption and photocatalytic degradation of Acid Orange 7 over hydrothermally synthesized mesoporous TiO2 nanotube. Colloids Surf. Physicochem. Eng. Asp. 2011, 379, 169–175. [Google Scholar] [CrossRef]
- Emanet, M.; Fakhrullin, R.; Çulha, M. Boron Nitride Nanotubes and Layer-By-Layer Polyelectrolyte Coating for Yeast Cell Surface Engineering. Chem. Nano. Mat. 2016, 2, 426–429. [Google Scholar] [CrossRef]
- Kalay, S.; Stetsyshyn, Y.; Lobaz, V.; Harhay, K.; Ohar, H.; Çulha, M. Water-dispersed thermo-responsive boron nitride nanotubes: Synthesis and properties. Nanotechnology 2016, 27, 035703. [Google Scholar] [CrossRef] [PubMed]
- Lazzara, G.; Cavallaro, G.; Panchal, A.; Fakhrullin, R.; Stavitskaya, A.; Vinokurov, V.; Lvov, Y. An assembly of organic-inorganic composites using halloysite clay nanotubes. Curr. Opin. Colloid Interface Sci. 2018, 35, 42–50. [Google Scholar] [CrossRef]
- Paineau, E.; Krapf, M.-E.M.; Amara, M.-S.; Matskova, N.V.; Dozov, I.; Rouzière, S.; Thill, A.; Launois, P.; Davidson, P. A liquid-crystalline hexagonal columnar phase in highly-dilute suspensions of imogolite nanotubes. Nat. Commun. 2016, 7, 10271. [Google Scholar] [CrossRef] [PubMed]
- Kalay, S.; Yilmaz, Z.; Sen, O.; Emanet, M.; Kazanc, E.; Çulha, M. Synthesis of boron nitride nanotubes and their applications. Beilstein J. Nanotechnol. 2015, 6, 84–102. [Google Scholar] [CrossRef] [PubMed]
- Massaro, M.; Amorati, R.; Cavallaro, G.; Guernelli, S.; Lazzara, G.; Milioto, S.; Noto, R.; Poma, P.; Riela, S. Direct chemical grafted curcumin on halloysite nanotubes as dual-responsive prodrug for pharmacological applications. Colloids Surf. B Biointerfaces 2016, 140, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, F.; Armata, N.; Lazzara, G. Modeling of the Halloysite Spiral Nanotube. J. Phys. Chem. C 2015, 119, 16700–16707. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Y.; Leng, F.; Huang, L.; Wang, Z.; Tian, W. Recent Advances on Surface Modification of Halloysite Nanotubes for Multifunctional Applications. Appl. Sci. 2017, 7, 1215. [Google Scholar] [CrossRef]
- Makaremi, M.; Pasbakhsh, P.; Cavallaro, G.; Lazzara, G.; Aw, Y.K.; Lee, S.M.; Milioto, S. Effect of Morphology and Size of Halloysite Nanotubes on Functional Pectin Bionanocomposites for Food Packaging Applications. ACS Appl. Mater. Interfaces 2017, 9, 17476–17488. [Google Scholar] [CrossRef] [PubMed]
- Kryuchkova, M.; Danilushkina, A.; Lvov, Y.; Fakhrullin, R. Evaluation of toxicity of nanoclays and graphene oxide in vivo: A Paramecium caudatum study. Environ. Sci. Nano 2016, 3, 442–452. [Google Scholar] [CrossRef]
- Fakhrullina, G.I.; Akhatova, F.S.; Lvov, Y.M.; Fakhrullin, R.F. Toxicity of halloysite clay nanotubes in vivo: A Caenorhabditis elegans study. Environ. Sci. Nano 2015, 2, 54–59. [Google Scholar] [CrossRef]
- Lvov, Y.; Abdullayev, E. Functional polymer–clay nanotube composites with sustained release of chemical agents. Prog. Polym. Sci. 2013, 38, 1690–1719. [Google Scholar] [CrossRef]
- Biddeci, G.; Cavallaro, G.; Di Blasi, F.; Lazzara, G.; Massaro, M.; Milioto, S.; Parisi, F.; Riela, S.; Spinelli, G. Halloysite nanotubes loaded with peppermint essential oil as filler for functional biopolymer film. Carbohydr. Polym. 2016, 152, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Bertolino, V.; Cavallaro, G.; Lazzara, G.; Merli, M.; Milioto, S.; Parisi, F.; Sciascia, L. Effect of the Biopolymer Charge and the Nanoclay Morphology on Nanocomposite Materials. Ind. Eng. Chem. Res. 2016, 55, 7373–7380. [Google Scholar] [CrossRef]
- Gorrasi, G.; Pantani, R.; Murariu, M.; Dubois, P. PLA/Halloysite Nanocomposite Films: Water Vapor Barrier Properties and Specific Key Characteristics. Macromol. Mater. Eng. 2014, 299, 104–115. [Google Scholar] [CrossRef]
- Massaro, M.; Lazzara, G.; Milioto, S.; Noto, R.; Riela, S. Covalently modified halloysite clay nanotubes: Synthesis, properties, biological and medical applications. J. Mater. Chem. B 2017, 5, 2867–2882. [Google Scholar] [CrossRef]
- Lvov, Y.M.; DeVilliers, M.M.; Fakhrullin, R.F. The application of halloysite tubule nanoclay in drug delivery. Expert Opin. Drug Deliv. 2016, 13, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Fakhrullin, R.F.; Lvov, Y.M. Halloysite clay nanotubes for tissue engineering. Nanomedicine 2016, 11, 2243–2246. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, Y.; Wu, C.; Xiong, S.; Zhou, C. Chitosan/halloysite nanotubes bionanocomposites: Structure, mechanical properties and biocompatibility. Int. J. Biol. Macromol. 2012, 51, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wu, C.; Jiao, Y.; Xiong, S.; Zhou, C. Chitosan-halloysite nanotubes nanocomposite scaffolds for tissue engineering. J. Mater. Chem. B 2013, 1, 2078–2089. [Google Scholar] [CrossRef]
- Cavallaro, G.; Lazzara, G.; Milioto, S.; Parisi, F.; Evtugyn, V.; Rozhina, E.; Fakhrullin, R. Nanohydrogel Formation within the Halloysite Lumen for Triggered and Sustained Release. ACS Appl. Mater. Interfaces 2018, 10, 8265–8273. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Abdullayev, E.; Vasiliev, A.; Lvov, Y. Halloysite nanotubule clay for efficient water purification. J. Colloid Interface Sci. 2013, 406, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, G.; Danilushkina, A.A.; Evtugyn, V.G.; Lazzara, G.; Milioto, S.; Parisi, F.; Rozhina, E.V.; Fakhrullin, R.F. Halloysite Nanotubes: Controlled Access and Release by Smart Gates. Nanomaterials 2017, 7, 199. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, G.; Lazzara, G.; Milioto, S.; Parisi, F.; Ruisi, F. Nanocomposites based on esterified colophony and halloysite clay nanotubes as consolidants for waterlogged archaeological woods. Cellulose 2017, 24, 3367–3376. [Google Scholar] [CrossRef]
- Cavallaro, G.; Lazzara, G.; Milioto, S.; Parisi, F.; Sparacino, V. Thermal and dynamic mechanical properties of beeswax-halloysite nanocomposites for consolidating waterlogged archaeological woods. Polym. Degrad. Stab. 2015, 120, 220–225. [Google Scholar] [CrossRef]
- Cavallaro, G.; Lazzara, G.; Milioto, S.; Parisi, F. Halloysite nanotubes as sustainable nanofiller for paper consolidation and protection. J. Therm. Anal. Calorim. 2014, 117, 1293–1298. [Google Scholar] [CrossRef]
- Cavallaro, G.; Lazzara, G.; Milioto, S.; Parisi, F. Halloysite Nanotubes for Cleaning, Consolidation and Protection. Chem. Rec. 2018. [Google Scholar] [CrossRef] [PubMed]
- Gorrasi, G. Dispersion of halloysite loaded with natural antimicrobials into pectins: Characterization and controlled release analysis. Carbohydr. Polym. 2015, 127, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Thill, A.; Maillet, P.; Guiose, B.; Spalla, O.; Belloni, L.; Chaurand, P.; Auffan, M.; Olivi, L.; Rose, J. Physico-chemical Control over the Single- or Double-Wall Structure of Aluminogermanate Imogolite-like Nanotubes. J. Am. Chem. Soc. 2012, 134, 3780–3786. [Google Scholar] [CrossRef] [PubMed]
- Rotoli, B.M.; Guidi, P.; Bonelli, B.; Bernardeschi, M.; Bianchi, M.G.; Esposito, S.; Frenzilli, G.; Lucchesi, P.; Nigro, M.; Scarcelli, V.; et al. Imogolite: An Aluminosilicate Nanotube Endowed with Low Cytotoxicity and Genotoxicity. Chem. Res. Toxicol. 2014, 27, 1142–1154. [Google Scholar] [CrossRef] [PubMed]
- Cradwick, P.D.G.; Farmer, V.C.; Russell, J.D.; Masson, C.R.; Wada, K.; Yoshinaga, N. Imogolite, a Hydrated Aluminium Silicate of Tubular Structure. Nat. Phys. Sci. 1972, 240, 187–189. [Google Scholar] [CrossRef]
- Amara, M.-S.; Paineau, E.; Bacia-Verloop, M.; Krapf, M.-E.M.; Davidson, P.; Belloni, L.; Levard, C.; Rose, J.; Launois, P.; Thill, A. Single-step formation of micron long (OH)3Al2O3Ge(OH) imogolite-like nanotubes. Chem. Commun. 2013, 49, 11284–11286. [Google Scholar] [CrossRef] [PubMed]
- Rubio, A.; Corkill, J.L.; Cohen, M.L. Theory of graphitic boron nitride nanotubes. Phys. Rev. B 1994, 49, 5081–5084. [Google Scholar] [CrossRef]
- Chopra, N.G.; Luyken, R.J.; Cherrey, K.; Crespi, V.H.; Cohen, M.L.; Louie, S.G.; Zettl, A. Boron Nitride Nanotubes. Science 1995, 269, 966–967. [Google Scholar] [CrossRef] [PubMed]
- Madani, M.S.; Monajjemi, M.; Aghaei, H. The Double Wall Boron Nitride Nanotube : Nano-Cylindrical Capacitor. Orient. J. Chem. 2017, 33, 1213–1222. [Google Scholar] [CrossRef]
- Chopra, N.G.; Zettl, A. Measurement of the elastic modulus of a multi-wall boron nitride nanotube. Solid State Commun. 1998, 105, 297–300. [Google Scholar] [CrossRef]
- Zhi, C.; Bando, Y.; Tang, C.; Xie, R.; Sekiguchi, T.; Golberg, D. Perfectly dissolved boron nitride nanotubes due to polymer wrapping. J. Am. Chem. Soc. 2005, 127, 15996–15997. [Google Scholar] [CrossRef] [PubMed]
- Terao, T.; Zhi, C.; Bando, Y.; Mitome, M.; Tang, C.; Golberg, D. Alignment of Boron Nitride Nanotubes in Polymeric Composite Films for Thermal Conductivity Improvement. J. Phys. Chem. C 2010, 114, 4340–4344. [Google Scholar] [CrossRef]
- Ciofani, G.; Danti, S.; Ricotti, L.; D’Alessandro, D.; Moscato, S.; Berrettini, S.; Mattoli, V.; Menciassi, A. Boron nitride nanotubes: Production, properties, biological interactions and potential applications as therapeutic agents in brain diseases. Curr. Nanosci. 2011, 7. [Google Scholar] [CrossRef]
- Gao, Z.; Zhi, C.; Bando, Y.; Golberg, D.; Serizawa, T. Chapter 2—Functionalization of boron nitride nanotubes for applications in nanobiomedicine. In Boron Nitride Nanotubes in Nanomedicine; Elsevier Inc.: New York, NY, USA, 2016; pp. 17–40. [Google Scholar]
- Ciofani, G.; Genchi, G.G.; Liakos, I.; Athanassiou, A.; Dinucci, D.; Chiellini, F.; Mattoli, V. A simple approach to covalent functionalization of boron nitride nanotubes. J. Colloid Interface Sci. 2012, 374. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hanagata, N.; Wang, X.; Yamaguchi, M.; Yi, W.; Bando, Y.; Golberg, D. Multimodal luminescent-magnetic boron nitride nanotubes@NaGdF(4):Eu structures for cancer therapy. Chem. Commun. 2014, 50, 4371–4374. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, T.H.; Hollanda, L.M.; Lancellotti, M.; de Sousa, E.M.B. Boron nitride nanotubes chemically functionalized with glycol chitosan for gene transfection in eukaryotic cell lines. J. Biomed. Mater. Res. Part A 2015, 103, 2176–2185. [Google Scholar] [CrossRef] [PubMed]
- Şen, Ö.; Çulha, M. Boron nitride nanotubes included thermally cross-linked gelatin-glucose scaffolds show improved properties. Colloids Surf. B 2016, 138, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Chen, H.; Liu, Y.; Li, L.H.; Chen, Y. Humidity sensing properties of single Au-decorated boron nitride nanotubes. Electrochem. Commun. 2013, 30, 29–33. [Google Scholar] [CrossRef]
- Leela, A.; Reddy, M.; Tanur, A.E.; Walker, G.C. Synthesis and hydrogen storage properties of different types of boron nitride nanostructures. Int. J. Hydrogen Energy 2010, 35, 4138–4143. [Google Scholar] [CrossRef]
- Cavallaro, G.; Grillo, I.; Gradzielski, M.; Lazzara, G. Structure of Hybrid Materials Based on Halloysite Nanotubes Filled with Anionic Surfactants. J. Phys. Chem. C 2016, 120, 13492–13502. [Google Scholar] [CrossRef]
- Luo, Z.; Song, H.; Feng, X.; Run, M.; Cui, H.; Wu, L.; Gao, J.; Wang, Z. Liquid Crystalline Phase Behavior and Sol-Gel Transition in Aqueous Halloysite Nanotube Dispersions. Langmuir 2013, 29, 12358–12366. [Google Scholar] [CrossRef] [PubMed]
- Bertolino, V.; Cavallaro, G.; Lazzara, G.; Milioto, S.; Parisi, F. Biopolymer-Targeted Adsorption onto Halloysite Nanotubes in Aqueous Media. Langmuir 2017, 33, 3317–3323. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Jung, G.-E.; Cho, S.J.; Geckeler, K.E.; Fuchs, H. Cellular interactions of doxorubicin-loaded DNA-modified halloysite nanotubes. Nanoscale 2013. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, G.; Lazzara, G.; Milioto, S. Exploiting the Colloidal Stability and Solubilization Ability of Clay Nanotubes/Ionic Surfactant Hybrid Nanomaterials. J. Phys. Chem. C 2012, 116, 21932–21938. [Google Scholar] [CrossRef]
- Cavallaro, G.; Lazzara, G.; Milioto, S.; Parisi, F. Steric stabilization of modified nanoclays triggered by temperature. J. Colloid Interface Sci. 2016, 461, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, G.; Lazzara, G.; Massaro, M.; Milioto, S.; Noto, R.; Parisi, F.; Riela, S. Biocompatible Poly(N-isopropylacrylamide)-halloysite Nanotubes for Thermoresponsive Curcumin Release. J. Phys. Chem. C 2015, 119, 8944–8951. [Google Scholar] [CrossRef]
- Cavallaro, G.; Lazzara, G.; Milioto, S.; Parisi, F.; Sanzillo, V. Modified Halloysite Nanotubes: Nanoarchitectures for Enhancing the Capture of Oils from Vapor and Liquid Phases. ACS Appl. Mater. Interfaces 2014, 6, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Derjaguin, B.V.; Landau, L.D. Theory of the Stability of Strongly Charged Lyophobic Sols and of the Adhesion of Strongly Charged Particles in Solutions of Electrolytes. Acta Physicochim. 1941, 14, 733–762. [Google Scholar] [CrossRef]
- Cavallaro, G.; Lazzara, G.; Milioto, S.; Palmisano, G.; Parisi, F. Halloysite nanotube with fluorinated lumen: Non-foaming nanocontainer for storage and controlled release of oxygen in aqueous media. J. Colloid Interface Sci. 2014, 417, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Lun, H.; Ouyang, J.; Yang, H. Enhancing dispersion of halloysite nanotubes via chemical modification. Phys. Chem. Miner. 2014, 41, 281–288. [Google Scholar] [CrossRef]
- Chang, P.R.; Xie, Y.; Wu, D.; Ma, X. Amylose wrapped halloysite nanotubes. Carbohydr. Polym. 2011, 84, 1426–1429. [Google Scholar] [CrossRef]
- Shamsi, M.H.; Geckeler, D.V. The first biopolymer-wrapped non-carbon nanotubes. Nanotechnology 2008, 19, 075604. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, G.; Lazzara, G.; Milioto, S.; Parisi, F. Hydrophobically Modified Halloysite Nanotubes as Reverse Micelles for Water-in-Oil Emulsion. Langmuir 2015, 31, 7472–7478. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Yuan, P.; Thill, A.; Annabi-Bergaya, F.; Liu, D.; Wang, S. Insights into the formation mechanism of imogolite from a full-range observation of its sol-gel growth. Appl. Clay Sci. 2017, 150, 115–124. [Google Scholar] [CrossRef]
- Kanji, K.; Nobuo, D.; Yuzuru, H.; Hiroshi, I. Lyotropic mesophase of imogolite, 1. Effect of polydispersity on phase diagram. Makromol. Chem. 1986, 187, 2883–2893. [Google Scholar] [CrossRef]
- Livolant, F.; Leforestier, A. Condensed phases of DNA: Structures and phase transitions. Prog. Polym. Sci. 1996, 21, 1115–1164. [Google Scholar] [CrossRef]
- Vroege, G.J.; Thies-Weesie, D.M.E.; Petukhov, A.V.; Lemaire, B.J.; Davidson, P. Smectic Liquid-Crystalline Order in Suspensions of Highly Polydisperse Goethite Nanorods. Adv. Mater. 2006, 18, 2565–2568. [Google Scholar] [CrossRef]
- Ramos, L.; Fabre, P. Swelling of a lyotropic hexagonal phase by monitoring the radius of the cylinders. Langmuir 1997, 13, 682–685. [Google Scholar] [CrossRef]
- Karube, J. Hysteresis of the Colloidal Stability of Imogolite. Clays Clay Miner. 1998, 46, 583–585. [Google Scholar] [CrossRef]
- Ma, Y.L.; Karube, J. Imogolite flocculation under alkaline conditions. Soil Sci. Plant Nutr. 2013, 59, 125–129. [Google Scholar] [CrossRef]
- Gao, Z.; Zhi, C.; Bando, Y.; Golberg, D.; Serizawa, T. Noncovalent Functionalization of Boron Nitride Nanotubes in Aqueous Media Opens Application Roads in Nanobiomedicine. Nanobiomedicine 2014, 1, 7. [Google Scholar] [CrossRef]
- Kim, D.; Sawada, T.; Zhi, C.Y.; Bando, Y.; Golberg, D.; Serizawa, T. Dispersion of Boron Nitride Nanotubes in Aqueous Solution by Simple Aromatic Molecules. J. Nanosci. Nanotechnol. 2014, 14, 3028–3033. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Fujioka, K.; Sawada, T.; Zhi, C.; Bando, Y.; Golberg, D.; Aizawa, M.; Serizawa, T. Noncovalent functionalization of boron nitride nanotubes using water-soluble synthetic polymers and the subsequent preparation of superhydrophobic surfaces. Polym. J. 2013, 45, 567–570. [Google Scholar] [CrossRef]
- Wu, X.; An, W.; Zeng, X.C. Chemical functionalization of boron-nitride nanotubes with NH3 and amino functional groups. J. Am. Chem. Soc. 2006, 128, 12001–12006. [Google Scholar] [CrossRef] [PubMed]
- Lau, Y.T.R.; Yamaguchi, M.; Li, X.; Bando, Y.; Golberg, D.; Winnik, F.M. Facile and mild strategy toward biopolymer-coated boron nitride nanotubes via a glycine-assisted interfacial process. J. Phys. Chem. C 2013, 117, 19568–19576. [Google Scholar] [CrossRef]
- Gao, Z.; Zhi, C.; Bando, Y.; Golberg, D.; Komiyama, M.; Serizawa, T. Efficient disentanglement of boron nitride nanotubes using water-soluble polysaccharides for protein immobilization. RSC Adv. 2012, 2, 6200–6208. [Google Scholar] [CrossRef]
- Gao, Z.; Zhi, C.; Bando, Y.; Golberg, D.; Serizawa, T. Isolation of individual boron nitride nanotubes via peptide wrapping. J. Am. Chem. Soc. 2010, 132, 4976–4977. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Zhi, C.; Bando, Y.; Golberg, D.; Serizawa, T. Noncovalent functionalization of disentangled boron nitride nanotubes with flavin mononucleotides for strong and stable visible-light emission in aqueous solution. ACS Appl. Mater. Interfaces 2011, 3, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Zhi, C.; Bando, Y.; Wang, W.; Tang, C.; Kuwahara, H.; Golberg, D. DNA-mediated assembly of boron nitride nanotubes. Chem. Asian J. 2007, 2, 1581–1585. [Google Scholar] [CrossRef] [PubMed]
- Emanet, M.; Şen, Ö.; Çulha, M. Evaluation of boron nitride nanotubes and hexagonal boron nitrides as nanocarriers for cancer drugs. Nanomedicine 2017, 12, 797–810. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Zhang, D.; Yap, Y. Functionalization, Dispersion, and Cutting of Boron Nitride Nanotubes in Water. J. Phys. Chem. C 2012, 116, 1798–1804. [Google Scholar] [CrossRef]
- Ikuno, T.; Sainsbury, T.; Okawa, D.; Frechet, J.M.J.; Zettl, A. Amine-functionalized boron nitride nanotubes. Solid State Commun. 2007, 142, 643–646. [Google Scholar] [CrossRef]
- Zhi, C.; Bando, Y.; Tang, C.; Honda, S.; Sato, K.; Kuwahara, H.; Golberg, D. Covalent functionalization: Towards soluble multiwalled boron nitride nanotubes. Angew. Chem. Int. Ed. 2005, 44, 7932–7935. [Google Scholar] [CrossRef] [PubMed]
- Emanet, M.; Şen, Ö.; Çobandede, Z.; Çulha, M. Interaction of carbohydrate modified boron nitride nanotubes with living cells. Colloids Surf. B 2015, 134, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Şen, Ö.; Çobandede, Z.; Emanet, M.; Bayrak, Ö.F.; Çulha, M. Boron nitride nanotubes for gene silencing. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 2391–2397. [Google Scholar] [CrossRef] [PubMed]
- Zhi, C.Y.; Bando, Y.; Terao, T.; Tang, C.C.; Kuwahara, H.; Golberg, D. Chemically activated boron nitride nanotubes. Chem. Asian J. 2009, 4, 1536–1540. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Ashrafi, B.; Laqua, K.; Kim, S. Nanotubes with peroxides and their application in polycarbonate composites. New J. Chem. 2017, 41, 7571–7577. [Google Scholar] [CrossRef]
- Ejaz, M.; Rai, S.C.; Wang, K.; Zhang, K.; Zhou, W.; Grayson, S.M. Surface-initiated atom transfer radical polymerization of glycidyl methacrylate and styrene from boron nitride nanotubes. J. Mater. Chem. C 2014, 2, 4073–4079. [Google Scholar] [CrossRef]
- Stetsyshyn, Y.; Raczkowska, J.; Lishchynskyi, O.; Bernasik, A.; Kostruba, A.; Harhay, K.; Ohar, H.; Marzec, M.M.; Budkowski, A. Temperature-Controlled Three-Stage Switching of Wetting, Morphology, and Protein Adsorption. ACS Appl. Mater. Interfaces 2017, 9, 12035–12045. [Google Scholar] [CrossRef] [PubMed]
- Raczkowska, J.; Stetsyshyn, Y.; Awsiuk, K.; Zemła, J.; Kostruba, A.; Harhay, K.; Marzec, M.; Bernasik, A.; Lishchynskyi, O.; Ohar, H.; Budkowski, A. Temperature-responsive properties of poly(4-vinylpyridine) coatings: Influence of temperature on the wettability, morphology, and protein adsorption. RSC Adv. 2016, 6, 87469–87477. [Google Scholar] [CrossRef]
- Lin, Y.; Williams, T.V.; Cao, W.; Elsayed-Ali, H.E.; Connell, J.W. Defect functionalization of hexagonal boron nitride nanosheets. J. Phys. Chem. C 2010, 114, 17434–17439. [Google Scholar] [CrossRef]
- Sundaram, R.; Scheiner, S.; Roy, A.K.; Kar, T. B=N bond cleavage and BN ring expansion at the surface of boron nitride nanotubes by iminoborane. J. Phys. Chem. C 2015, 119, 3253–3259. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lisuzzo, L.; Cavallaro, G.; Lazzara, G.; Milioto, S.; Parisi, F.; Stetsyshyn, Y. Stability of Halloysite, Imogolite, and Boron Nitride Nanotubes in Solvent Media. Appl. Sci. 2018, 8, 1068. https://doi.org/10.3390/app8071068
Lisuzzo L, Cavallaro G, Lazzara G, Milioto S, Parisi F, Stetsyshyn Y. Stability of Halloysite, Imogolite, and Boron Nitride Nanotubes in Solvent Media. Applied Sciences. 2018; 8(7):1068. https://doi.org/10.3390/app8071068
Chicago/Turabian StyleLisuzzo, Lorenzo, Giuseppe Cavallaro, Giuseppe Lazzara, Stefana Milioto, Filippo Parisi, and Yurij Stetsyshyn. 2018. "Stability of Halloysite, Imogolite, and Boron Nitride Nanotubes in Solvent Media" Applied Sciences 8, no. 7: 1068. https://doi.org/10.3390/app8071068
APA StyleLisuzzo, L., Cavallaro, G., Lazzara, G., Milioto, S., Parisi, F., & Stetsyshyn, Y. (2018). Stability of Halloysite, Imogolite, and Boron Nitride Nanotubes in Solvent Media. Applied Sciences, 8(7), 1068. https://doi.org/10.3390/app8071068