Synthesis and Characterization of Polystyrene-Montmorillonite Nanocomposite Particles Using an Anionic-Surfactant-Modified Clay and Their Friction Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
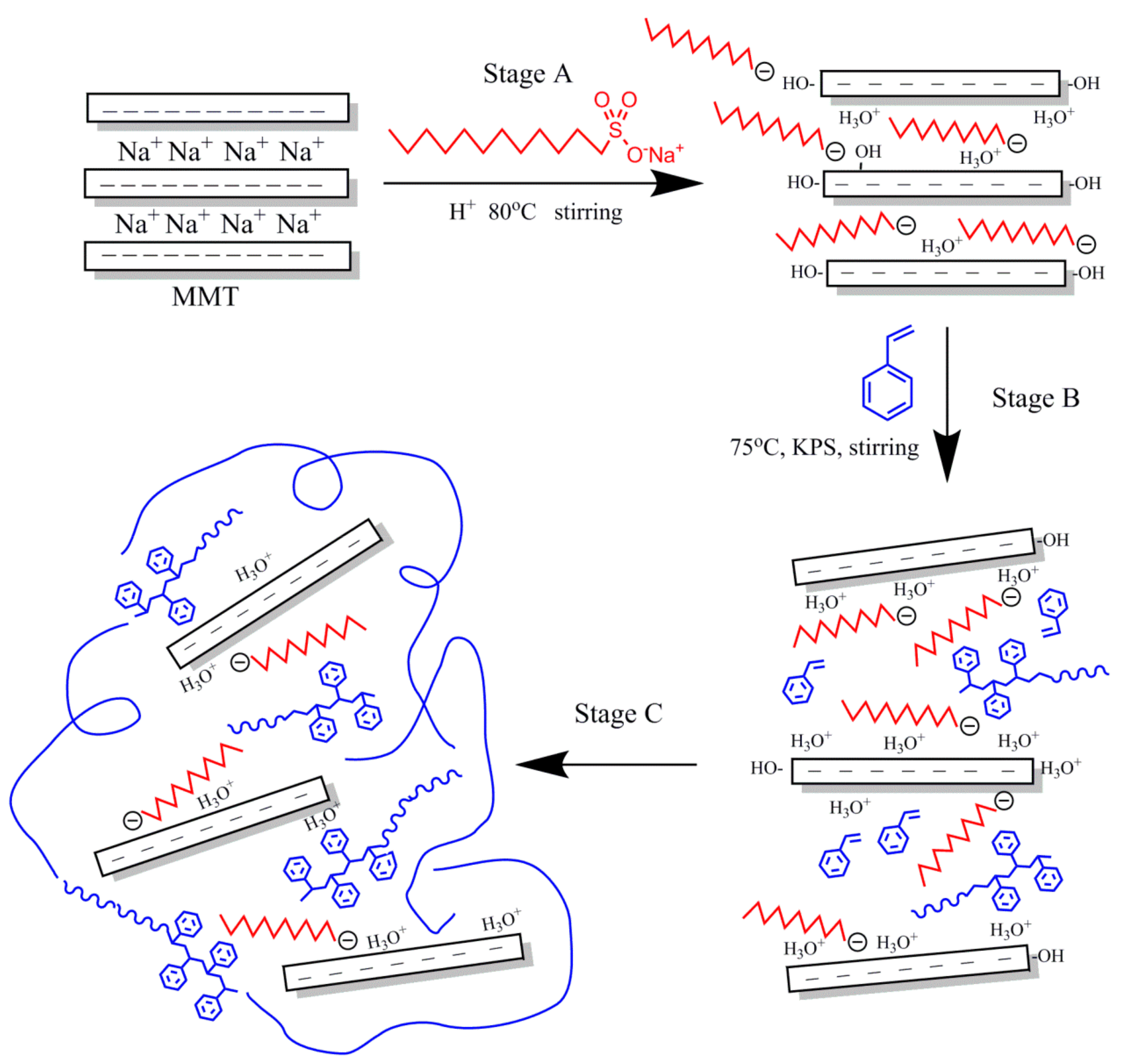
2.2. Preparation of OMMT
2.3. Preparation of PS-OMMT Nanocomposites
2.4. Preparation of PAO-PS-OMMT Lubricant
2.5. Characterization
3. Result and Discussion
3.1. Fourier Transform Infrared Spectroscopy
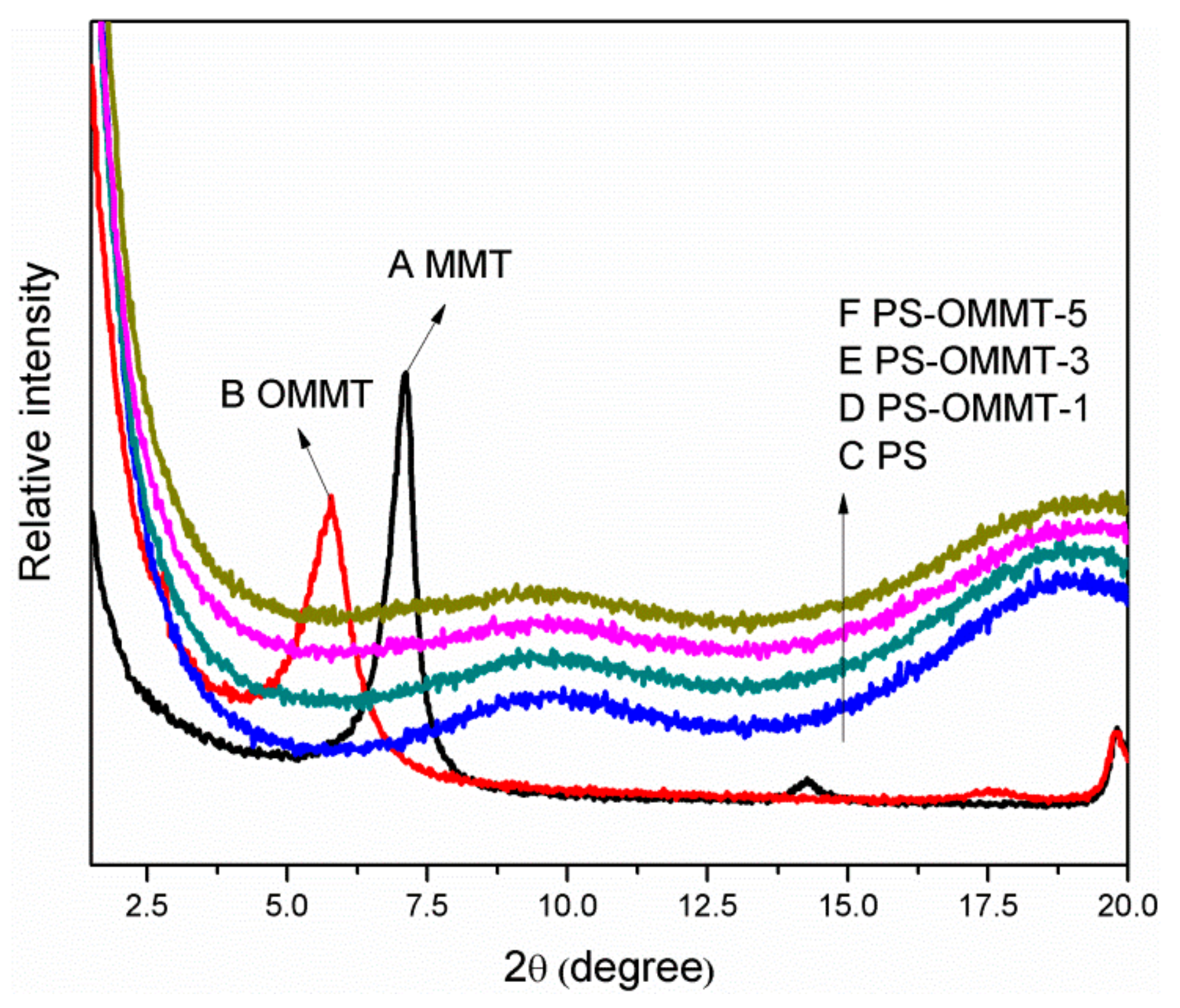
3.2. X-Ray Diffraction
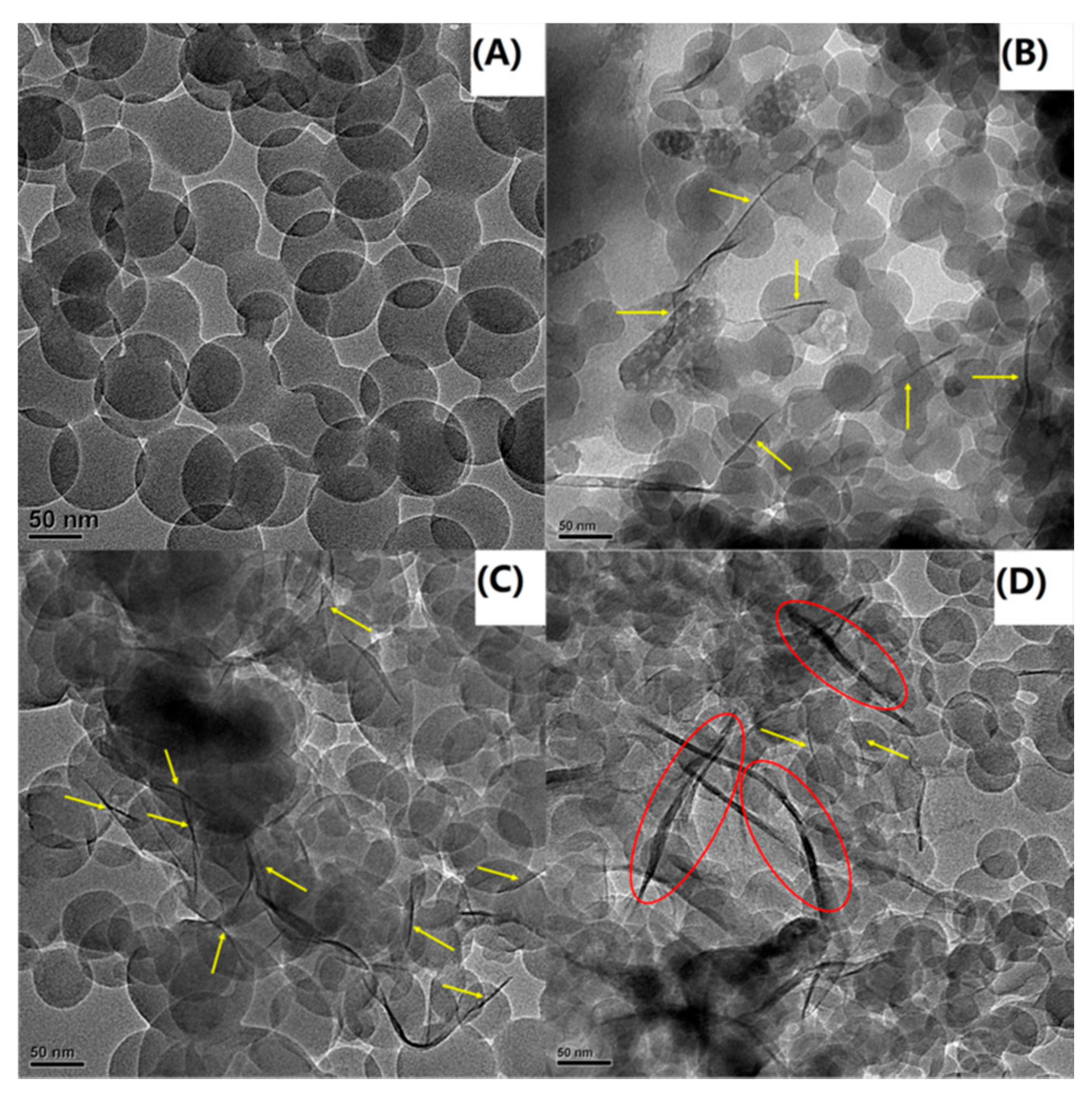
3.3. Transmission Electron Microscopy
3.4. Gel Permeation Chromatography
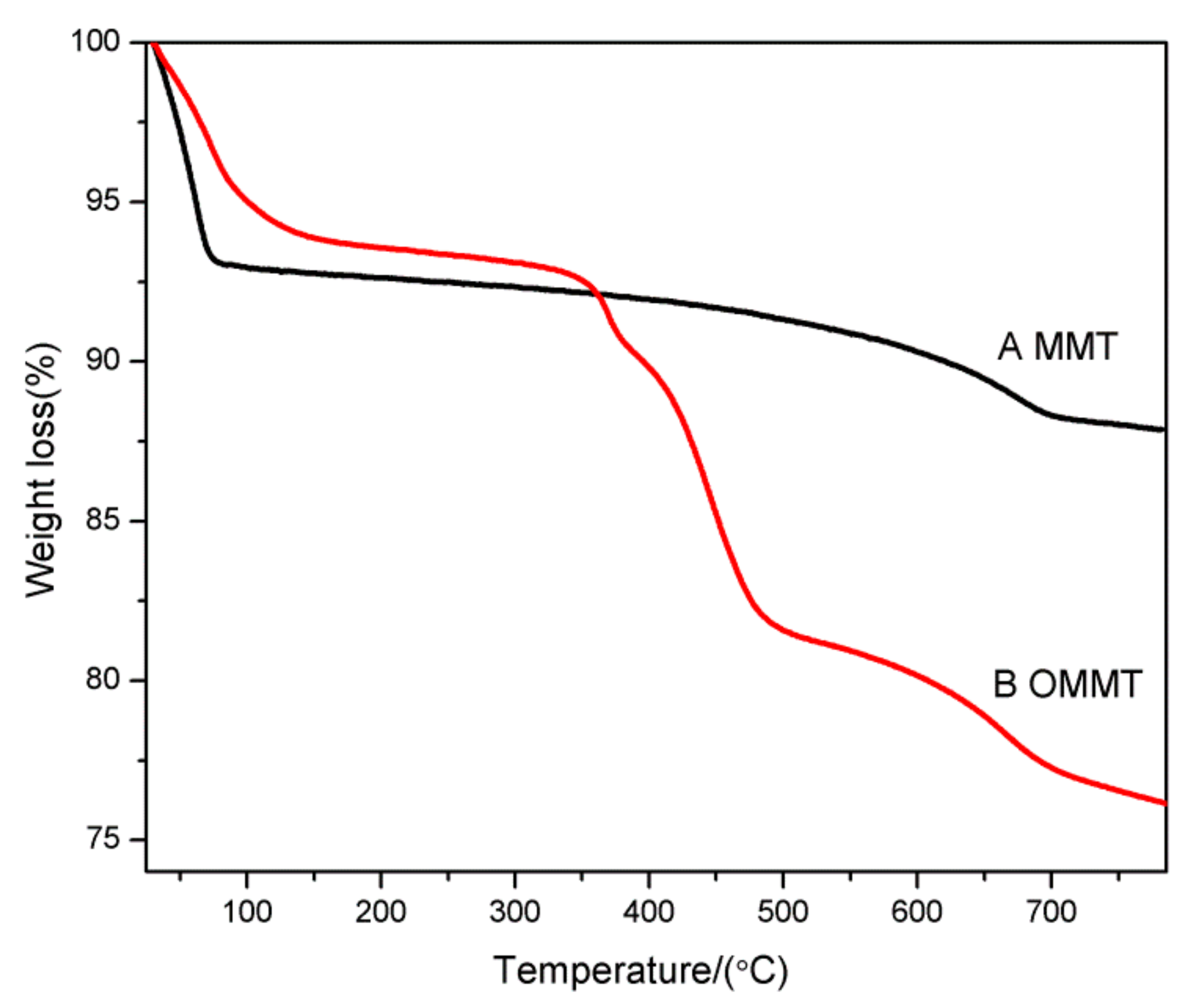
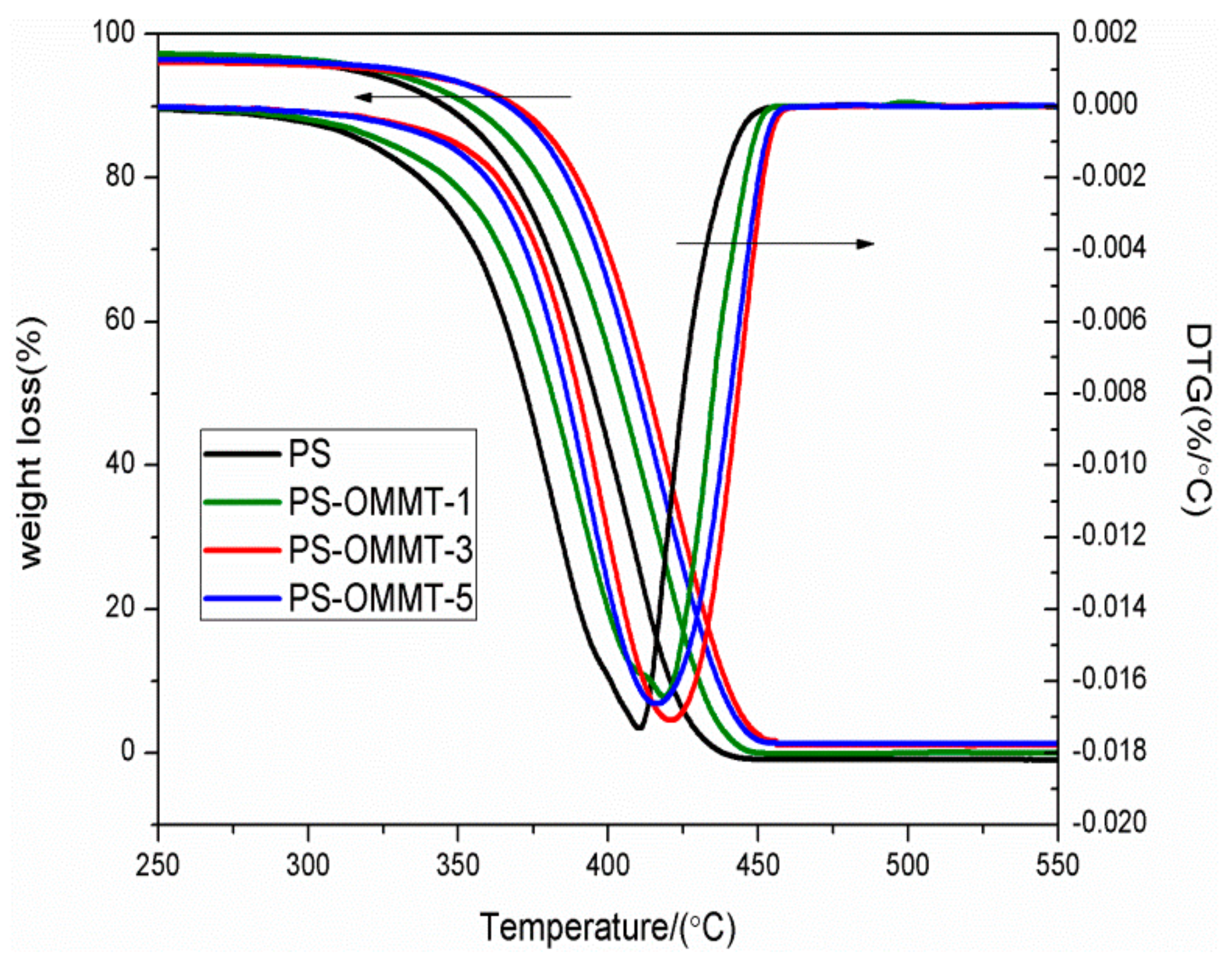
3.5. Thermal Stability
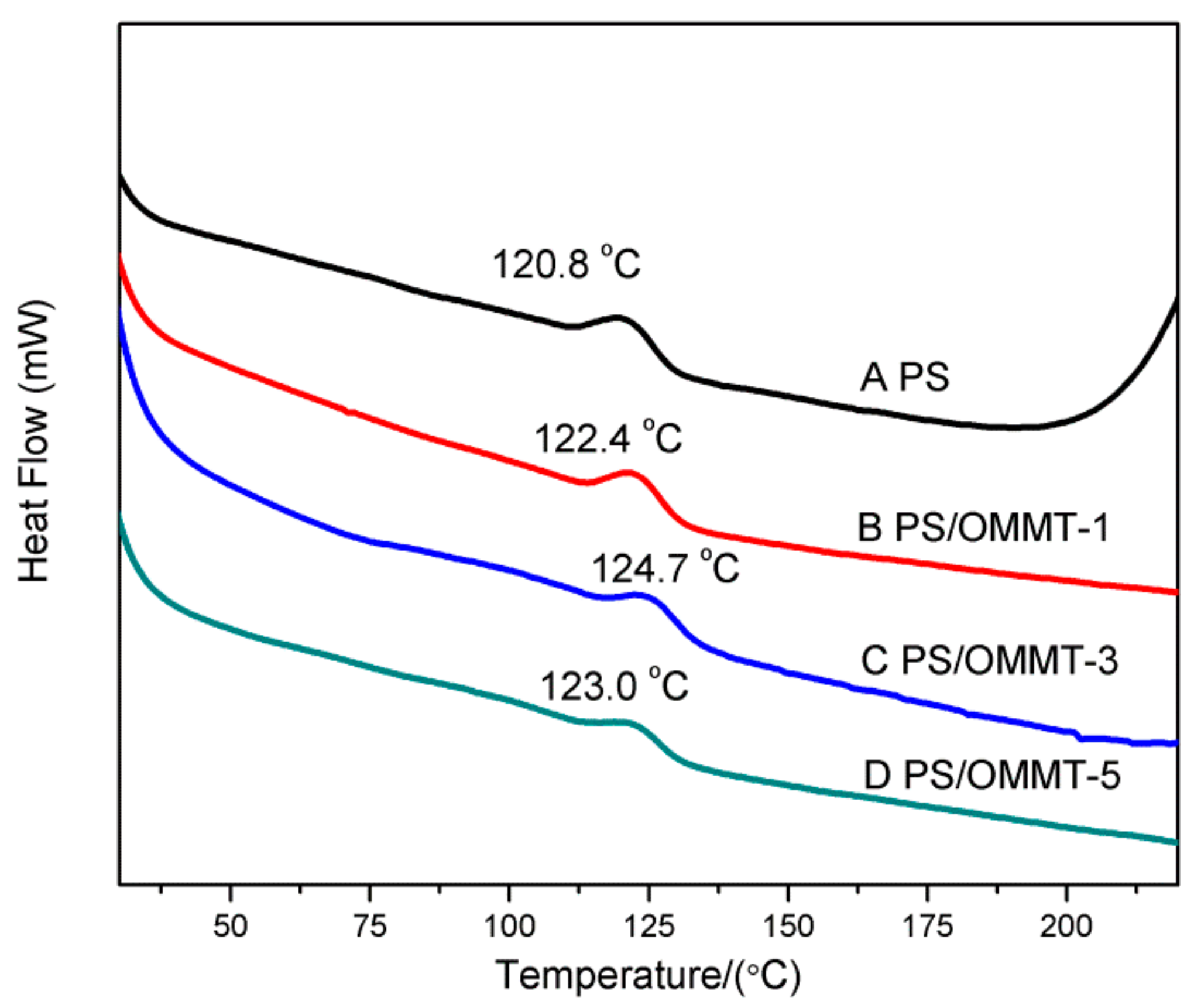
3.6. Differential Scanning Calorimetry
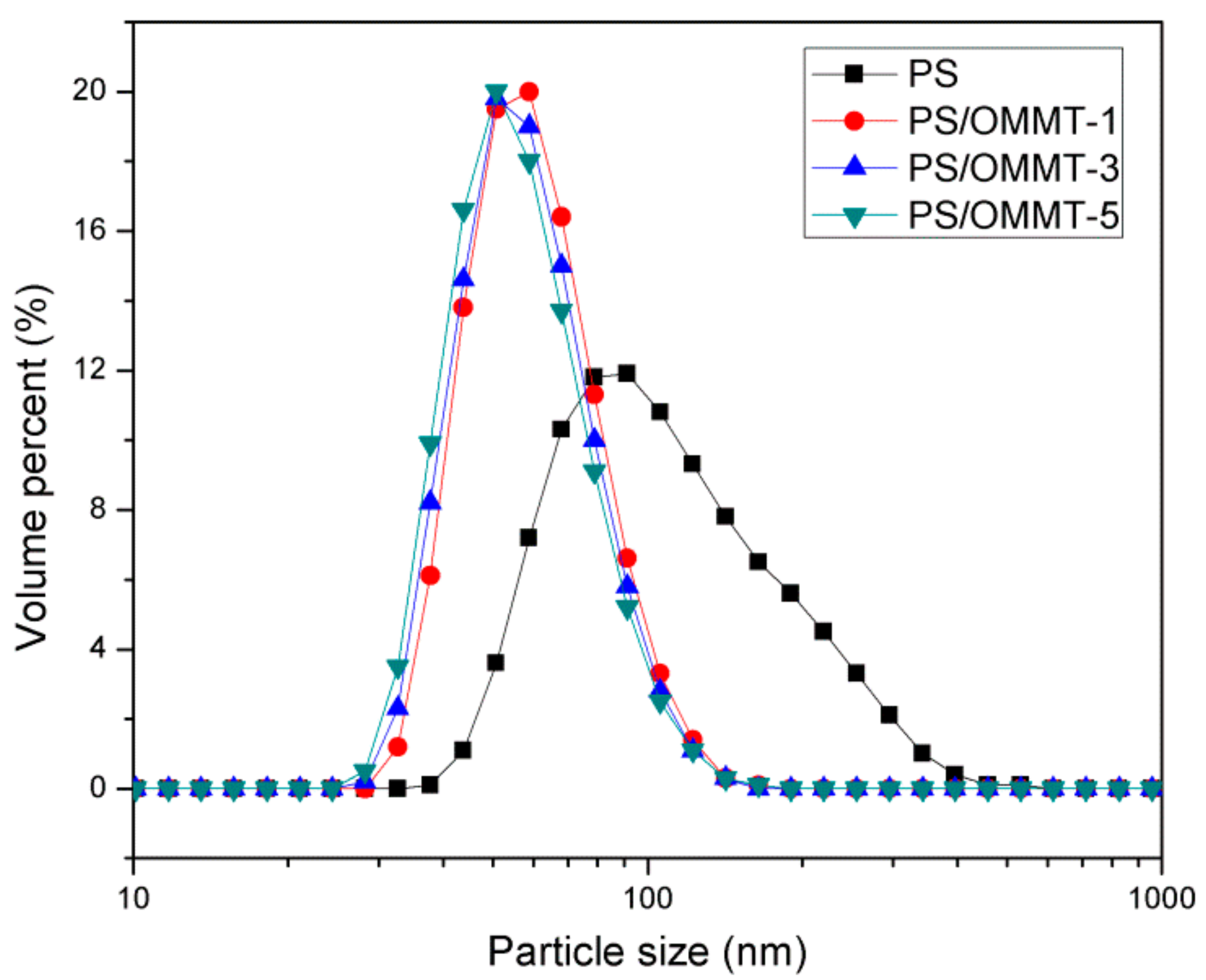
3.7. Particle Size Analysis
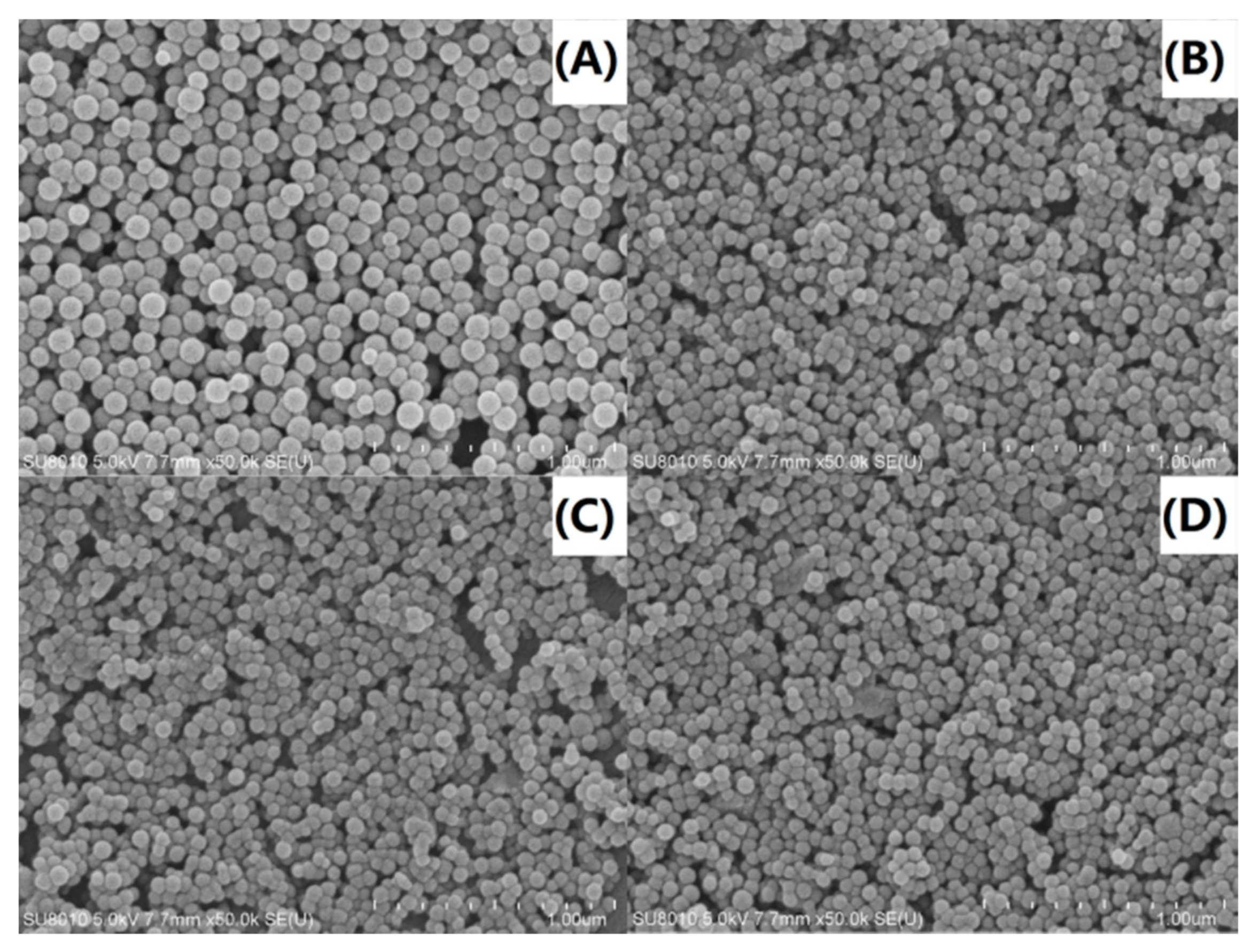
3.8. Scanning Electron Microscopy
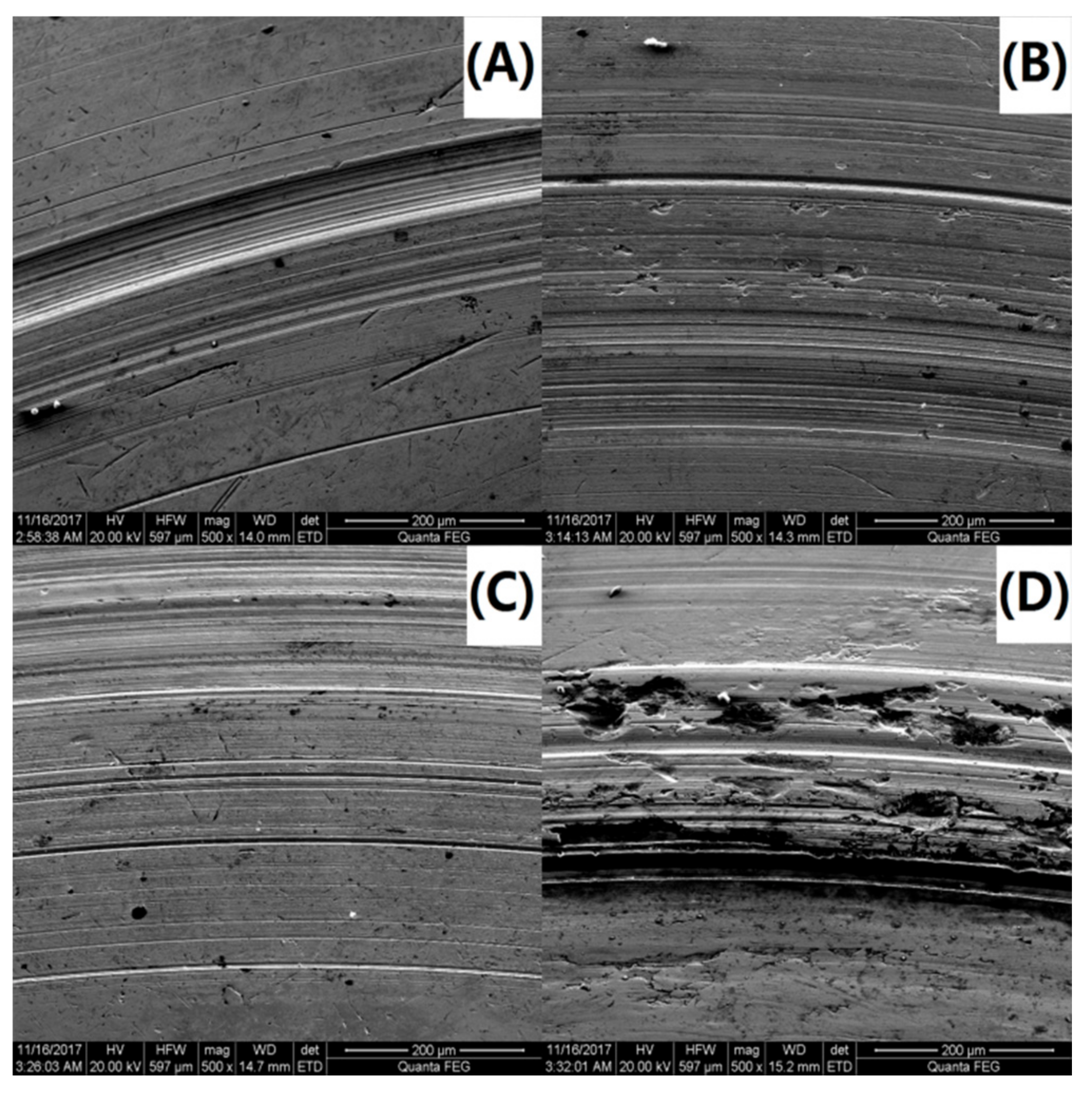
3.9. Friction Performance Analysis
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Moraes, R.P.; Valera, T.S.; Pereira, A.M.C.; Demarquette, N.R.; Santos, A.M. Influence of the type of quaternary ammonium salt used in the organic treatment of montmorillonite on the properties of poly(styrene-co-butyl acrylate)/layered silicate nanocomposites prepared by in situminiemulsion polymerization. J. Appl. Polym. Sci. 2011, 119, 3658–3669. [Google Scholar] [CrossRef]
- Kawasumi, M. The discovery of polymer-clay hybrids. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 819–824. [Google Scholar] [CrossRef]
- Zhang, G.; Ke, Y.; He, J.; Qin, M.; Shen, H.; Lu, S.; Xu, J. Effects of organo-modified montmorillonite on the tribology performance of bismaleimide-based nanocomposites. Mater. Des. 2015, 86, 138–145. [Google Scholar] [CrossRef]
- EI-Sigeny, S.; Mohamed, S.K.; Taleb, M.F.A. Radiation synthesis and characterization of styrene/acrylic acid/organophilic montmorillonite hybrid nanocomposite for sorption of dyes from aqueous solutions. Polym. Compos. 2014, 35, 2353–2364. [Google Scholar] [CrossRef]
- Huang, H.; Liu, H. Synthesis of the Raspberry-Like PS/PAN Particles with Anisotropic properties via seeded emulsion polymerization initiated by γ-Ray radiation. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 5198–5205. [Google Scholar] [CrossRef]
- Palanisamy, A. Water-blown polyurethane–clay nanocomposite foams from biopolyol—Effect of nanoclay on the properties. Polym. Compos. 2013, 34, 1306–1312. [Google Scholar] [CrossRef]
- Chen, Y.N.; Chung, P.Y.; Yen, S.C. Conductivity and methanol permeability of sulfonated polystyrene membrane with dispersed montmorillonite nanoclay. Polym. Compos. 2012, 33, 2105–2113. [Google Scholar] [CrossRef]
- Dawson, J.I.; Oreffo, R.O. Clay: New opportunities for tissue regeneration and biomaterial design. Adv. Mater. 2013, 25, 4069–4086. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.J.; Wang, H.Y.; Chen, K.; Song, Y.M.; Wei, Z.; Xue, M. The modification of lanthanum-exchanged montmorillonite with anionic surfactants to enhance the thermal stability of polyvinyl chloride. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Huang, J.C.; Zhu, Z.K.; Yin, J.; Qian, X.F.; Sun, Y.Y. Poly(etherimide)/montmorillonite nanocomposites prepared by melt intercalation: Morphology, solvent resistance properties and thermal properties. Polymer 2001, 42, 873–877. [Google Scholar] [CrossRef]
- Ji, J.Q.; Ke, Y.C.; Pei, Y.; Zhang, G.L. Effects of highly exfoliated montmorillonite layers on the properties of clay reinforced terpolymer nanocomposite plugging microspheres. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Momani, B.; Sen, M.; Endoh, M.; Wang, X.L.; Koga, T.; Winter, H.H. Temperature dependent intercalation and self–exfoliation of clay/polymer nanocomposite. Polymer 2016, 93, 204–212. [Google Scholar] [CrossRef]
- Soulestin, J.; Rashmi, B.J.; Bourbigot, S.; Lacrampe, M.F.; Krawczak, P. Mechanical and optical properties of polyamide 6/clay nanocomposite cast films: Influence of the degree of exfoliation. Macromol. Mater. Eng. 2012, 297, 444–454. [Google Scholar] [CrossRef]
- Hwang, S.J.; Yong, L.J.; Lee, S.J. Properties of High-Impact Polystyrene/Organoclay Nanocomposites Synthesized via In Situ Polymerization. J. Appl. Polym. Sci. 2008, 110, 1141–1450. [Google Scholar] [CrossRef]
- Liu, L.; Wei, S.L.; Lai, X.J. In situ synthesis and characterization of polypropylene/polyvinyl acetate-organophilic montmorillonite nanocomposite. J. Appl. Polym. Sci. 2012, 124, 4107–4113. [Google Scholar] [CrossRef]
- Chen, G.M.; Liu, S.H.; Chen, S.J.; Qi, Z.N. FTIR spectra, thermal properties, and dispersibility of a polystyrene/montmorillonite nanocomposite. Macromol. Chem. Phys. 2001, 202, 1189–1193. [Google Scholar] [CrossRef]
- Simons, R.; Qiao, G.G.; Powell, C.E.; Bateman, S.A. Effect of surfactant architecture on the properties of polystyrene-montmorillinite nanocomposites. Langmuir 2010, 26, 9023–9031. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Morgan, A.B.; Lamelas, F.J.; Wilkie, C.A. Fire properties of polystyrene-clay nanocomposites. Chem. Mater. 2001, 13, 3774–3780. [Google Scholar] [CrossRef]
- Zhang, Z.; Liao, L.; Xia, Z. Ultrasound-assisted preparation and characterization of anionic surfactants modified montmorillonites. Appl. Clay. Sci. 2010, 50, 576–581. [Google Scholar] [CrossRef]
- Paiva, L.B.D.; Morales, A.R.; Diaz, F.R.V. Organoclays: Properties, preparation and applications. Appl. Clay. Sci. 2008, 42, 8–24. [Google Scholar] [CrossRef]
- Alansi, A.M.; Alkayali, W.Z.; Al-Qunaibit, M.H.; Qahtan, T.F.; Saleh, T.A. Synthesis of exfoliated polystyrene/anionic clay MgAl-layered double hydroxide: Structural and thermal properties. RSC Adv. 2015, 5, 71441–71448. [Google Scholar] [CrossRef]
- Zhu, L.; Guo, J.; Liu, P. Effects of length and organic modification of attapulgite nanorods on attapulgite/polystyrene nanocomposite via in-situ radical bulk polymerization. Appl. Clay. Sci. 2016, 119, 87–95. [Google Scholar] [CrossRef]
- Ianchis, R.; Donescu, D.; Petcu, C.; Ghiurea, M.; Anghel, D.F.; Stanga, G.; Marcu, A. Surfactant-free emulsion polymerization of styrene in the presence of silylated montmorillonite. Appl. Clay. Sci. 2009, 45, 164–170. [Google Scholar] [CrossRef]
- Qian, Z.; Zhou, H.; Xu, X.; Ding, Y.; Zhang, S.; Yang, M. Effect of the grafted silane on the dispersion and orientation of clay in polyethylene nanocomposites. Polym. Compos. 2009, 30, 1234–1242. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Chen, Z.; Ma, P. Effect of doubly organo-modified vermiculite on the properties of vermiculite/polystyrene nanocomposites. Appl. Clay. Sci. 2013, 75–76, 74–81. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, H.; Chen, X.; Chen, Z.; Cui, Z.; Yang, B. Monodisperse silica-polymer core-shell microspheres via surface grafting and emulsion polymerization. Macromol. Mater. Eng. 2003, 288, 380–385. [Google Scholar] [CrossRef]
- Li, H.; Yu, Y.; Yang, Y. Synthesis of exfoliated polystyrene/montmorillonite nanocomposite by emulsion polymerization using a zwitterion as the clay modifier. Eur. Polym. J. 2005, 41, 2016–2022. [Google Scholar] [CrossRef]
- Wang, J.; Hu, Y.; Wang, S.; Chen, Z. Sonochemical one-directional growth of montmorillonite–polystyrene nanocomposite. Ultrason. Sonochem. 2005, 12, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.T.; Ko, T.H.; Wang, S.C.; Shih, P.I.; Chang, M.J.; Jiang, G.J. Preparation of polystyrene/clay nanocomposite by suspension and emulsion polymerization. Polym. Compos. 2010, 29, 409–414. [Google Scholar] [CrossRef]
- Yi, D.; Yang, H.; Zhao, M.; Huang, L.; Camino, G.; Frache, A.; Yang, R. A novel, low surface charge density, anionically modified montmorillonite for polymer nanocomposites. RSC Adv. 2017, 7, 5980–5988. [Google Scholar] [CrossRef]
- Yang, L.; Ke, Y. Synthesis of polystyrene nanolatexes via emulsion polymerization using sodium dodecyl sulfonate as the emulsifier. High Perform. Polym. 2014, 26, 900–905. [Google Scholar] [CrossRef]
- Piscitelli, F.; Posocco, P.; Toth, R.; Fermeglia, M.; Pricl, S.; Mensitieri, G.; Lavorgna, M. Sodium montmorillonite silylation: Unexpected effect of the aminosilane chain length. J. Colloid. Interf. Sci. 2010, 351, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Bhanvase, B.A.; Pinjari, D.V.; Gogate, P.R.; Sonawane, S.H.; Pandit, A.B. Synthesis of exfoliated poly(styrene-co-methyl methacrylate)/montmorillonite nanocomposite using ultrasound assisted in situ emulsion copolymerization. Chem. Eng. J. 2012, 181–182, 770–778. [Google Scholar] [CrossRef]
- Ezquerro, C.S.; Ric, G.I.; Miñana, C.C.; Bermejo, J.S. Characterization of montmorillonites modified with organic divalent phosphonium cations. Appl. Clay. Sci. 2015, 111, 1–9. [Google Scholar] [CrossRef]
- Faucheu, J.; Gauthier, C.; Chazeau, L.; Cavaille, J.Y.; Mellon, V.; Lami, E.B. Miniemulsion polymerization for synthesis of structured clay/polymer nanocomposites: Short review and recent advances. Polymer 2010, 51, 6–17. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, J.; Zhao, H. Functional colloidal particles stabilized by layered silicate with hydrophilic face and hydrophobic polymer brushes. J. Polym. Sci. Polym. Chem. 2009, 47, 1535–1543. [Google Scholar] [CrossRef]
- Hu, J.; Chen, M.; Wu, L. Organic-inorganic nanocomposites synthesized via miniemulsion polymerization. Polym. Chem. 2011, 2, 760–772. [Google Scholar] [CrossRef]
- Greesh, N.; Sanderson, R.; Hartmann, P. Prepatation of polystyrene-clay nanocomposites via dispersion polymerization using oligomeric styrene-montmorillonite as stabilizer. Polym. Int. 2012, 61, 834–843. [Google Scholar] [CrossRef]
- Patel, H.A.; Somani, R.S.; Bajaj, H.C.; Jasra, R.V. Preparation and characterization of phosphonium montmorillonite with enhanced thermal stability. Appl. Clay. Sci. 2007, 35, 194–200. [Google Scholar] [CrossRef]
- Bee, S.L.; Abdullah, M.A.A.; Mamat, M.; Bee, S.T.; Sin, L.T.; Hui, D.; Rahmat, A.R. Characterization of silylated modified clay nanoparticles and its functionality in PMMA. Compos. Part B Eng. 2017, 110, 83–95. [Google Scholar] [CrossRef]
- Periyayya, U.; Joo, K.H.; Hong, C.; Eun-Kyung, S.; Youn-Sik, L. Preparation of exfoliated high-impact polystyrene/MMT nanocomposites via in situ polymerization under controlling viscosity of the reaction medium. Polym. Compos. 2010, 29, 142–148. [Google Scholar]
- Greesh, N.; Hartmann, P.C.; Cloete, V.; Sanderson, R.D. Impact of the clay organic modifier on the morphology of polymer–clay nanocomposites prepared by in situ free-radical polymerization in emulsion. J. Polym. Sci. Polym. Chem. 2008, 46, 3619–3628. [Google Scholar] [CrossRef]
- Dong, Z.; Liu, Z.; Zhang, J.; Han, B.; Sun, D.; Wang, Y.; Huang, Y.J. Synthesis of montmorillonite/polystyrene nanocomposites in supercritical carbon dioxide. Appl. Polym. Sci. 2004, 94, 1194–1197. [Google Scholar] [CrossRef]
- Chen, S.; Lu, X.; Zhang, Z.; Wang, T.; Pan, F. Preparation and characterization of poly (methyl methacrylate)/reactive montmorillonite nanocomposites. Polym. Compos. 2016, 37, 2396–2403. [Google Scholar] [CrossRef]
- Fu, X.; Qutubuddin, S. Synthesis of polystyrene–clay nanocomposites. Mater. Lett. 2000, 42, 12–15. [Google Scholar] [CrossRef]



| Sample Code | Mn (×105) | Mw (×105) | PDI |
|---|---|---|---|
| PS | 3.65 | 15.09 | 4.14 |
| PS-OMMT-1 | 1.76 | 7.56 | 4.31 |
| PS-OMMT-3 | 1.56 | 5.57 | 3.57 |
| PS-OMMT-5 | 1.55 | 7.35 | 4.74 |
| Sample Code | Z-Average (nm) | PDI |
|---|---|---|
| PS | 129.5 ± 6.5 | 0.204 |
| PS-OMMT-1 | 64.58 ± 2.91 | 0.082 |
| PS-OMMT-3 | 62.83 ± 2.19 | 0.079 |
| PS-OMMT-5 | 63.38 ± 2.92 | 0.102 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, C.; Ke, Y.; Deng, Q.; Lu, S.; Ji, J.; Hu, X.; Zhao, Y. Synthesis and Characterization of Polystyrene-Montmorillonite Nanocomposite Particles Using an Anionic-Surfactant-Modified Clay and Their Friction Performance. Appl. Sci. 2018, 8, 964. https://doi.org/10.3390/app8060964
Yu C, Ke Y, Deng Q, Lu S, Ji J, Hu X, Zhao Y. Synthesis and Characterization of Polystyrene-Montmorillonite Nanocomposite Particles Using an Anionic-Surfactant-Modified Clay and Their Friction Performance. Applied Sciences. 2018; 8(6):964. https://doi.org/10.3390/app8060964
Chicago/Turabian StyleYu, Chengcheng, Yangchuan Ke, Qingchun Deng, Shichao Lu, Jingqi Ji, Xu Hu, and Yi Zhao. 2018. "Synthesis and Characterization of Polystyrene-Montmorillonite Nanocomposite Particles Using an Anionic-Surfactant-Modified Clay and Their Friction Performance" Applied Sciences 8, no. 6: 964. https://doi.org/10.3390/app8060964
APA StyleYu, C., Ke, Y., Deng, Q., Lu, S., Ji, J., Hu, X., & Zhao, Y. (2018). Synthesis and Characterization of Polystyrene-Montmorillonite Nanocomposite Particles Using an Anionic-Surfactant-Modified Clay and Their Friction Performance. Applied Sciences, 8(6), 964. https://doi.org/10.3390/app8060964






