An Effective Delivery System of Sitagliptin Using Optimized Mucoadhesive Nanoparticles
Abstract
1. Introduction
2. Material and Methods
2.1. Formulation of Sitagliptin Nanoparticles
2.2. Characterization of Nanoparticles
2.2.1. Scanning Electron Microscopy (SEM) of Nanoparticles
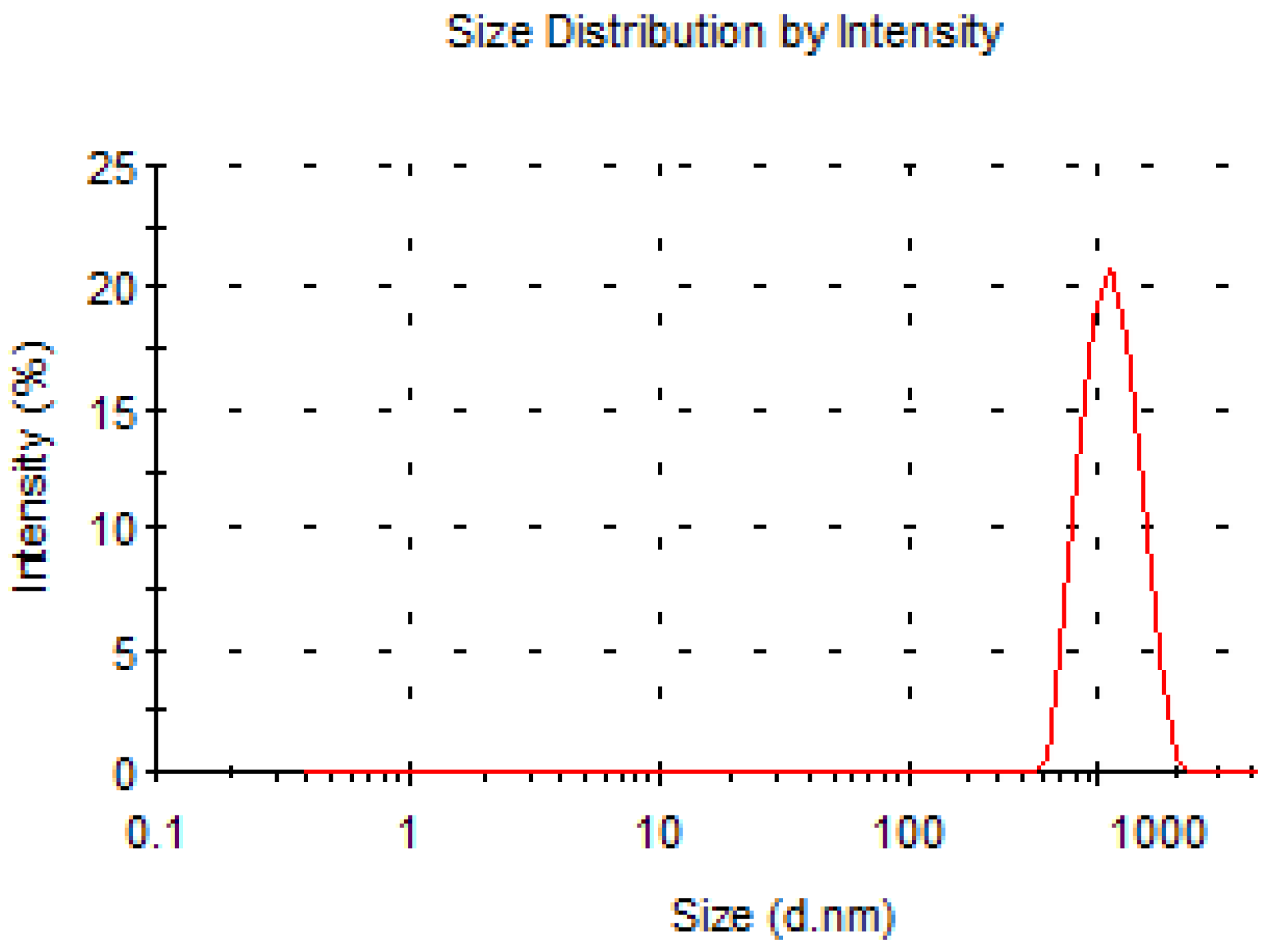
2.2.2. Analysis of Particle Size of Nanoparticles
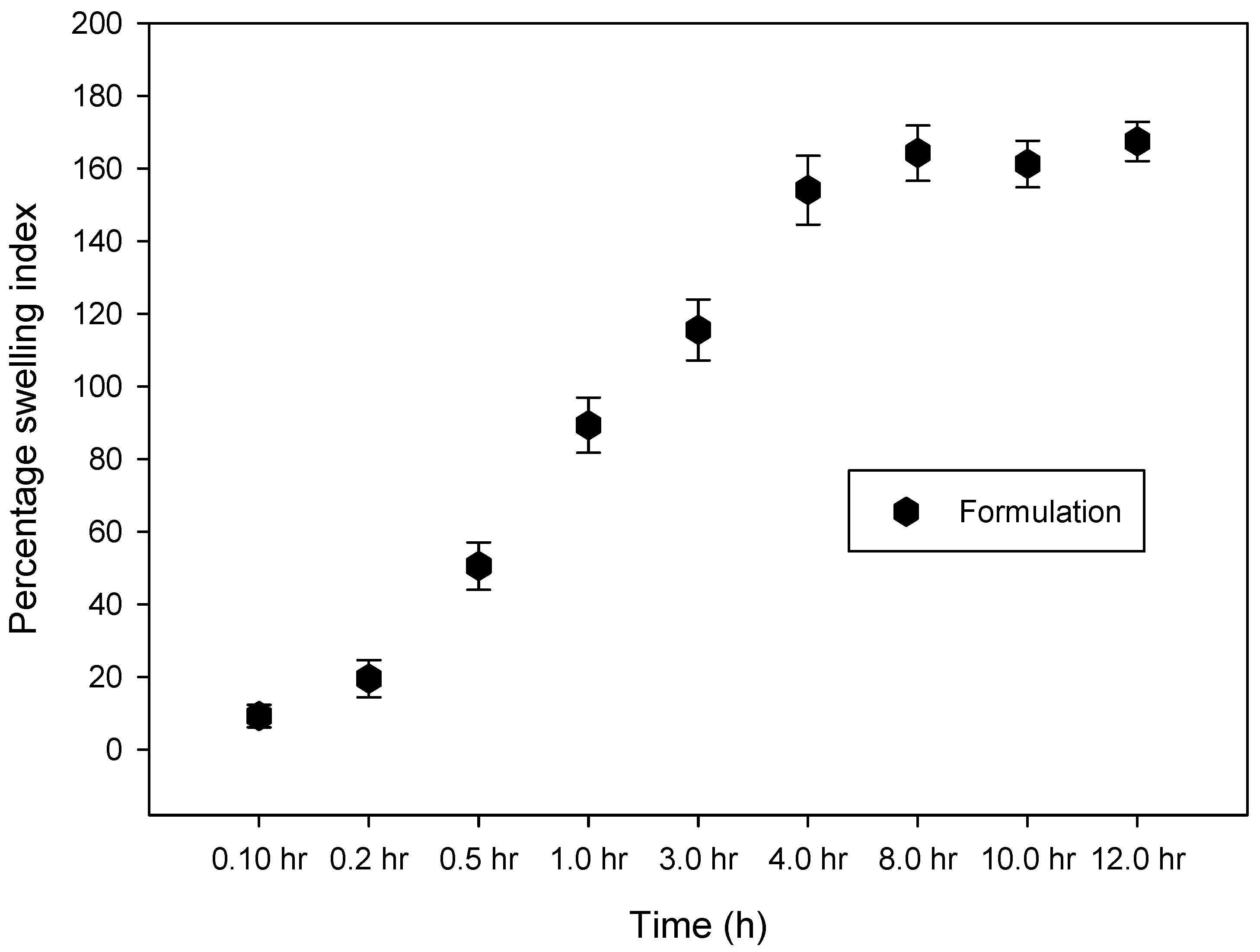
2.2.3. Swelling Measurement
2.2.4. Production Yield
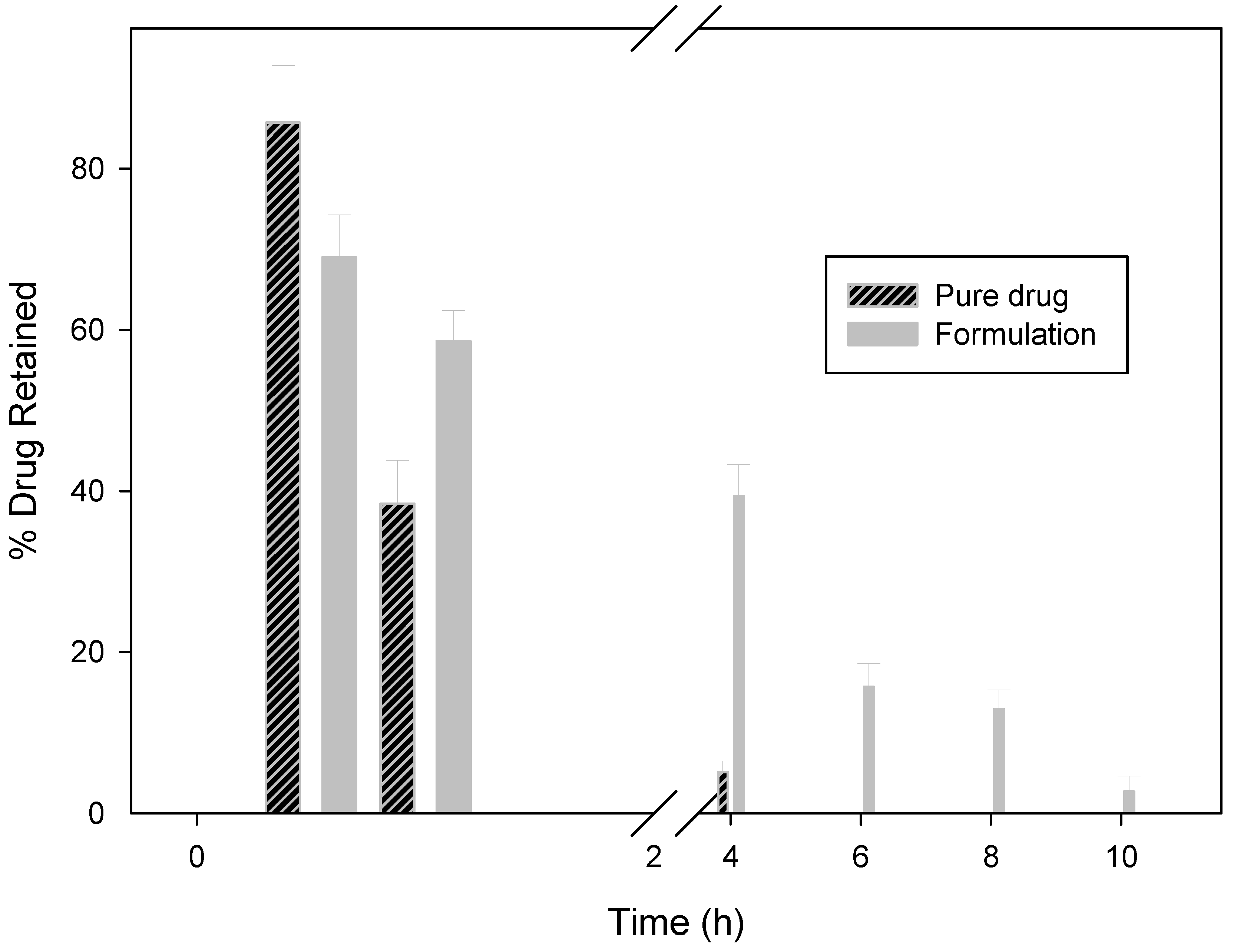
2.2.5. In Vitro Wash-off Test for Nanoparticles
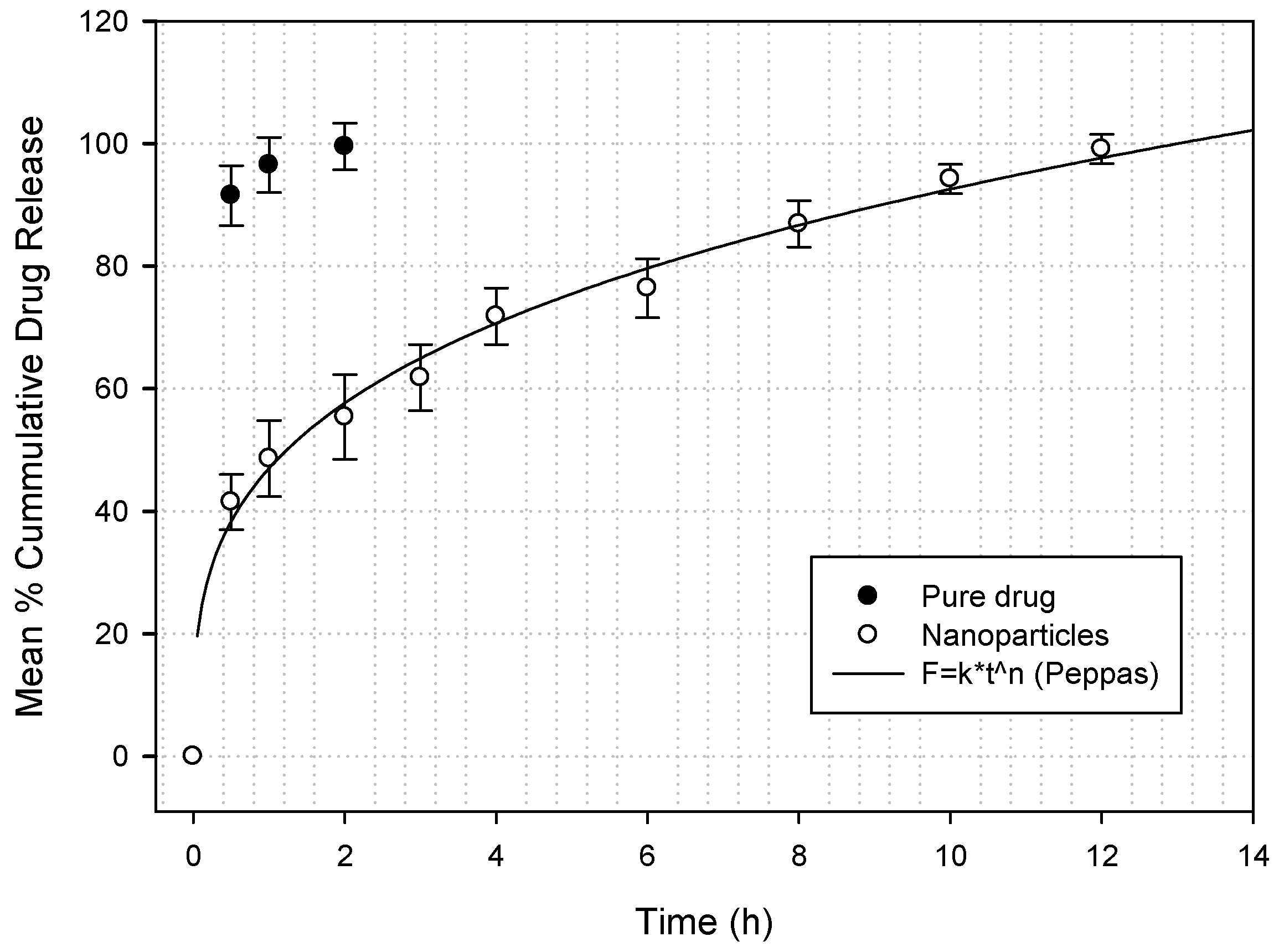
2.2.6. Nanoparticle Sitagliptin Drug Release
2.2.7. Drug Distribution in GIT
2.2.8. Data Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Acknowledgement
Conflicts of Interest
References
- Sivaprasad, S.; Gupta, B.; Crosby-Nwaobi, R.; Evans, J. Prevalence of Diabetic Retinopathy in Various Ethnic Groups: A Worldwide Perspective. Surv. Ophthalmol. 2012, 57, 347–370. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.-Q.; Li, M.; Zhu, F.; Liu, F.-L.; Huang, J.-B. Inhibitory potential of trilobatin from Lithocarpus polystachyus Rehd against α-glucosidase and α-amylase linked to type 2 diabetes. Food Chem. 2011, 130, 261–266. [Google Scholar] [CrossRef]
- Kikuchi, M.; Haneda, M.; Koya, D.; Tobe, K.; Onishi, Y.; Couturier, A.; Mimori, N.; Inaba, Y.; Goodman, M. Efficacy and tolerability of vildagliptin as an add-on to glimepiride in Japanese patients with Type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2010, 89, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Boutayeb, A.; Boutayeb, S. The burden of non communicable diseases in developing countries. Int. J. Equity Health 2005, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Farag, Y.M.K.; Gaballa, M.R. Diabesity: An overview of a rising epidemic. Nephrol. Dial. Transpl. 2011, 26, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Kheir, N.; Zaidan, M.; Younes, H.; Hajj, M.E.; Wilbur, K.; Jewesson, P.J. Pharmacy Education and Practice in 13 Middle Eastern Countries. Am. J. Pharm. Educ. 2009, 72, 133. [Google Scholar] [CrossRef]
- Elhadd, T.A.; Al-Amoudi, A.A.; Alzahrani, A.S. Epidemiology, clinical and complications profile of diabetes in Saudi Arabia: A review. Am. J. Pharm. Educ. 2007, 27, 241–250. [Google Scholar]
- Al-Khader, A.A. Impact of diabetes in renal diseases in Saudi Arabia. Nephrol Dial Transplant. 2001, 16, 2132–2135. [Google Scholar]
- Kikuchi, M.; Abe, N.; Kato, M.; Terao, S.; Mimori, N.; Tachibana, H. Vildagliptin dose-dependently improves glycemic control in Japanese patients with type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2009, 83, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Ahrén, B.; Schweizer, A.; Dejager, S.; Villhauer, E.B.; Dunning, B.E.; Foley, J.E. Mechanisms of action of the dipeptidyl peptidase-4 inhibitor vildagliptin in humans. Diabetes Obes. Metab. 2011, 13, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Noh, Y.-H.; Lim, H.-S.; Jin, S.-J.; Kim, M.J.; Kim, Y.H.; Sung, H.R.; Choi, H.Y.; Bae, K.-S. Effects of Ketoconazole and Rifampicin on the Pharmacokinetics of Gemigliptin, a Dipeptidyl Peptidase-IV Inhibitor: A Crossover Drug-Drug Interaction Study in Healthy Male Korean Volunteers. Clin. Ther. 2012, 34, 1182–1194. [Google Scholar] [CrossRef] [PubMed]
- Holst, J.J.; Deacon, C.F. Inhibition of the activity of dipeptidyl-peptidase IV as a treatment for type 2 diabetes. Diabetes 1998, 47, 1663–1670. [Google Scholar] [CrossRef] [PubMed]
- Seewoodhary, J.; Bain, S.C. Novel treatments for type 2 diabetes. Br. J. Gen. Pract. 2011, 61, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Samanta, M.; Raichur, A. Dual-Drug Delivery System Based on In Situ Gel-Forming Nanosuspension of Forskolin to Enhance Antiglaucoma Efficacy. AAPS PharmSciTech 2010, 11, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Chakkal, S.K.; Ahuja, N. Formulation and Optimization of Controlled Release Mucoadhesive Tablets of Atenolol Using Response Surface Methodology. AAPS Pharm Sci Tech 2006, 7, E19–E28. [Google Scholar] [CrossRef] [PubMed]
- Ponchel, G.; Irache, J.-M. Specific and non-specific bioadhesive particulate systems for oral delivery to the gastrointestinal tract. Adv. Drug Deliv. Rev. 1998, 34, 191–219. [Google Scholar] [CrossRef]
- Harsha, N.S.; Rani, R.H.S. Drug targeting to lungs by way of microspheres. Arch. Pharm. Res. 2006, 29, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Harsha, S.; Attimard, M.; Khan, T.A.; Nair, A.B.; Aldhubiab, B.E.; Sangi, S.; Shariff, A. Design and formulation of mucoadhesive microspheres of sitagliptin. J. Microencapsul. 2013, 30, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Khonsari, F.; Zakeri-Milani, P.; Jelvehgari, M. Formulation and Evaluation of In-vitro Characterization of Gastic-Mucoadhesive Microparticles/Discs Containing Metformin Hydrochloride. Iran. J. Pharm. Res. 2014, 13, 67–80. [Google Scholar] [PubMed]
- Singh, R.; Lillard, J.W. Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol. 2009, 86, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Dhaliwal, S.; Jain, S.; Hardevinder, P.S.; Tiwary, A.K. Mucoadhesive Microspheres for Gastroretentive Delivery of Acyclovir In Vitro and In Vivo Evaluation. AAPS J. 2004, 10, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Lu, Y.; Sun, Y.; Gu, B.; Lu, W.; Pan, J. Development of mucoadhesive microspheres of acyclovir with enhanced bioavailability. Int. J. Pharm. 2009, 378, 30–36. [Google Scholar] [CrossRef] [PubMed]
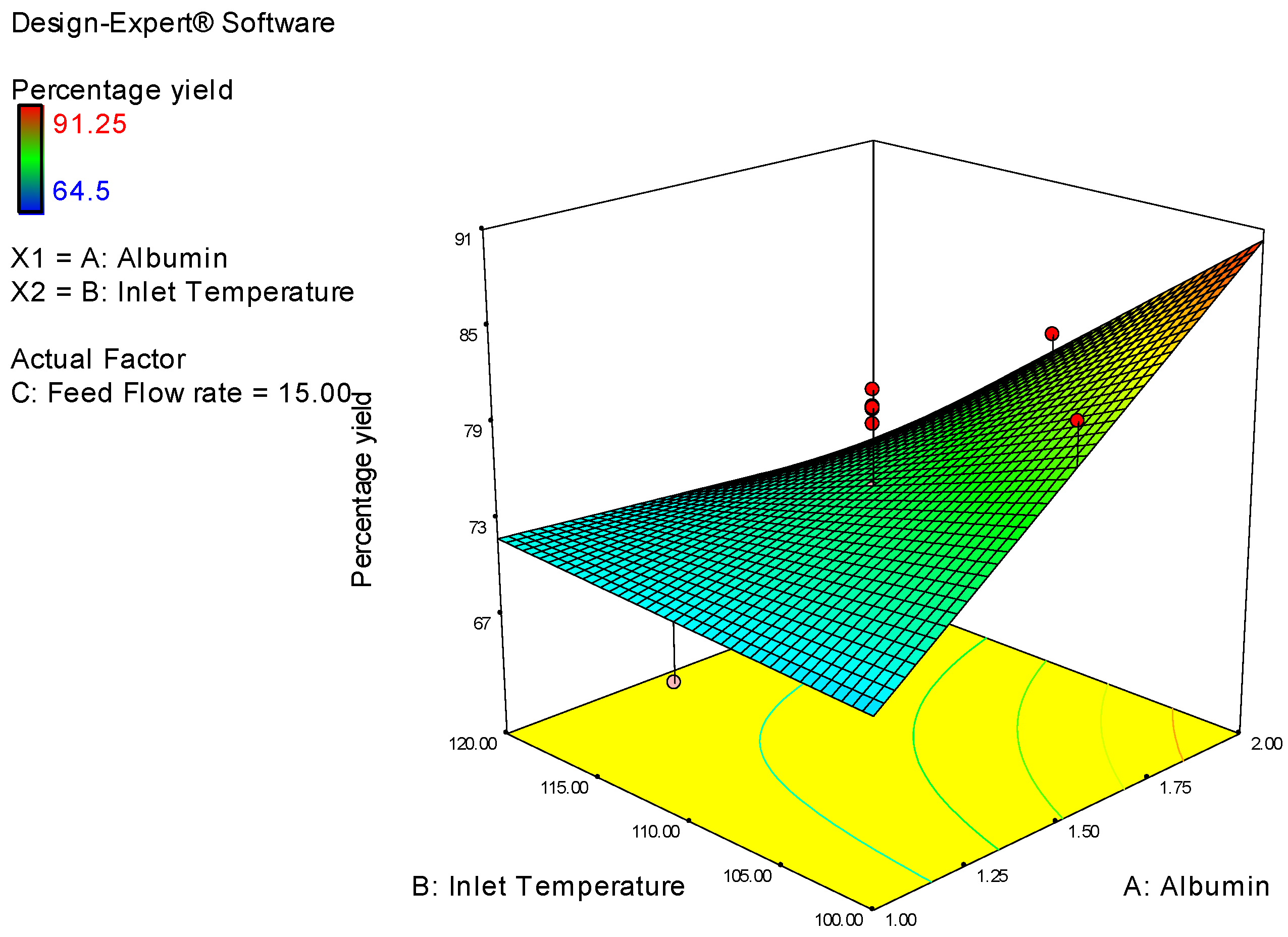
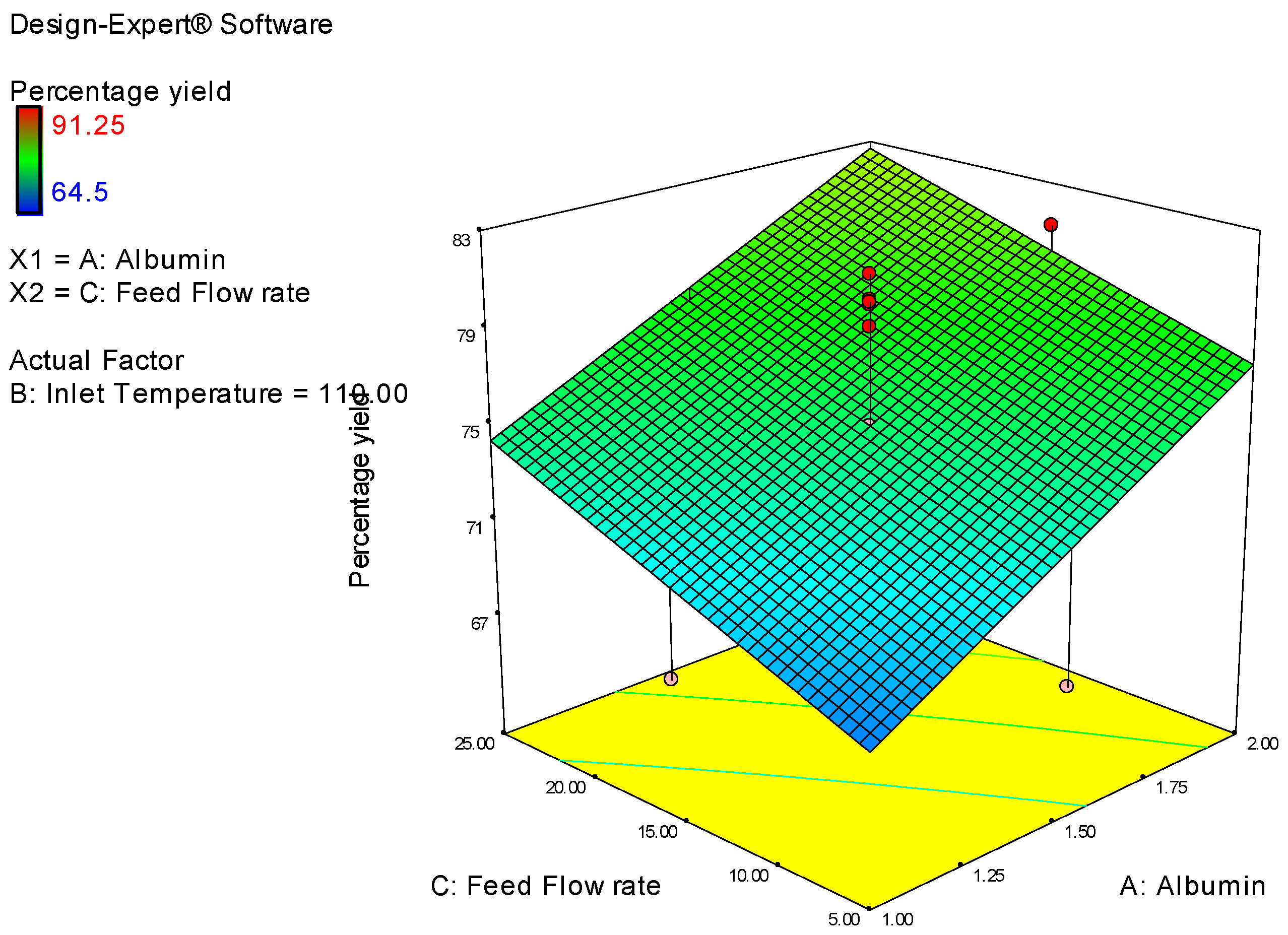
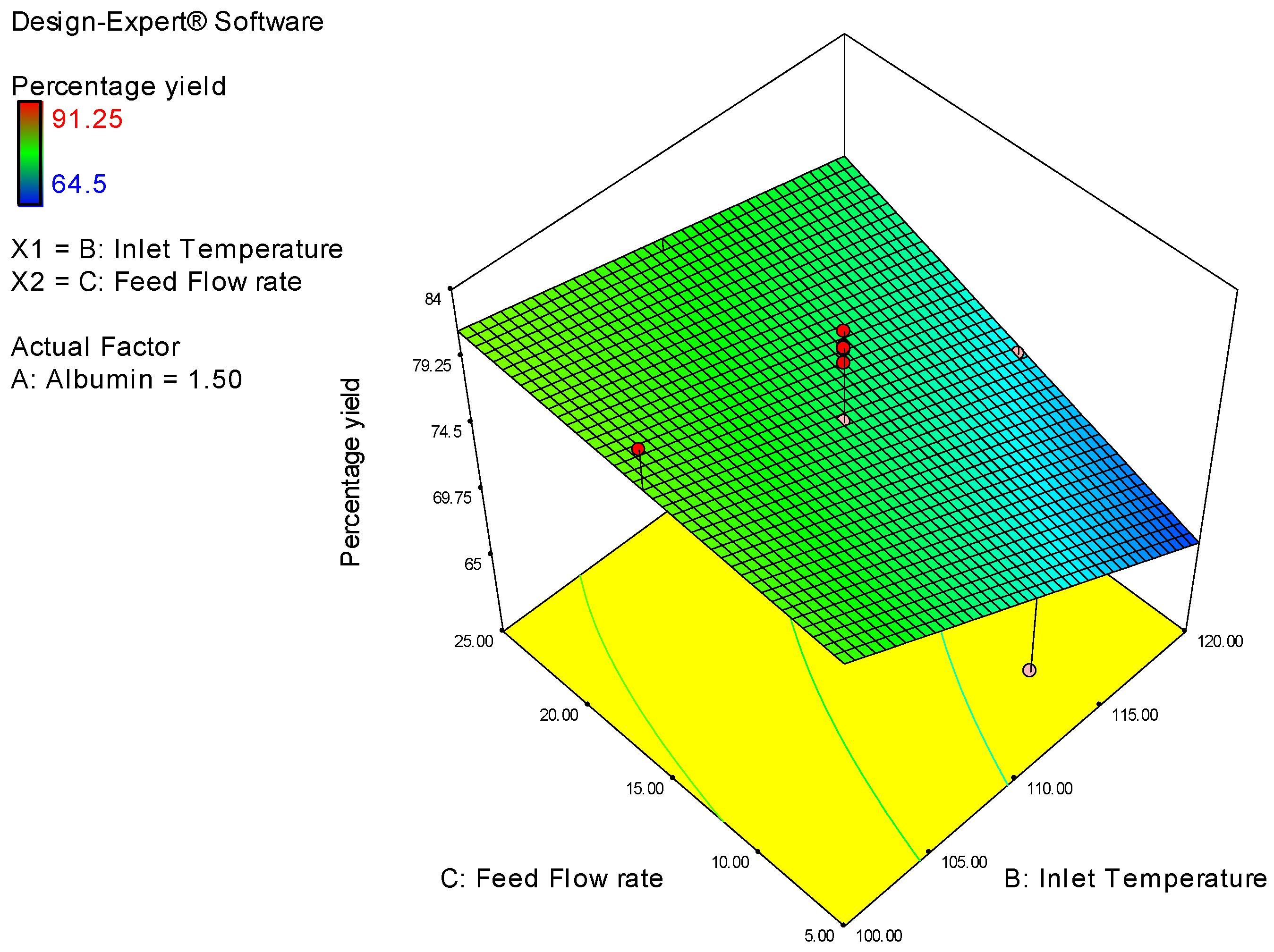

| Study Type | Response Surface | Runs | 20 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Initial Design | Central Composite | Blocks | No Blocks | ||||||
| Design Model | Quadratic | ||||||||
| Factor | Name | Units | Type | Low Actual | High Actual | Low Coded | High Coded | Mean | Std. Dev. |
| A | Albumin Conc | % | Numeric | 1 | 2 | −1 | 1 | 1.5 | 0.353 |
| B | Inlet Temperature | degree | Numeric | 100 | 120 | −1 | 1 | 110 | 7.071 |
| C | Feed Flow rate | % | Numeric | 5 | 25 | −1 | 1 | 15 | 7.071 |
| Response | Name | Units | Obs | Analysis | Minimum | Maximum | Mean | Std. Dev. | Ratio |
| Y1 | Percentage yield | % | 20 | Polynomial | 64.5 | 91.25 | 75.658 | 7.25 | 1.41 |
| Run | Design Point | Albumin Conc. | Inlet Temp. | Flow rate | Percentage Yield |
|---|---|---|---|---|---|
| 1 | Fact | 1 | 100 | 5 | 70.15 |
| 5 | Fact | 1 | 120 | 5 | 64.5 |
| 8 | Fact | 1 | 100 | 25 | 69.5 |
| 19 | Fact | 1 | 120 | 25 | 79.5 |
| 15 | Axial | 1 | 110 | 15 | 67.4 |
| 18 | Axial | 1.5 | 100 | 15 | 83.1 |
| 10 | Axial | 1.5 | 120 | 15 | 70.32 |
| 14 | Axial | 1.5 | 110 | 5 | 67.1 |
| 6 | Axial | 1.5 | 110 | 25 | 70.1 |
| 20 | Center | 1.5 | 110 | 15 | 75 |
| 3 | Center | 1.5 | 110 | 15 | 80 |
| 7 | Center | 1.5 | 110 | 15 | 81.24 |
| 12 | Center | 1.5 | 110 | 15 | 79.1 |
| 2 | Center | 1.5 | 110 | 15 | 80.2 |
| 11 | Center | 1.5 | 110 | 15 | 80.1 |
| 4 | Fact | 2 | 100 | 5 | 85.7 |
| 13 | Fact | 2 | 120 | 5 | 65.8 |
| 16 | Fact | 2 | 100 | 25 | 91.25 |
| 9 | Fact | 2 | 120 | 25 | 71.9 |
| 17 | Axial | 2 | 110 | 15 | 81.2 |
| Percentage Yield | = |
|---|---|
| 75.658 | |
| 4.48 | * A |
| −4.768 | * B |
| 2.9 | * C |
| −5.45 | * A * B |
| −0.3375 | * A * C |
| 2.025 | * B * C |
| Albumin Conc. g | Inlet Temp. °C | Flow Rate (Rounded to 0.5) mL/h | Predicted | Actual | |
|---|---|---|---|---|---|
| Suggestion-1 | 2 | 106.65 | 25 | 85.45 | 84.51 |
| Suggestion-2 | 2 | 106.56 | 25 | 85.52 | 85.12 |
| Suggestion-3 | 2 | 106.75 | 25 | 85.37 | 84.91 |
| Suggestion-4 | 2 | 106.56 | 24.71 | 85.46 | 84.60 |
| Suggestion-5 | 1.99 | 106.69 | 25 | 85.35 | 86.1 |
| Suggestion-6 | 1.99 | 106.59 | 25 | 85.42 | 87.1 |
| Suggestion-7 | 2 | 106.73 | 24.44 | 85.27 | 85.12 |
| Suggestion-8 | 1.99 | 106.43 | 25 | 85.49 | 85.46 |
| Suggestion-9 | 2 | 107.71 | 25 | 84.57 | 83.98 |
| Suggestion-10 | 2 | 106.66 | 24.08 | 85.27 | 84.69 |
| Source | Sum of Squares | df | Mean Square | F Value | Prob > F |
|---|---|---|---|---|---|
| Model | 783.4785 | 6 | 130.5797 | 6.313662 | 0.0027 |
| A-Albumin Conc | 200.704 | 1 | 200.704 | 9.70424 | 0.0082 |
| B-Inlet Temperature | 227.3382 | 1 | 227.3382 | 10.99203 | 0.0056 |
| C-Feed Flow Rate | 84.1 | 1 | 84.1 | 4.066319 | 0.0649 |
| AB | 237.62 | 1 | 237.62 | 11.48917 | 0.0048 |
| AC | 0.91125 | 1 | 0.91125 | 0.04406 | 0.8370 |
| BC | 32.805 | 1 | 32.805 | 1.586155 | 0.2300 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haq Asif, A.; Harsha, S.; Hodalur Puttaswamy, N.; E. Al-Dhubiab, B. An Effective Delivery System of Sitagliptin Using Optimized Mucoadhesive Nanoparticles. Appl. Sci. 2018, 8, 861. https://doi.org/10.3390/app8060861
Haq Asif A, Harsha S, Hodalur Puttaswamy N, E. Al-Dhubiab B. An Effective Delivery System of Sitagliptin Using Optimized Mucoadhesive Nanoparticles. Applied Sciences. 2018; 8(6):861. https://doi.org/10.3390/app8060861
Chicago/Turabian StyleHaq Asif, Afzal, Sree Harsha, Niranjan Hodalur Puttaswamy, and Bandar E. Al-Dhubiab. 2018. "An Effective Delivery System of Sitagliptin Using Optimized Mucoadhesive Nanoparticles" Applied Sciences 8, no. 6: 861. https://doi.org/10.3390/app8060861
APA StyleHaq Asif, A., Harsha, S., Hodalur Puttaswamy, N., & E. Al-Dhubiab, B. (2018). An Effective Delivery System of Sitagliptin Using Optimized Mucoadhesive Nanoparticles. Applied Sciences, 8(6), 861. https://doi.org/10.3390/app8060861






