Microstructure and Mechanical Properties of the Dactylopodites of the Chinese Mitten Crab (Eriocheir sinensis)
Abstract
:1. Introduction
2. Experimental Methods
2.1. Preparation of Dactylopodite Samples from Chinese Mitten Crabs
2.2. Biological Characteristics of Chinese Mitten Crabs
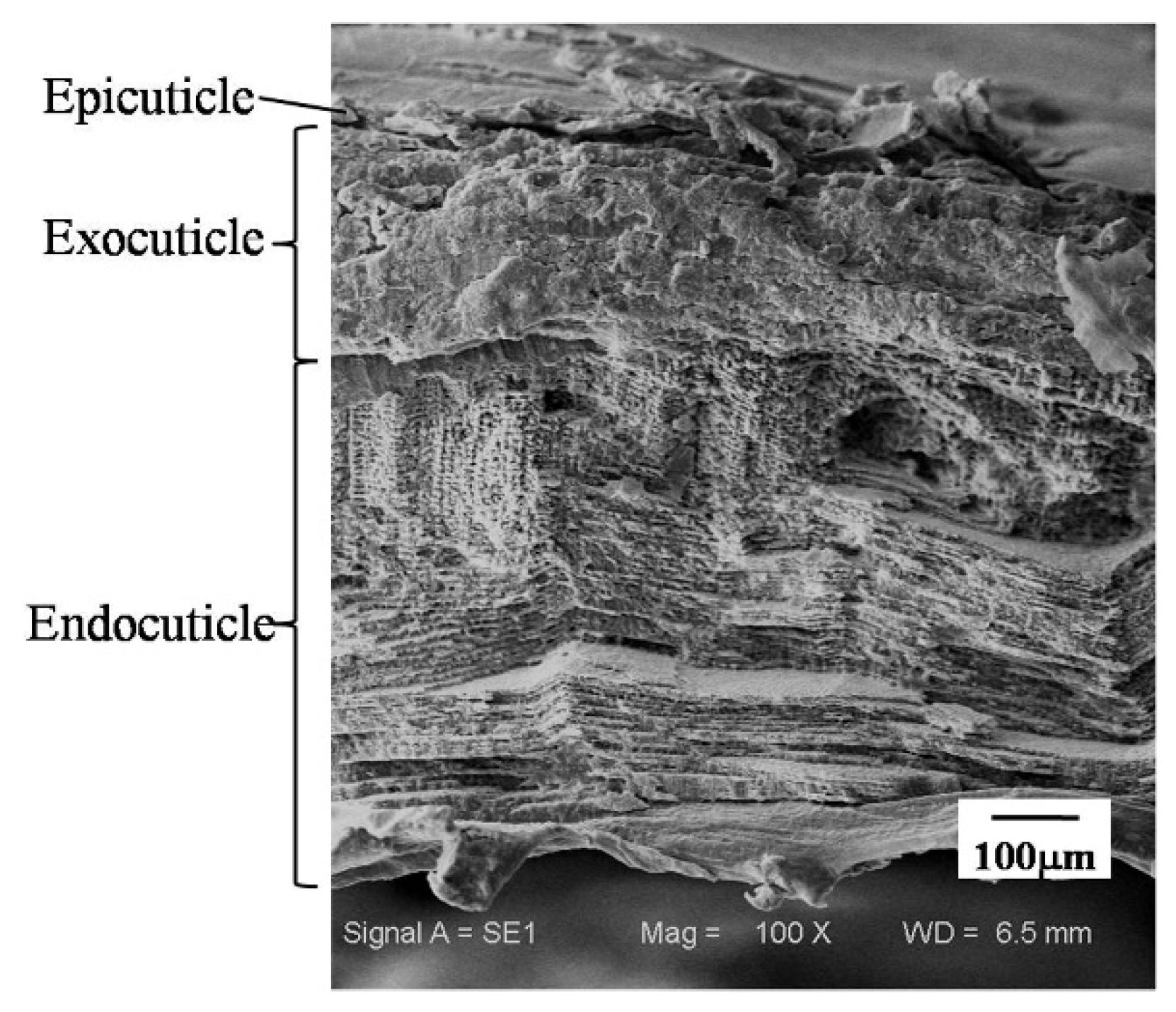
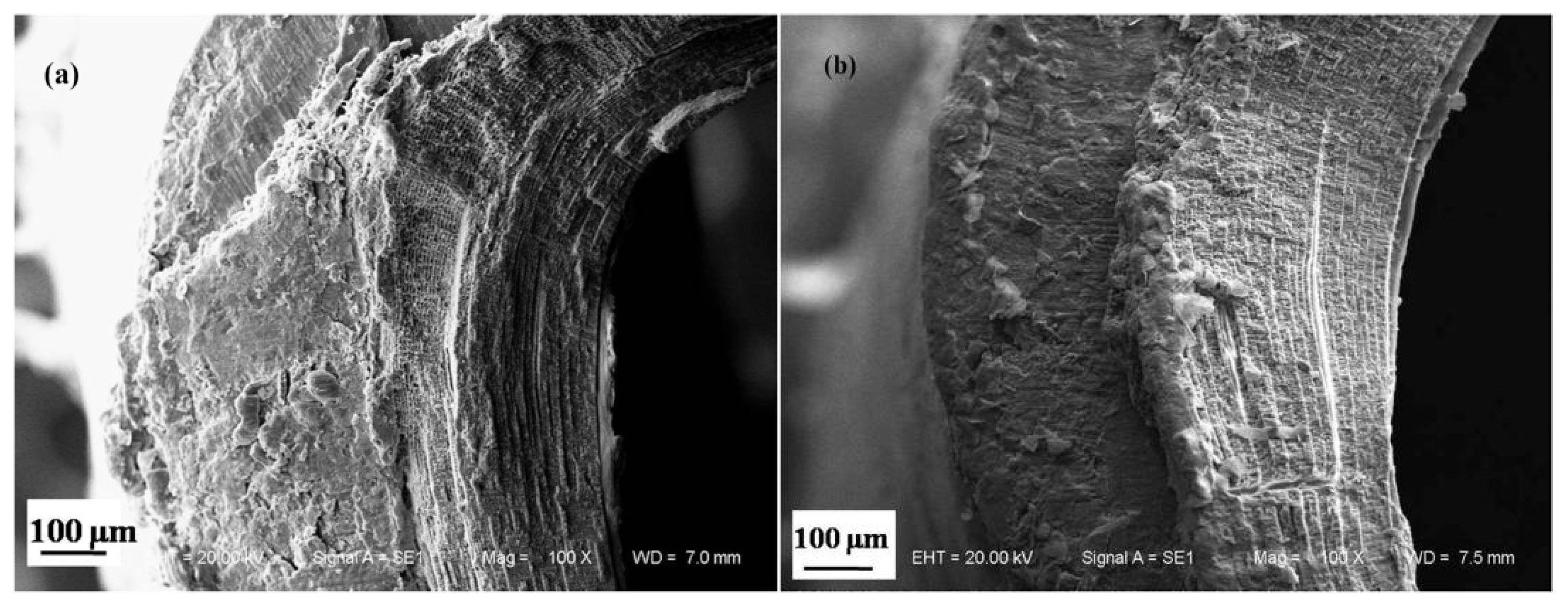
2.2.1. Microstructure of the Dactylopodites
2.2.2. Composition of the Dactylopodites
2.3. Three-Point Bending Tests
3. Results and Discussion
3.1. Structural Property
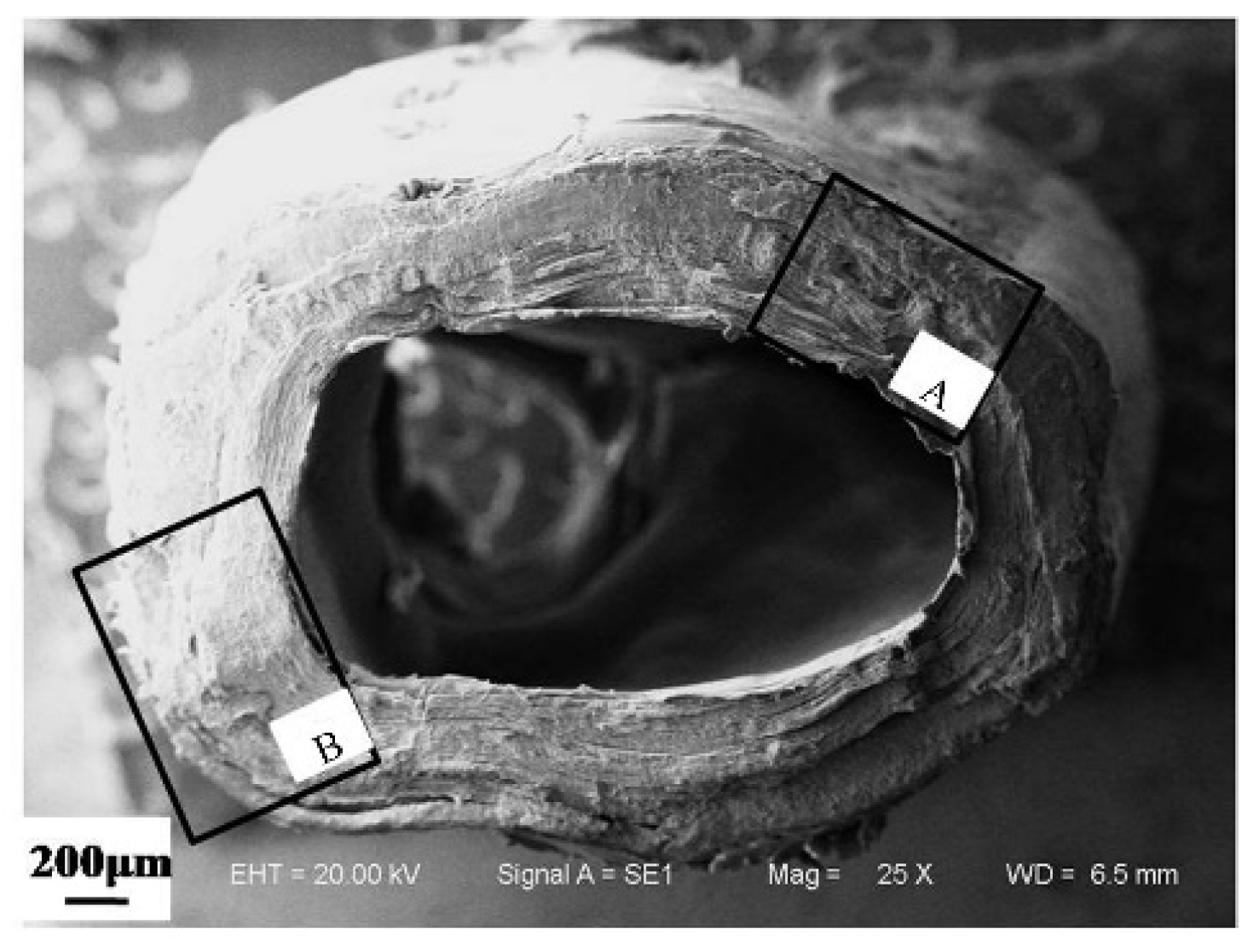
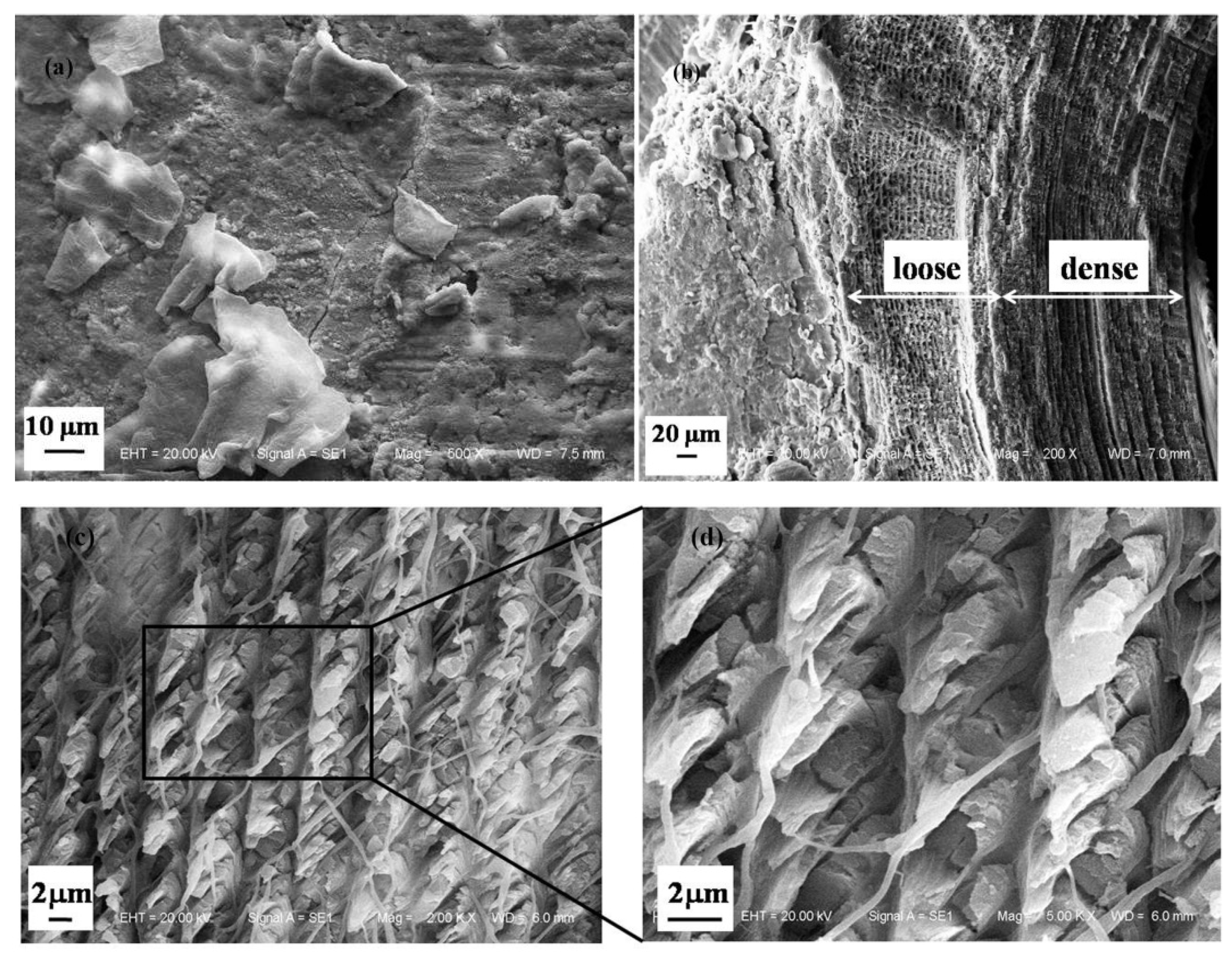
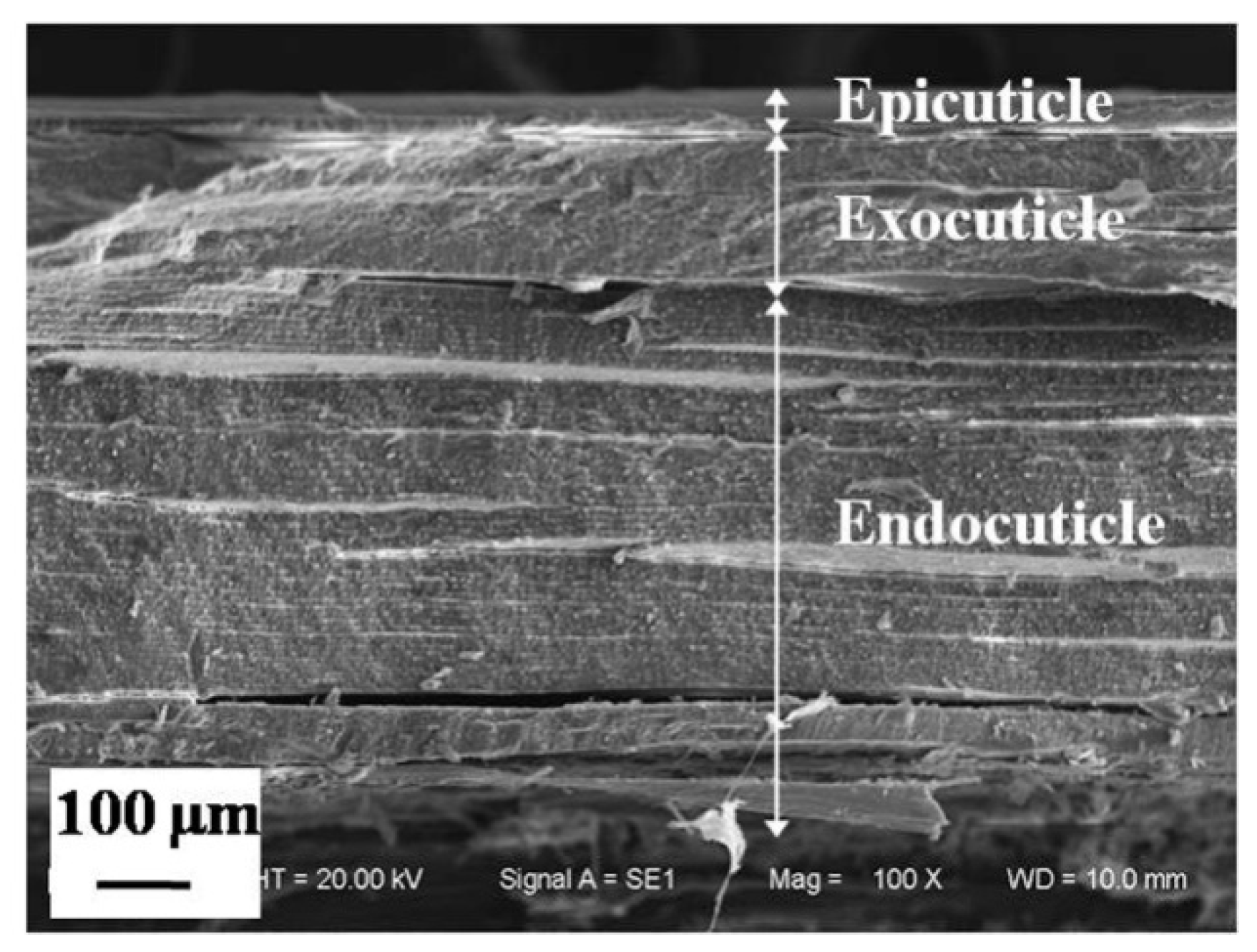
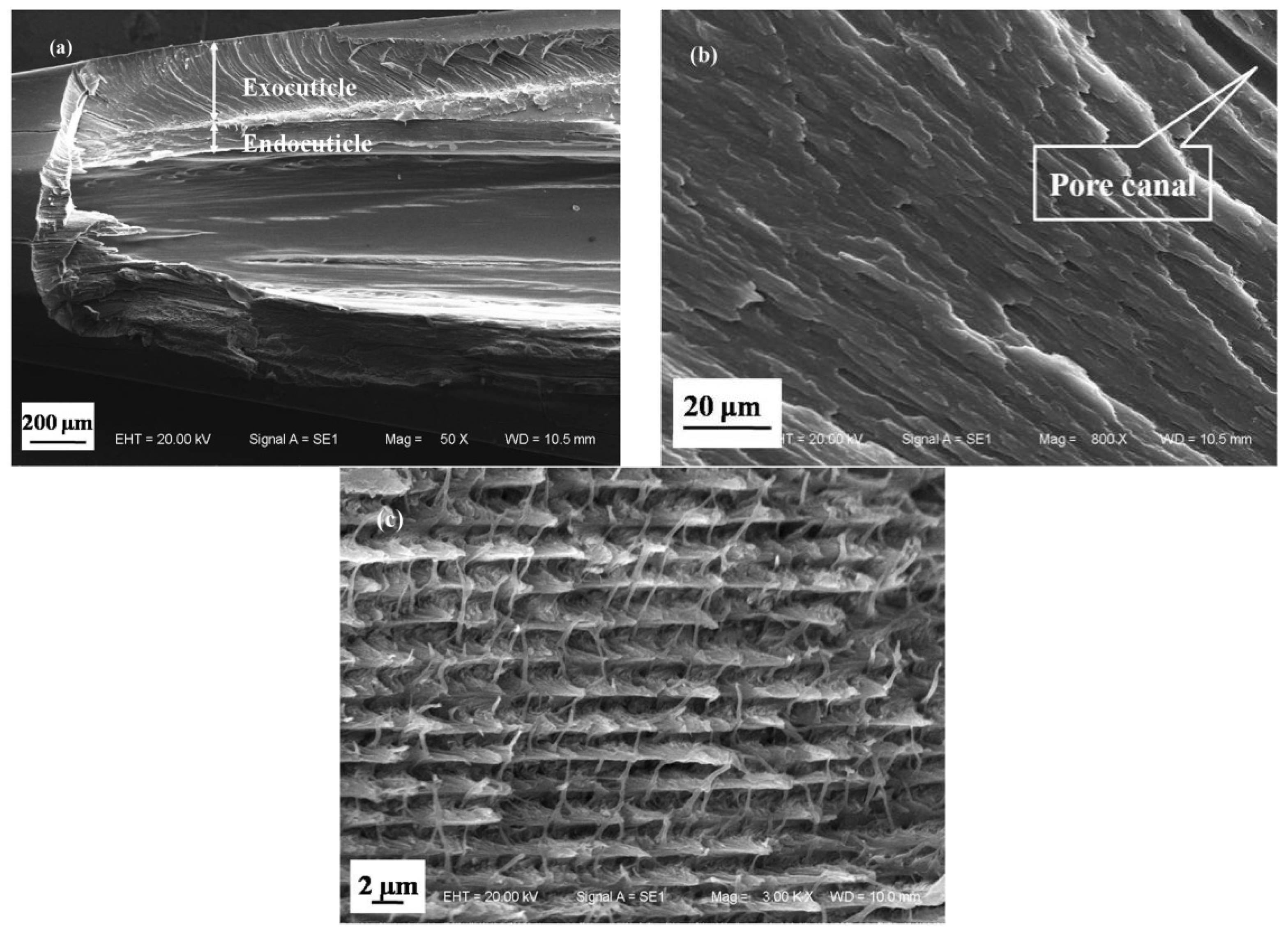
3.1.1. Microstructure of Cross Section of a Dactylopodite
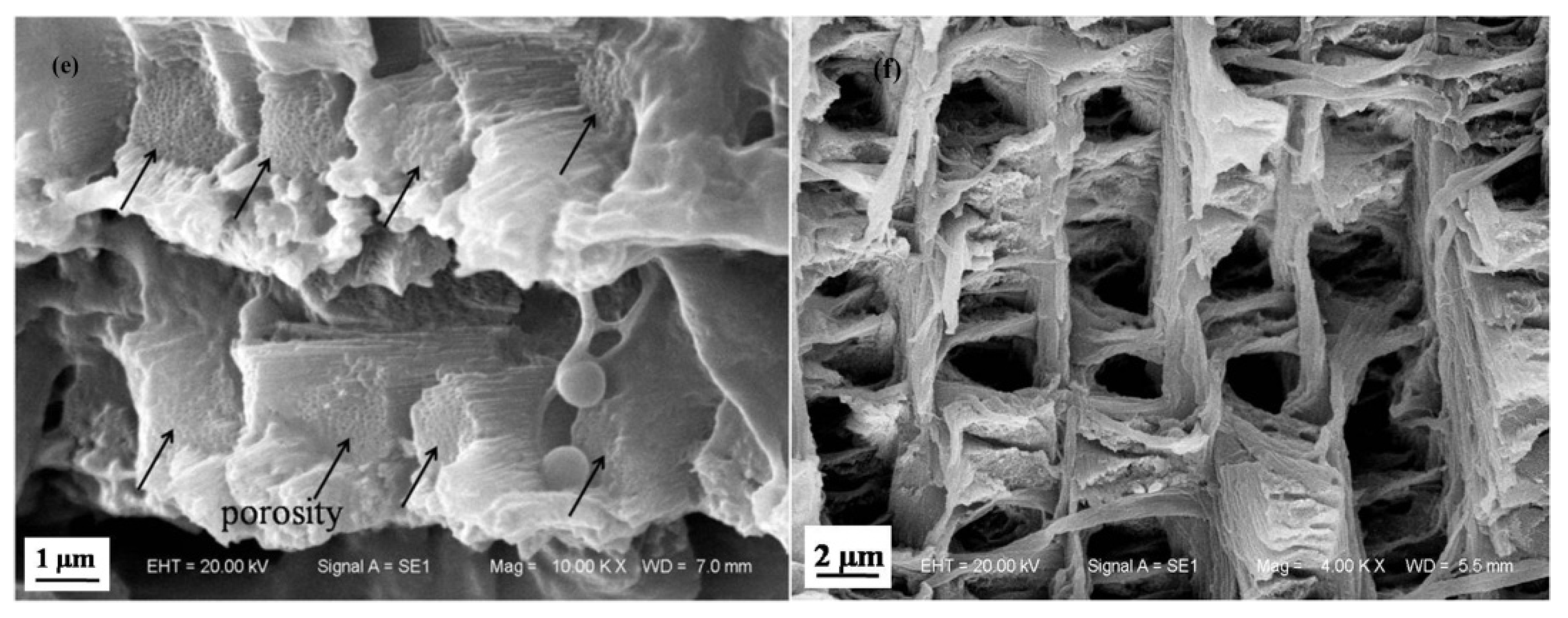
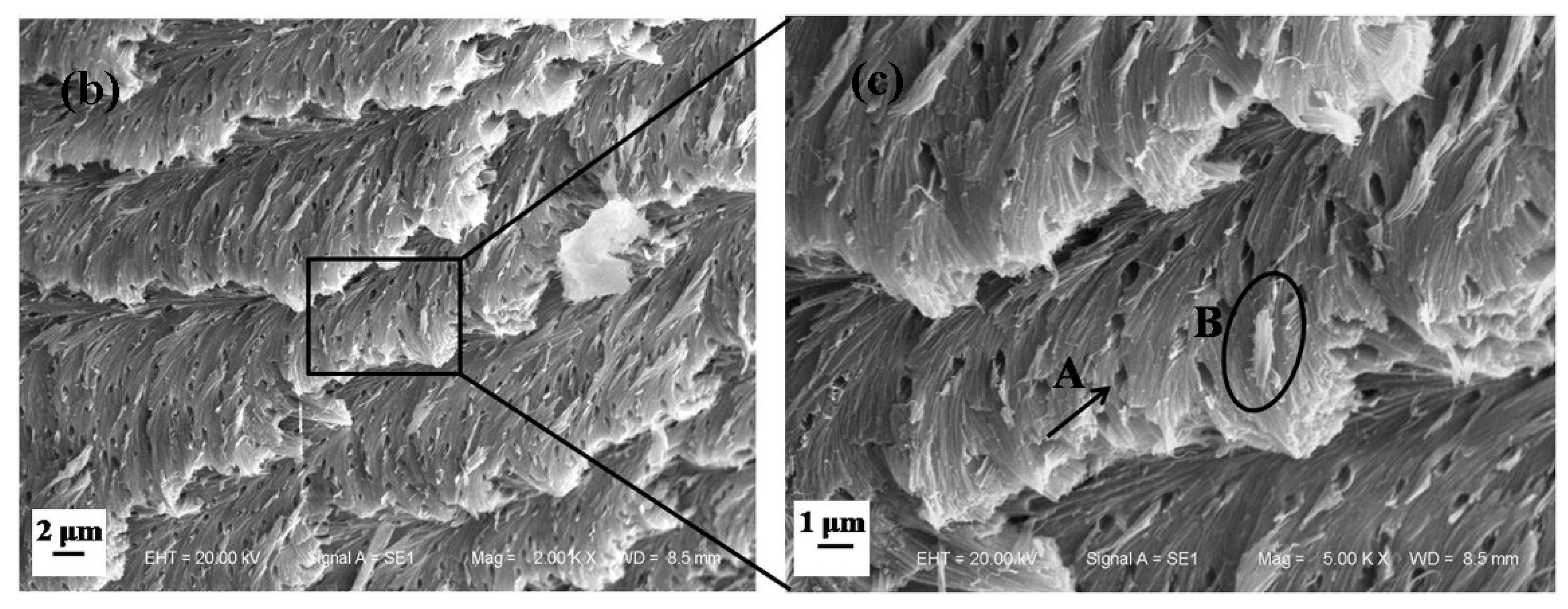
3.1.2. Microstructure of Longitudinal Section of Dactylopodite
3.2. Material Properties
3.3. Mechanical Properties
4. Conclusions
- The exoskeleton of the dactylopodites of Chinese mitten crab is multilayered, with an epicuticle, exocuticle and endocuticle. The thickness ratio of each layer is related to the section diameter and the concave and convex aspects of the profile. As the cross-sectional diameter decreases, the thicknesses of the exocuticle and endocuticle reduce and increase, respectively. For a given section, the thickness of the concave area is less than that of the convex area.
- Cross sections and longitudinal sections of the endocuticle of the exoskeleton of the dactylopodites reveal a Bouligand structure, which benefits their mechanical properties. Each layer of the fibrous layer consists of several regularly arranged fiber bundles. Many pore canals are distributed regularly in the fiber layer, in which pore canal tubules transport nutrients while increasing the toughness of the inner epidermis.
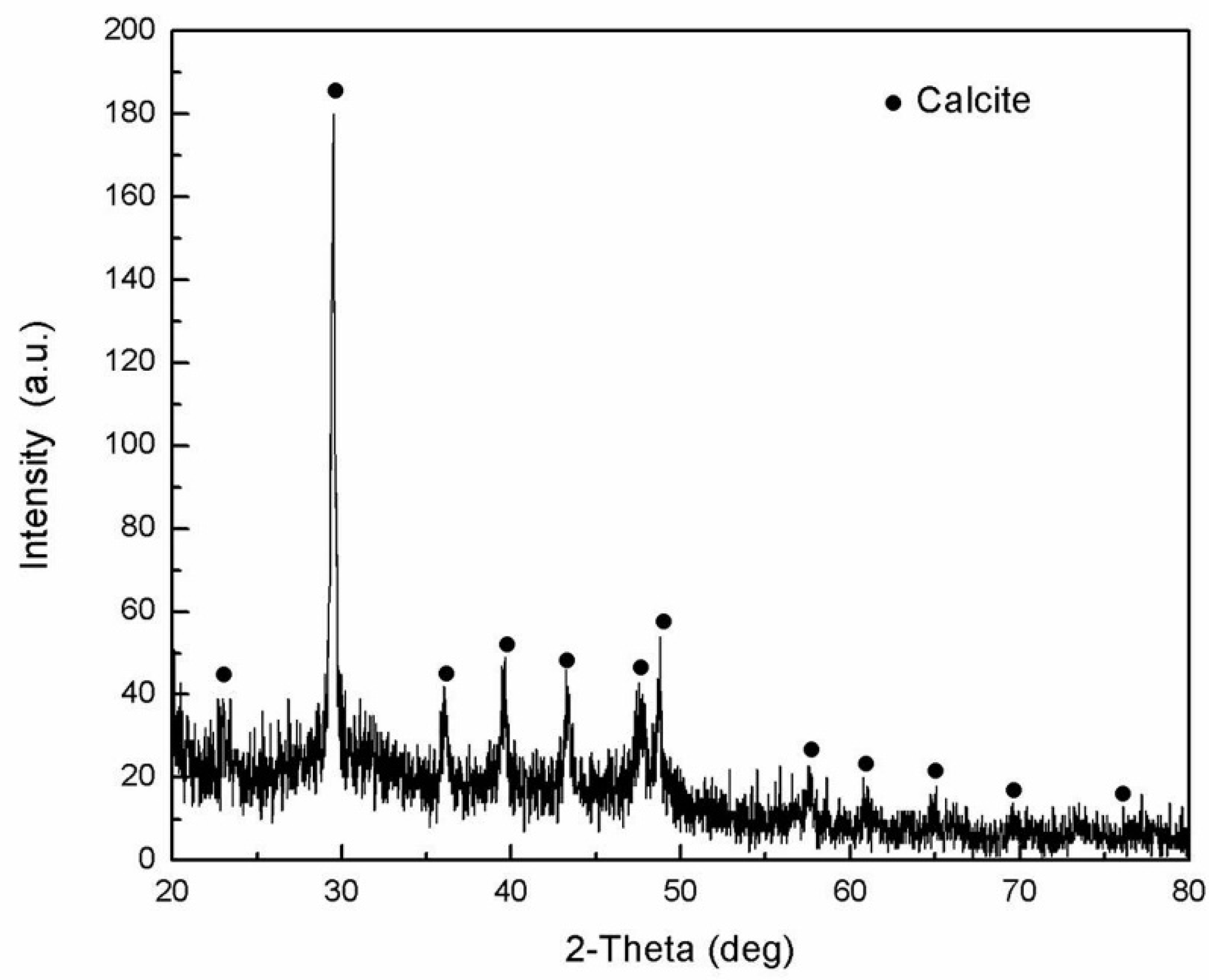
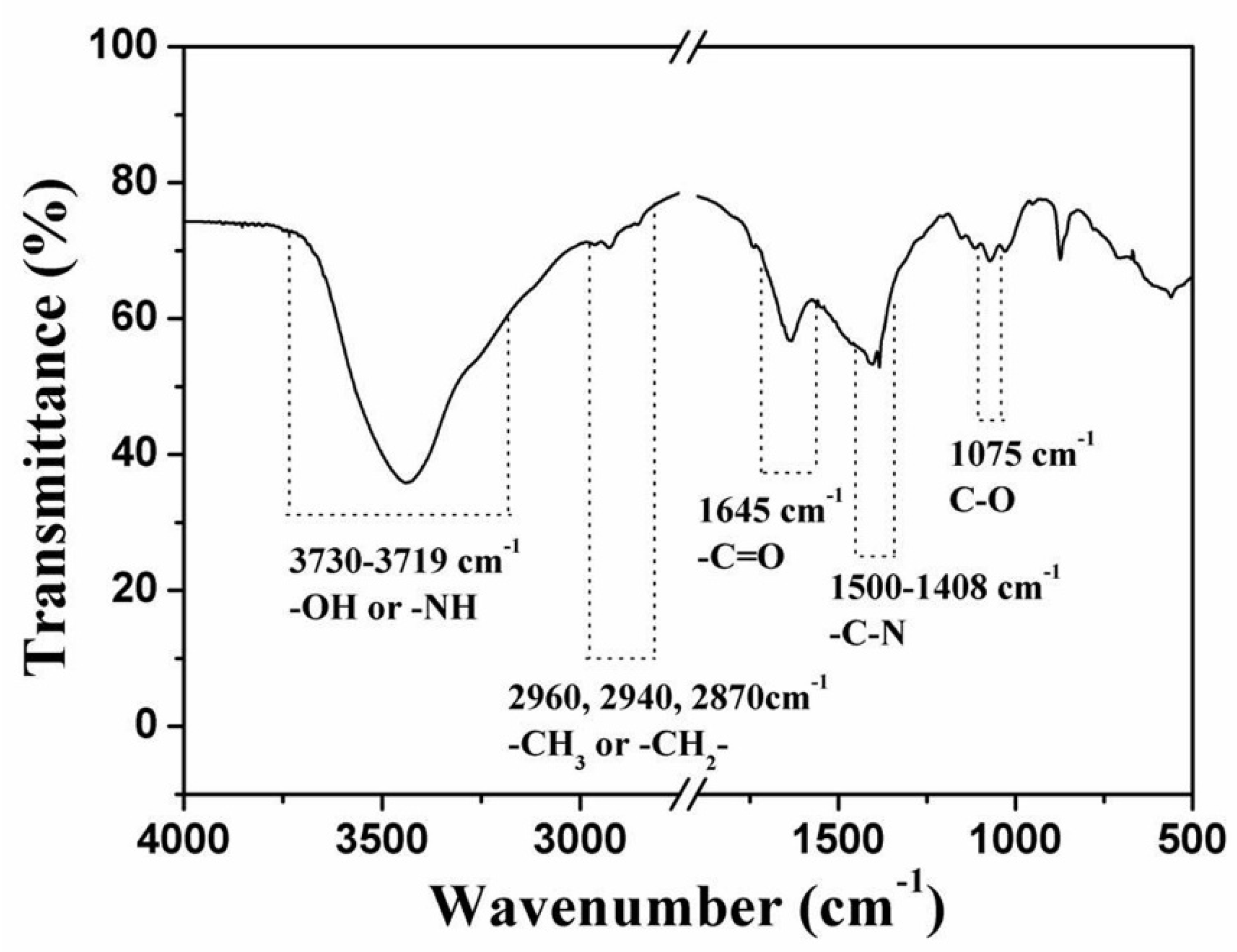
- The main organic constituents of the exoskeleton of the dactylopodites are chitin and protein, and the major inorganic compound is CaCO3 crystallized as calcite.
- Due to their structure and composition, the dry dactylopodites had characteristics of brittle materials, whereas the wet dactylopodites had characteristics of ductile materials.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, G.B.; Chen, D.S.; Chen, K.W.; Zhang, Z.Q. The current research status and development strategy on biomimetic robot. J. Mech. Eng. 2015, 51, 27–44. [Google Scholar] [CrossRef]
- Zhuang, H.C.; Gao, H.B.; Deng, Z.Q. Analysis method of articulated torque of heavy-duty six-legged robot under its quadrangular gait. Appl. Sci. 2016, 6, 323. [Google Scholar] [CrossRef]
- Yang, W.X.; Zhou, H. Primary study on the histology of exoskeleton of female pleopod in Eriocheir sinensis. Dong Hai Mar. Sci. 2000, 9, 29–33. [Google Scholar]
- Neville, A.C. Biology of the Arthropod Cuticle; Springer: New York, NY, USA, 1975. [Google Scholar]
- Vincent, J.F. Structural Biomaterials; Princet on University Press: Princeton, NJ, USA, 1991. [Google Scholar]
- Vincent, J.F. Arthropod cuticle: A natural composite shell system. Composites A 2002, 33, 1311–1315. [Google Scholar] [CrossRef]
- Herborg, L.M.; Rushton, S.P.; Clare, A.S.; Bentley, M.G. Spread of the Chinese mitten crab (Eriocheir sinensis H. Milne Edwards) in Continental Europe: Analysis of a historical data set. Hydrobiologia 2003, 503, 21–28. [Google Scholar] [CrossRef]
- Clark, P.F.; Rainbow, P.S.; Robbins, R.S. The alien Chinese mitten crab, Eriocheir sinensis (Crustacea: Decapoda: Brachyura), in the thames catchent. J. Mar. Biol. Assoc. UK 1998, 78, 1215–1221. [Google Scholar] [CrossRef]
- Li, J.Q.; Zhang, X.D.; Zou, M.; Zhang, R.; Chirende, B.; Shi, R.Y.; Wei, C.G. An experimental study on the gait patterns and kinematics of Chinese mitten Crabs. J. Bionic Eng. 2013, 10, 305–315. [Google Scholar] [CrossRef]
- Zhang, X.D.; Li, J.Q.; Zou, M.; Zhang, R.; Li, Y.W. Three-dimensional observation and dynamics analysis of Chinese mitten crab’s locomotion on smooth terrain. Trans. Chin. Soc. Agric. Eng. 2013, 29, 30–37. [Google Scholar]
- Li, J.Q.; Zhang, X.D.; Zou, M.; Li, H.; Wang, Y.; Shi, R.Y. 3-D Surveying and gaits analysis of Chinese mitten crab in smooth road. Trans. Chin. Soc. Agric. Eng. 2012, 43, 335–338. [Google Scholar]
- Wang, G.; Zhang, L.X.; Wang, L.Q. Research on a gait planning method for a crab-like octopod robot. J. Harbin Eng. Univ. 2011, 32, 486–491. [Google Scholar]
- Wang, Y.; Li, J.Q.; Li, X.J.; Huang, H.; Qiu, F. Bionic Walking Foot and Mechanical Performance on Soil. Appl. Sci. 2017, 7, 575. [Google Scholar] [CrossRef]
- Elices, M. Structural Biological Materials: Design and Structure–Property Relationships; Pergamon: Elmsford, NY, USA, 2000. [Google Scholar]
- Raabe, D.; Romano, P.; Sachs, C.; Fabritius, H.; Sawalmih, A.; Yi, S.B.; Servos, G.; Hartwig, H.G. Microstructure and crystallographic texture of the chitin–protein network in the biological composite material of the exoskeleton of the lobster Homarus americanus. Mater. Sci. Eng. A 2006, 421, 143–153. [Google Scholar] [CrossRef]
- James, C.; Weaver, G.W.; Milliron, A.M.; Kenneth, E.L.; Steven, H.I.; William, J.M.; Brook, S.P.; Zavattieri, E.D.; David, K. The Stomatopod Dactyl Club: A Formidable Damage-Tolerant Biological Hammer. Science 2012, 336, 8. [Google Scholar]
- Compere, P.; Goffinet, G. Elaboration and ultrastructural-changes in the pore canal system of the mineralized cuticle of Carcinus maenas during the molting cycle. Tissue Cell 1987, 19, 859–875. [Google Scholar] [CrossRef]
- Hegdahl, T.; Silness, J.; Gustavsen, F. The structure and mineralization of the carapace of the crab (Cancer pagurus L)—1. The endocuticle. Zool. Scr. 1977, 6, 89–99. [Google Scholar]
- Roer, R.D. Mechanisms of resorption and deposition of calcium in the carapace of the crab Carcinus maenas. J. Exp. Biol. 1980, 88, 205–218. [Google Scholar]
- Sawalmih, A.; Li, C.; Siegel, S.; Fabritius, H.; Yi, S.; Raabe, D.; Fratzl, P.; Paris, O. Microtexture and chitin/calcite orientation relationship in the mineralized exoskeleton of the American lobster. Adv. Funct. Mater. 2008, 18, 3307–3314. [Google Scholar] [CrossRef]
- Tian, X.M.; Han, Z.W.; Li, X.J.; Pu, Z.G.; Ren, L.Q. Biological coupling anti-wear properties of three typical molluscan shells Scapharca subcrenata, Rapana venosa and Acanthochiton rubrolineatus. Sci. China Technol. Sci. 2010, 53, 2905–2913. [Google Scholar] [CrossRef]
- Liang, Y.H.; Zhao, Q.; Li, X.J.; Zhang, Z.H.; Ren, L.Q. Study of the microstructure and mechanical properties of white clam shell. Micron 2016, 87, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Karaarslan, M.; Baran, T.; Can, E.; Ekemen, G.; Bitim, B.; Duman, F. The quick extraction of chitin from an epizoic crustacean species (Chelonibia patula). Nat. Prod. Res. 2014, 28, 2186–2190. [Google Scholar] [CrossRef] [PubMed]
- Bolat, Y.; Bilgin, S.; Günlü, A.; Izci, L.; Bahadır, K.S.; Çetinkaya, S. Chitin-chitosan yield of freshwater crab (Potamon potamios, Olivier 1804) shell. Pak. Vet. J. 2010, 30, 227–231. [Google Scholar]
- Kaya, M.; Dudakli, F.; Asan, O.M.; Cakmak, Y.S.; Baran, T.; Mentes, A.; Erdogan, S. Porous and nanofiber a-chitosan obtained from blue crab (Callinectes sapidus) tested for antimicrobial and antioxidant activities. LWT-Food Sci. Technol. 2016, 65, 1109–1117. [Google Scholar] [CrossRef]
- Sagheer, F.A.; Sughayer, M.A.; Muslim, S.; Elsabee, M.Z. Extraction and characterization of chitin and chitosan from marine sources in ArabianGulf. Carbohydr. Polym. 2009, 77, 410–419. [Google Scholar] [CrossRef]
- Kaya, M.; Tozak, K.O.; Baran, T.; Sezen, G.; Sargin, I. Natural porous and nano fiber chitin structure from Gammarus argaeus (Gammaridae Crustacea). EXCLI J. 2013, 12, 503–510. [Google Scholar] [PubMed]
- Jiao, D.; Liu, Z.Q.; Qu, R.T.; Zhang, Z.F. Anisotropic mechanical behaviors and their structural dependences of crossed-lamellar structure in a bivalve shell. Mater. Sci. Eng. C 2016, 59, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Hepburn, H.R.; Joffe, I.; Green, N.; Nelson, K.J. Mechanical properties of a crab shell. Comp. Biochem. Physiol. 1975, 50, 551–554. [Google Scholar] [CrossRef]
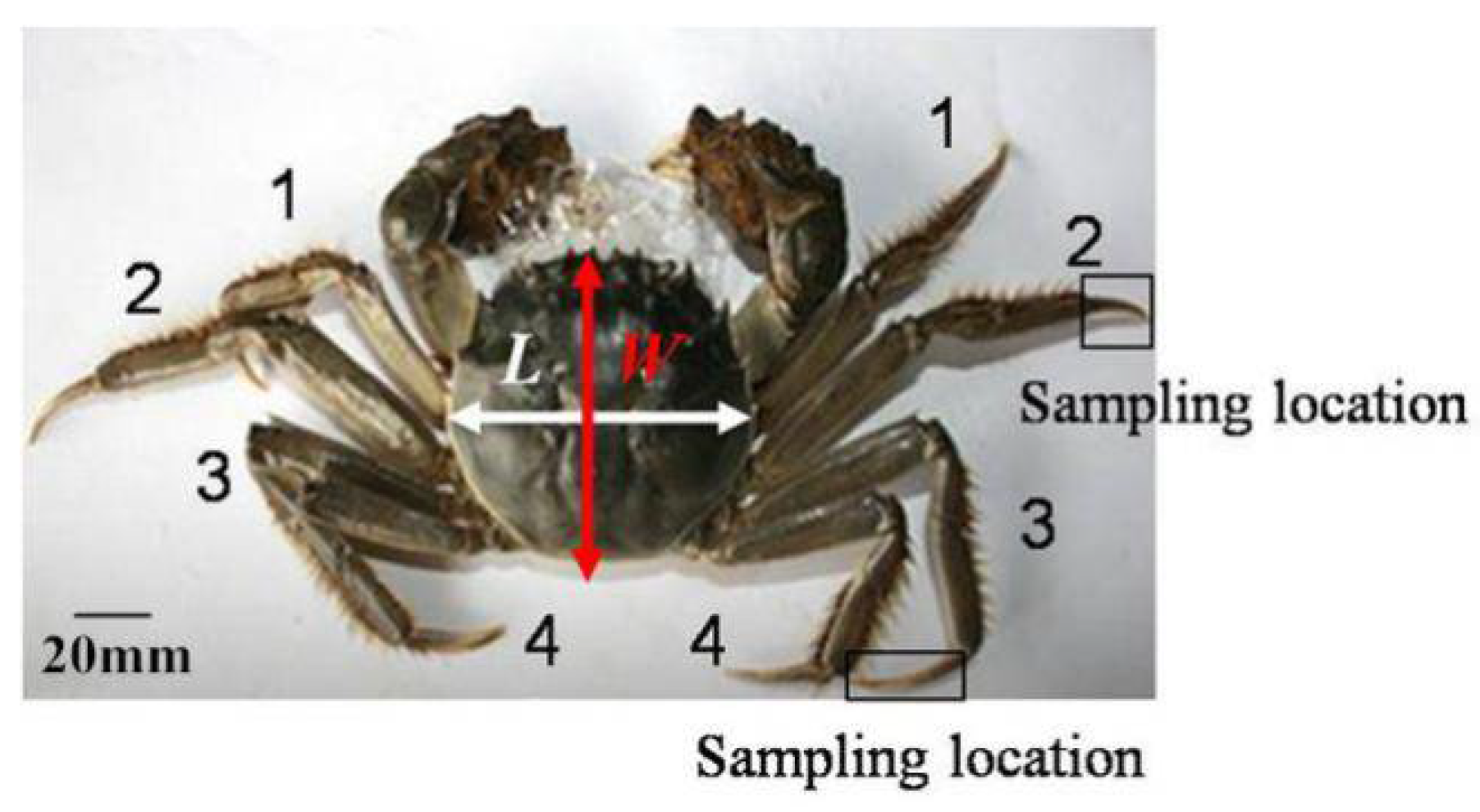
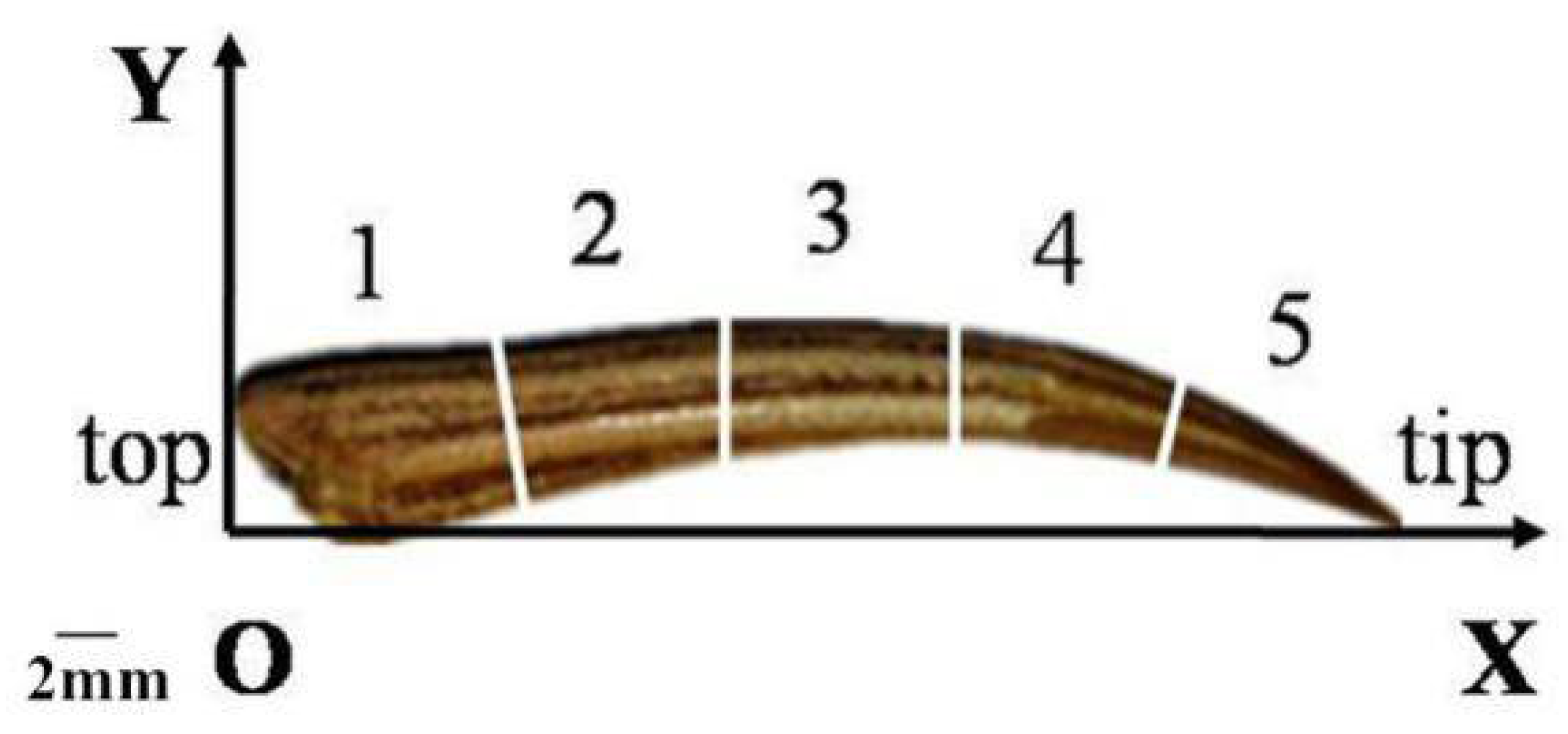

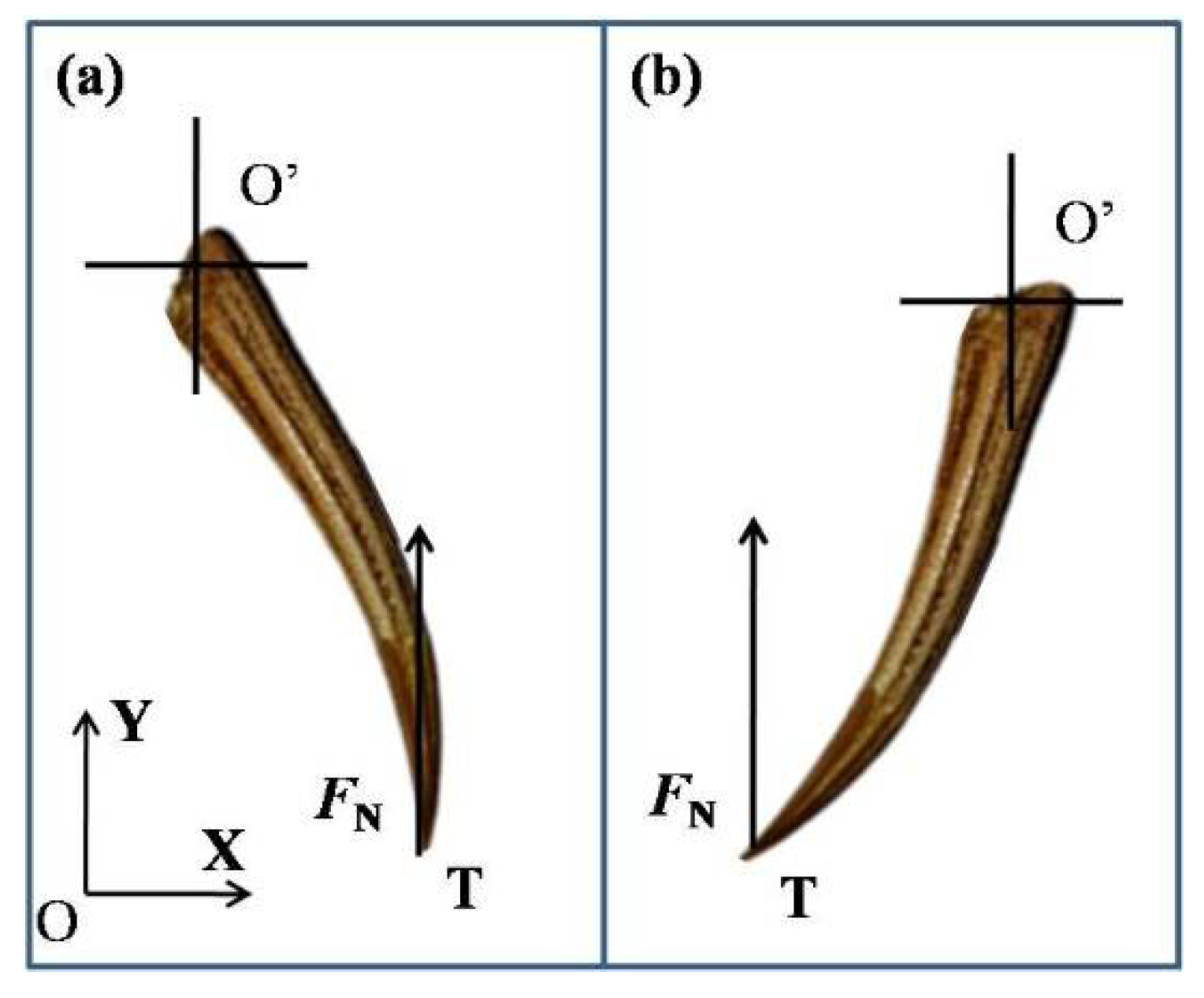
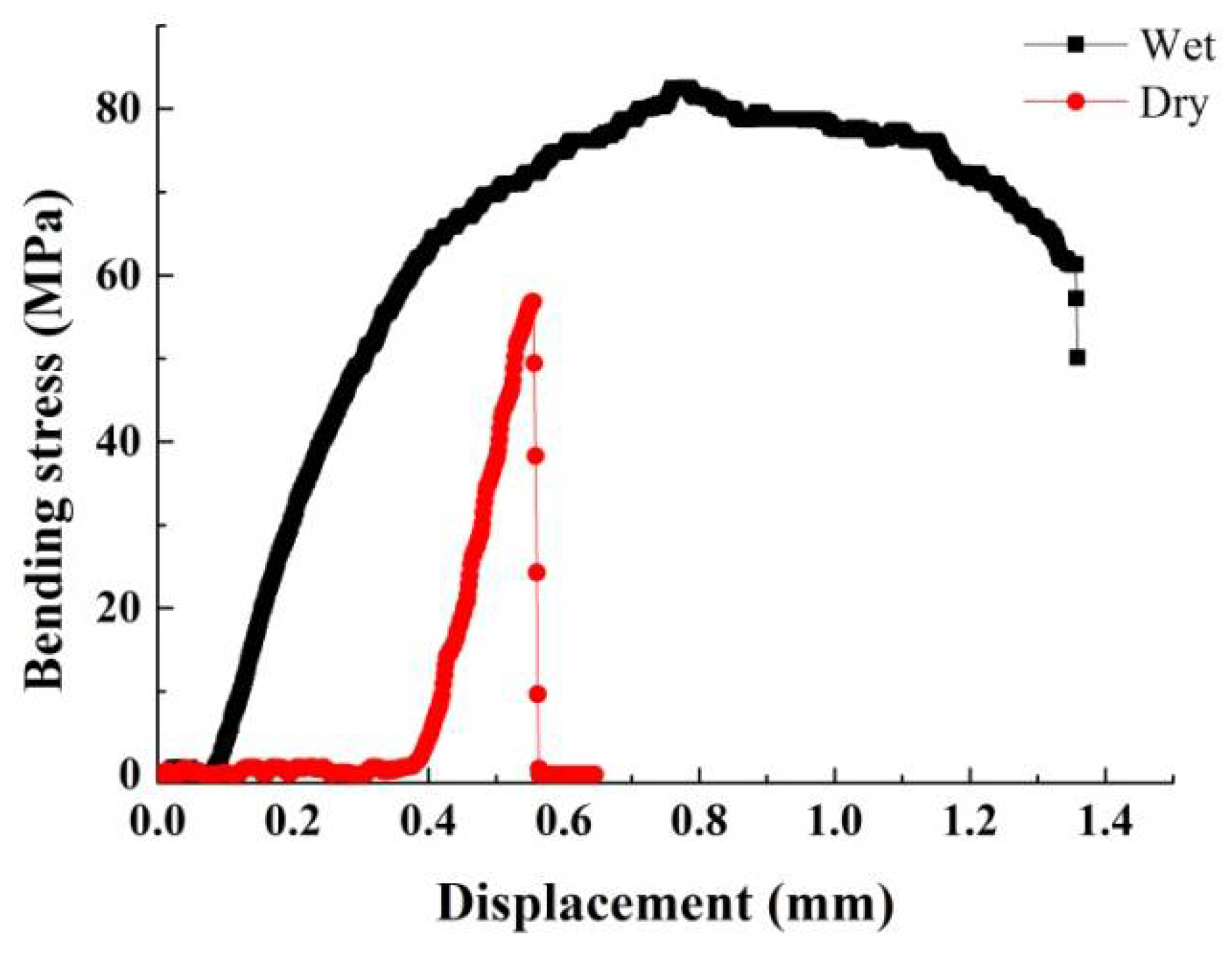
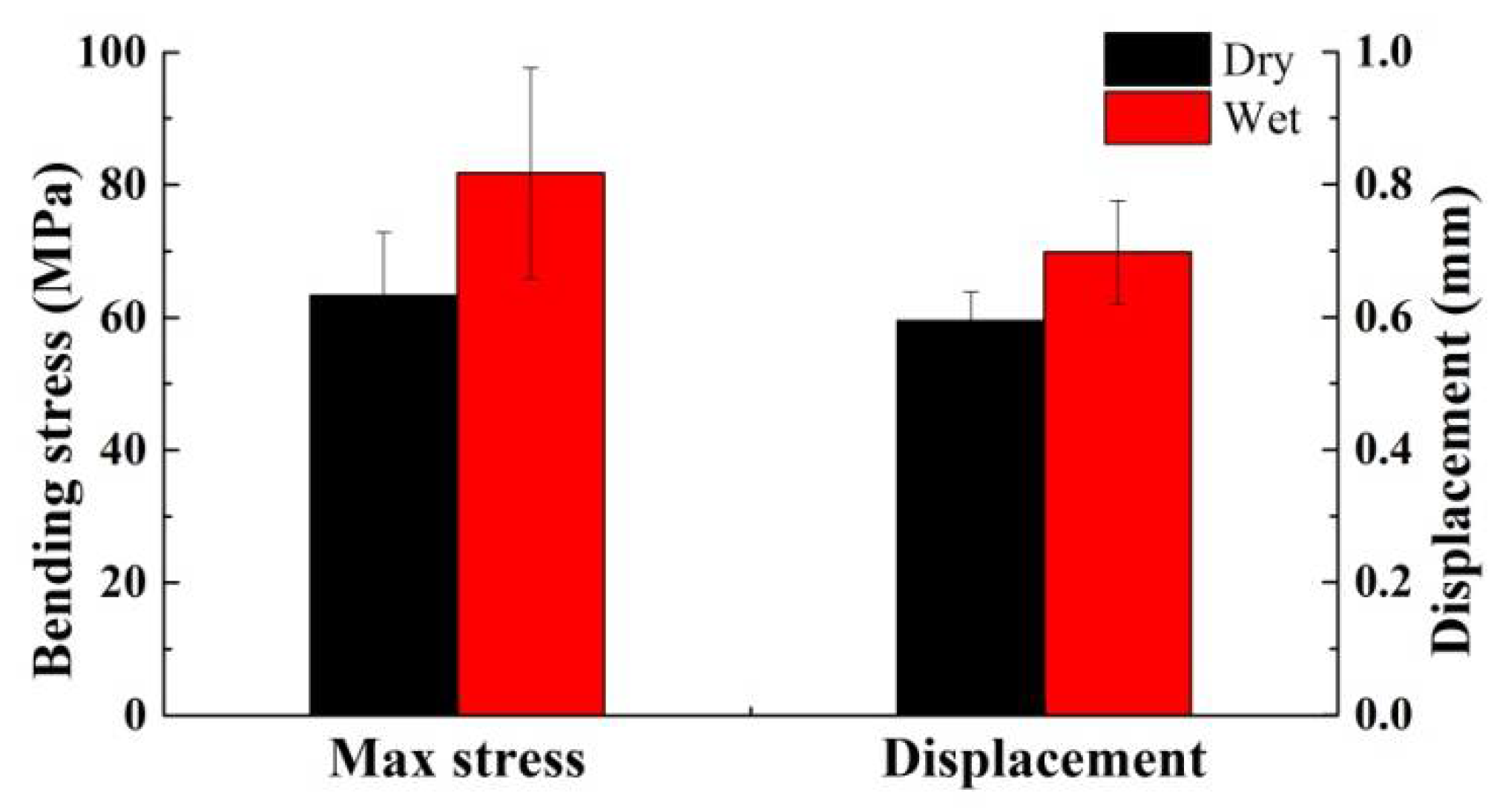
| Specimen | Dimension | Weight (g) | |
|---|---|---|---|
| Length (mm) | Width (mm) | ||
| 1 (male) | 71.21 | 64.03 | 167 |
| 2 (male) | 68.42 | 62.38 | 174 |
| 3 (male) | 57.46 | 52.73 | 92 |
| 4 (male) | 55.74 | 51.61 | 81 |
| 5 (female) | 54.81 | 51.32 | 77 |
| 6 (female) | 56.12 | 51.18 | 75 |
| 7 (female) | 51.50 | 49.22 | 63 |
| Mean ± SD | 59.32 ± 7.44 | 54.64 ± 5.96 | 104.14 ± 46.18 |
| Samples | Parameters | |||
|---|---|---|---|---|
| Moisture Content ϕ (%) | Max. Outer Diameter D (mm) | Inner Diameter D (mm) | Length l (mm) | |
| Dry | 0 | 2.71 ± 0.28 | 1.39 ± 0.046 | 32.62 ± 1.95 |
| Wet | 41.06 ± 4.64 | 2.50 ± 0.15 | 1.31 ± 0.005 | 30.88 ± 1.16 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Li, X.; Li, J.; Qiu, F. Microstructure and Mechanical Properties of the Dactylopodites of the Chinese Mitten Crab (Eriocheir sinensis). Appl. Sci. 2018, 8, 674. https://doi.org/10.3390/app8050674
Wang Y, Li X, Li J, Qiu F. Microstructure and Mechanical Properties of the Dactylopodites of the Chinese Mitten Crab (Eriocheir sinensis). Applied Sciences. 2018; 8(5):674. https://doi.org/10.3390/app8050674
Chicago/Turabian StyleWang, Ying, Xiujuan Li, Jianqiao Li, and Feng Qiu. 2018. "Microstructure and Mechanical Properties of the Dactylopodites of the Chinese Mitten Crab (Eriocheir sinensis)" Applied Sciences 8, no. 5: 674. https://doi.org/10.3390/app8050674
APA StyleWang, Y., Li, X., Li, J., & Qiu, F. (2018). Microstructure and Mechanical Properties of the Dactylopodites of the Chinese Mitten Crab (Eriocheir sinensis). Applied Sciences, 8(5), 674. https://doi.org/10.3390/app8050674






