Effects of Electrode Structure and Electron Energy on Abatement of NO in Dielectric Barrier Discharge Reactor
Abstract
Featured Application
Abstract
1. Introduction
2. Experiment and Methods
2.1. Experiment
2.2. Numerical Simulation
3. Results
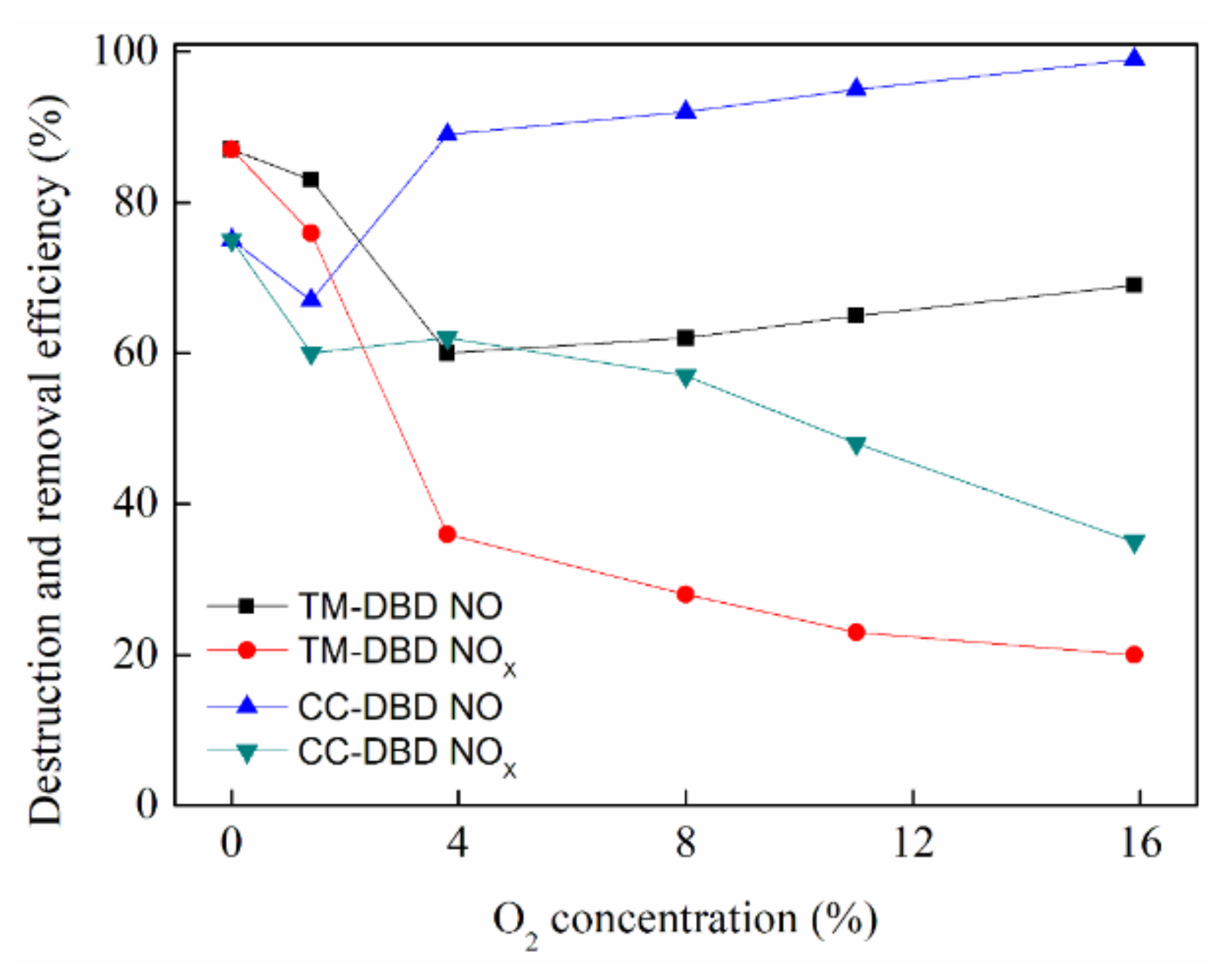
3.1. Effect of O2 Concentration on DRE of NO and NOx
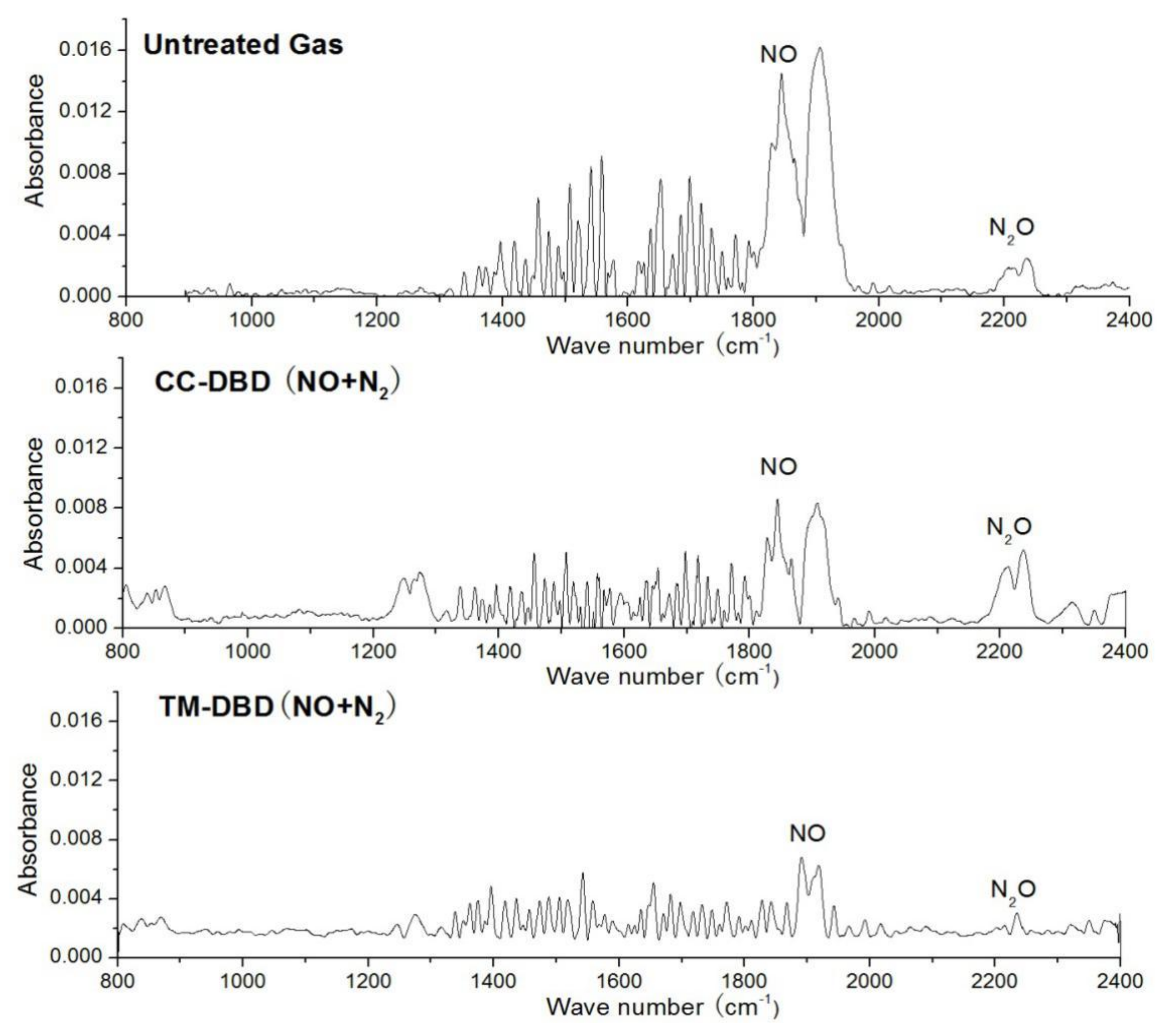
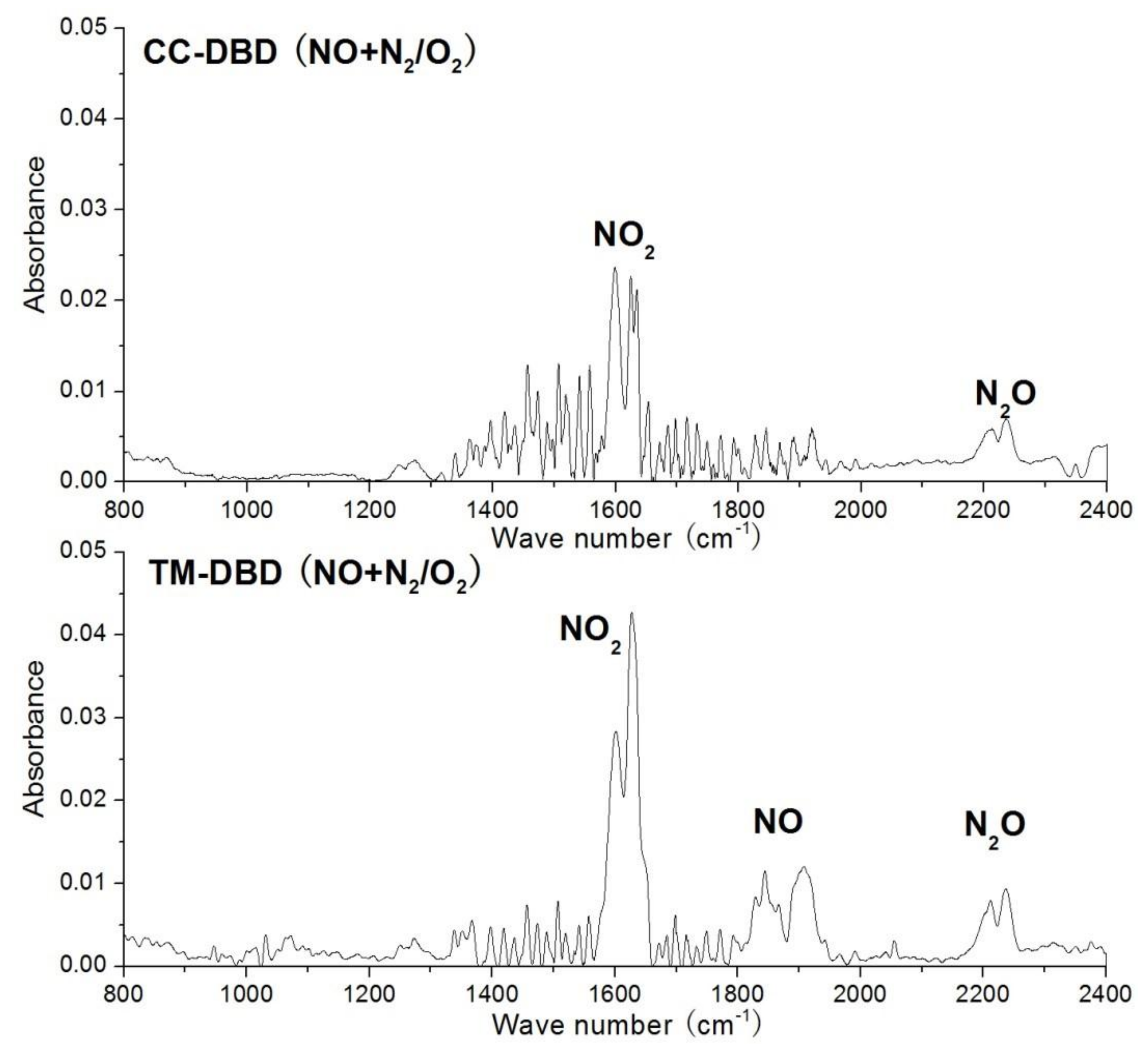
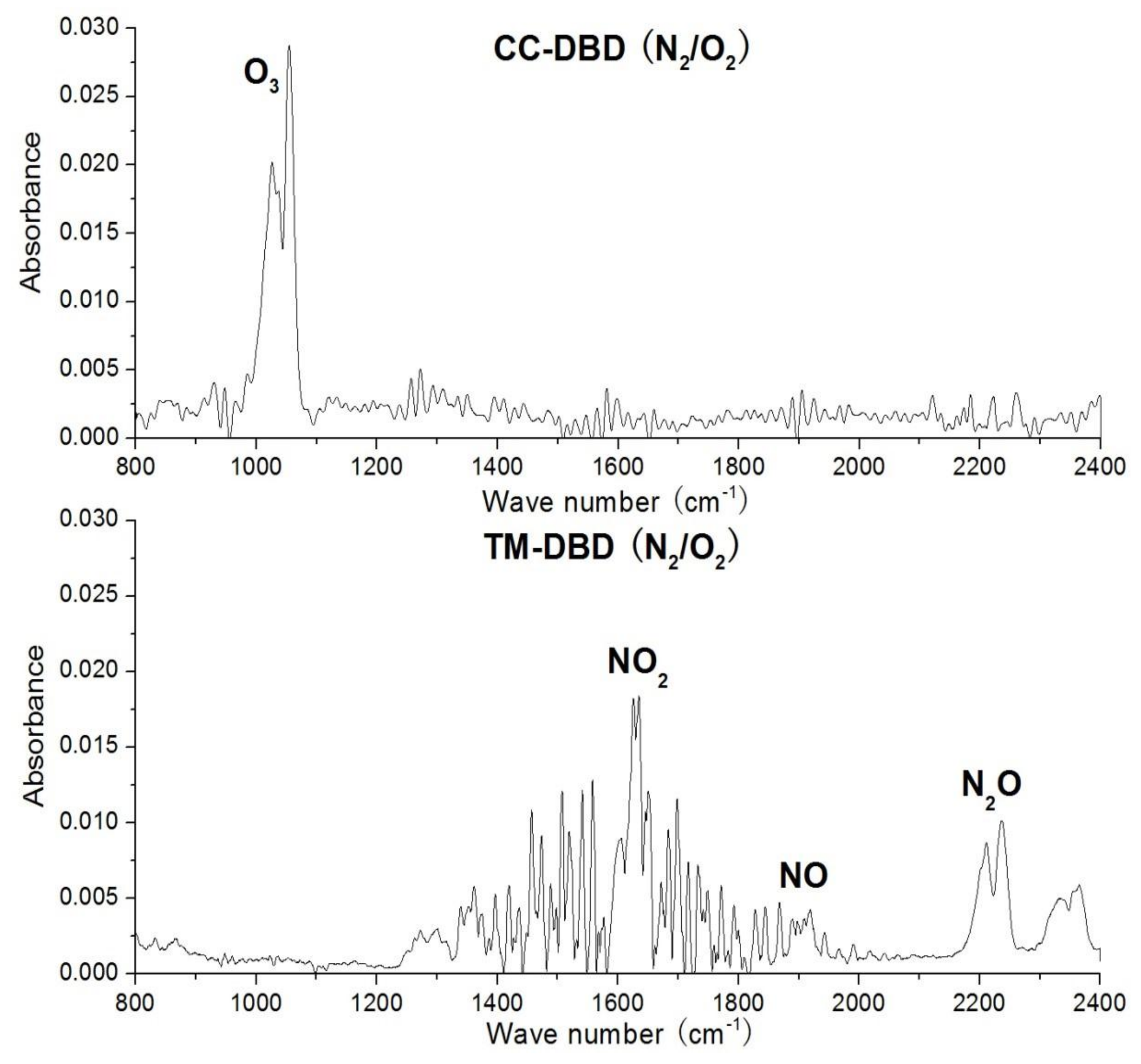
3.2. Final Product Analysis by FTIR
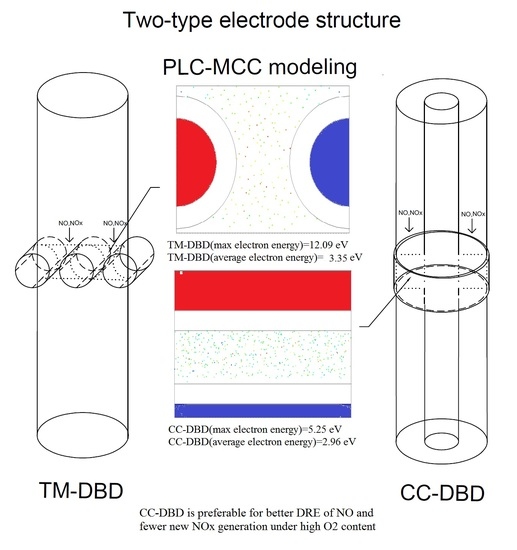
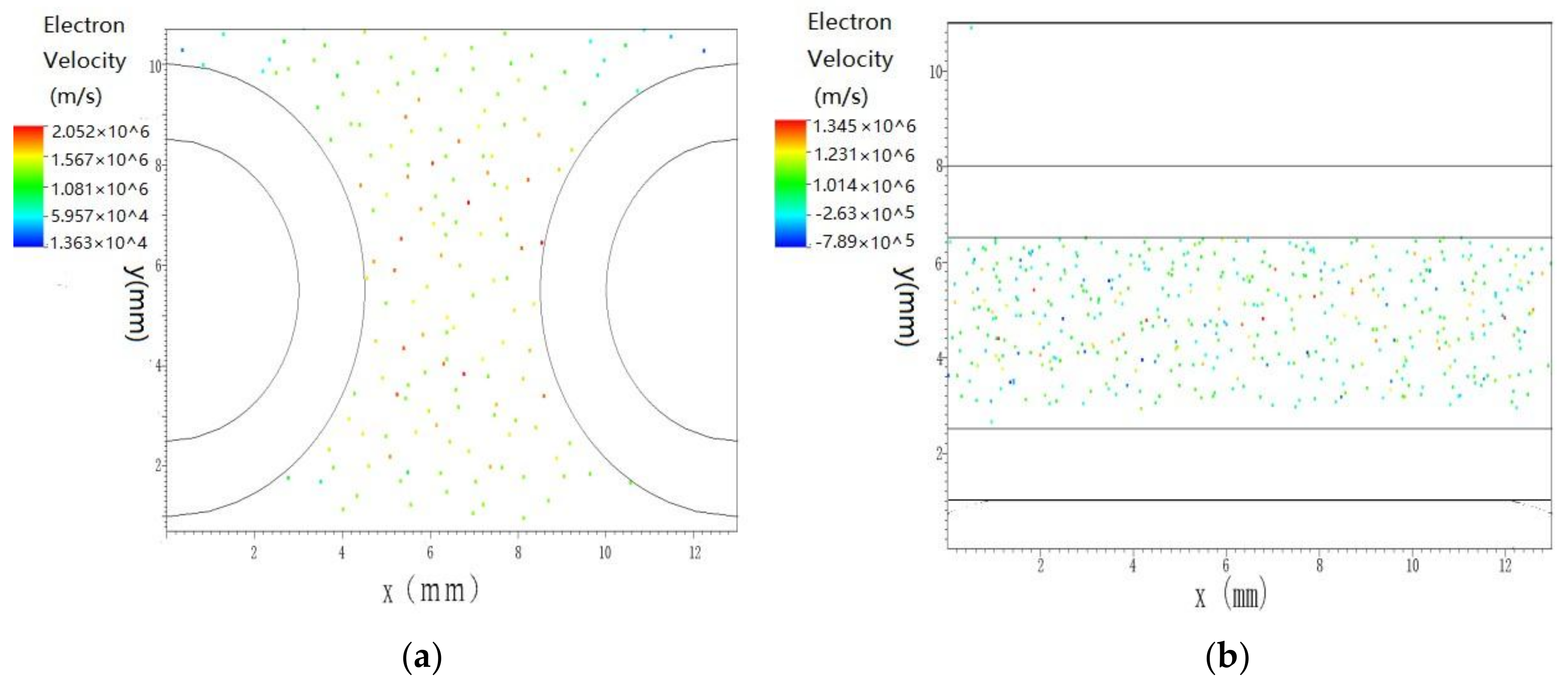
3.3. Numerical Simulation Result
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Devahasdin, S.; Fan, C, Jr.; Li, K.; Chen, D. TiO2, photocatalytic oxidation of nitric oxide: Transient behavior and reaction kinetics. J. Photochem. Photobiol. A Chem. 2003, 156, 161–170. [Google Scholar] [CrossRef]
- Vestreng, V.; Ntziachristos, L.; Semb, A.; Reis, S.; Isaksen, I.S.A.; Tarrason, L. Evolution of NOx emissions in Europe with focus on road transport control measures. Atmos. Chem. Phys. 2009, 9, 1503–1520. [Google Scholar] [CrossRef]
- Forzatti, P. Present status and perspectives in de-NOx, SCR catalysis. Appl. Catal. A Gen. 2001, 222, 221–236. [Google Scholar] [CrossRef]
- Zhao, L.; Li, C.; Wang, Y.; Wu, H.; Gao, L.; Zhang, J.; Zeng, G. Simultaneous removal of elemental mercury and NO from simulated flue gas using a CeO2 modified V2O5–WO3/TiO2 catalyst. Catal. Sci. Technol. 2016, 6, 420–430. [Google Scholar] [CrossRef]
- Boulter, P.G.; Borken-Kleefeld, J.; Ntziachristos, L. The evolution and control of NOx, emissions from road transport in Europe. In Urban Air Quality in Europe; Springer: Berlin, Germany, 2012; pp. 31–53. [Google Scholar]
- Vasilets, V.N. Nitric oxide plasma sources for bio-decontamination and plasma therapy. In NATO Science for Peace and Security Series A: Chemistry and Biology; Springer: Berlin, Germany, 2012; pp. 393–402. [Google Scholar]
- Collet, G.; Robert, E.; Lenoir, A.; Vandamme, M.; Darny, T.; Dozias, S.; Kieda, C.; Pouvesle, J.M. Plasma jet-induced tissue oxygenation: Potentialities for new therapeutic strategies. Plasma Sour. Sci. Technol. 2014, 23, 184–195. [Google Scholar] [CrossRef]
- Guo, Y.; Liao, X.; Fu, M.; Huang, H.; Ye, D. Toluene decomposition performance and NOx, by-product formation during a DBD-catalyst process. J. Environ. Sci. 2015, 28, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cai, Y.; Wang, J.; Zhang, L.; Li, X. Research on the Effect of C3H6 on NO Conversion Rate in a NTP Reactor. In Proceedings of the 2010 International Conference on Optoelectronics and Image Processing (ICOIP 2010), Haiko, China, 11–12 November 2010; IEEE: Piscataway, NJ, USA, 2010; pp. 297–300. [Google Scholar]
- Anaghizi, S.J.; Talebizadeh, P.; Rahimzadeh, H.; Ghomi, H. The Configuration Effects of Electrode on the Performance of Dielectric Barrier Discharge Reactor for NOx, Removal. IEEE Trans. Plasma Sci. 2015, 43, 1944–1953. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, W.; Long, X.; Liang, P. NOx Formation Mechanism and Control Technology in Process of Orimulsion Combustion. Pollut. Control Technol. 2005, 3, 243–247. [Google Scholar]
- Lieplapa, L.; Blumberga, D. Assessing methods of PM10 and NOx emission for EIA of roads. Manag. Environ. Qual. 2012, 23, 163–172. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, J.; Zhu, Y.; Wen, Z.; Liu, J.; Cen, K. Simultaneous removal of NOx, SO2 and Hg in nitrogen flow in a narrow reactor by ozone injection: Experimental results. Fuel Process. Technol. 2007, 88, 817–823. [Google Scholar] [CrossRef]
- Dell’Acqua, S.; Pauleta, S.R.; Monzani, E.; Pereira, A.S.; Casella, L.; Moura, J.J.G.; Moura, I. Electron transfer complex between nitrous oxide reductase and cytochrome c552 from Pseudomonas nautica: Kinetic, nuclear magnetic resonance, and docking studies. Biochemistry 2014, 47, 10852–10862. [Google Scholar] [CrossRef] [PubMed]
- Jani, M.A.; Toda, K.; Takaki, K.; Fujiwara, T. An experimental comparison between electrode shapes for NOx treatment using a dielectric barrier discharge. J. Phys. D Appl. Phys. 2000, 33, 3078. [Google Scholar] [CrossRef]
- Chen, G.; Sun, Q.; Shi, L.; Song, Z. Effect of Combination Way of Dielectric Barrier Discharge and CuZSM-5 on NOx Removal. Chin. J. Catal. 2010, 31, 817–821. [Google Scholar]
- Penetrante, B.M.; Hsiao, M.C.; Merritt, B.T.; Vogtlin, G.E.; Wallman, P.H.; Neiger, M.; Wolf, O.; Hammer, T.; Broer, S. Pulsed corona and dielectric-barrier discharge processing of NO in N2. Appl. Phys. Lett. 1996, 68, 3719–3721. [Google Scholar] [CrossRef]
- Li, X.H.; Wei, X.; Han, W.H.; Cai, Y.X.; Shi, Y.X. Effects of argon on NO conversion by NTP in NO/N2/O2 mixtures through emission spectroscopy analysis. J. Chem. Eng. Chin. Univ. 2015, 29, 346–351. [Google Scholar]
- Bai, M.; Leng, B.; Mao, S.; Li, C. Flue Gas Desulfurization by Dielectric Barrier Discharge. Plasma Chem. Plasma Process. 2016, 36, 511–521. [Google Scholar] [CrossRef]
- Sun, B.M.; Gao, X.D.; Xiao, H.P.; Du, X.; Duan, E.; Zeng, J. Influence of power frequency on energy consumption of NOx removal by DBD technology. J. Chin. Soc. Power Eng. 2012, 32, 47–51. [Google Scholar]
- Rajasekaran, P.; Opländer, C.; Hoffmeister, D.; Bibinov, N.; Suschek, C.V.; Wandke, D.; Awakowicz, P. Characterization of Dielectric Barrier Discharge (DBD) on Mouse and Histological Evaluation of the Plasma Treated Tissue. Plasma Processes Polym. 2011, 8, 246–255. [Google Scholar] [CrossRef]
- Li, X.; Wei, X.; Han, W.; Cai, Y.; Wang, J.; Li, K. Experimental study on NO conversion in N2/NO mixtures by non-thermal plasma based on spectroscopy diagnosis. In Proceedings of the 2012 Asia-Pacific Power and Energy Engineering Conference, Shanghai, China, 27–29 March 2012; pp. 1–4. [Google Scholar]
- Penetrante, B.M.; Hsiao, M.C.; Merritt, B.T.; Vogtlin, G.E.; Wallman, P.H. Comparison of electrical discharge techniques for nonthermal plasma processing of NO in N2. IEEE Trans. Plasma Sci. 1995, 23, 679–687. [Google Scholar] [CrossRef]
- Sun, Q.; Zhu, A.; Yang, X.; Niu, J.; Xu, Y. Formation of NOx from N2 and O2 in catalyst-pellet filled dielectric barrier discharges at atmospheric pressure. Chem. Commun. 2003, 12, 1418–1419. [Google Scholar] [CrossRef]
- Kim, H.H.; Teramoto, Y.; Ogata, A.; Takagi, H.; Nanba, T. Plasma Catalysis for Environmental Treatment and Energy Applications. Plasma Chem. Plasma Process. 2016, 36, 45–72. [Google Scholar] [CrossRef]
- Wang, J.; Cao, X.; Zhang, R.; Gong, T.; Hou, H.; Chen, S.; Zhang, R. Effect of Water Vaporon Toluene Removal in Catalysis-DBD Plasma Reactors. Plasma Sci. Technol. 2016, 18, 370–375. [Google Scholar] [CrossRef]
- Mahammadunnisa, S.; Reddy, E.L.; Reddy, P.R.M.K.; Subrahmanyam, C. A Facile Approach for Direct Decomposition of Nitrous Oxide Assisted by Non-Thermal Plasma. Plasma Process. Polym. 2013, 10, 444–450. [Google Scholar] [CrossRef]
- Mohapatro, S.; Rajanikanth, B.S. Cascaded cross flow DBD-adsorbent technique for NOx, abatement in diesel engine exhaust. IEEE Trans. Dielectr. Electr. Insul. 2010, 17, 1543–1550. [Google Scholar] [CrossRef]
- Song, C.; Bin, F.; Tao, Z.; Li, F.; Huang, Q. Simultaneous removals of NOx, HC and PM from diesel exhaust emissions by dielectric barrier discharges. J. Hazard. Mater. 2009, 166, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Sun, B.; Xiao, H.; Zeng, J.; Duan, E.; Xin, J.; Li, C. Effect of Reactor Structure in DBD for Nonthermal Plasma Processing of NO in N2, at Ambient Temperature. Plasma Chem. Plasma Process. 2012, 32, 1189–1201. [Google Scholar] [CrossRef]
- Ma, S.; Zhao, Y.; Yang, J.; Zhang, S.; Zhang, J.; Zheng, C. Research progress of pollutants removal from coal-fired flue gas using non-thermal plasma. Renew. Sustain. Energy Rev. 2017, 67, 791–810. [Google Scholar] [CrossRef]
- Xia, L.; Huang, L.; Shu, X.; Zhang, R.; Dong, W.; Hou, H. Removal of ammonia from gas streams with dielectric barrier discharge plasmas. J. Hazard. Mater. 2008, 152, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Pawłat, J.; Diatczyk, J.; Stryczewska, H.D. Low-temperature plasma for exhaust gas purification from paint shop—A case study. Prz. Elektrotech. 2011, 87, 245–248. [Google Scholar]
- Kim, H.H. Nonthermal Plasma Processing for Air-Pollution Control: A Historical Review, Current Issues, and Future Prospects. Plasma Process. Polym. 2004, 1, 91–110. [Google Scholar] [CrossRef]
- Ran, J.; Luo, H.; Wang, X. Experimental study of homogeneous dielectric barrier discharge in air at atmospheric pressure. In Proceedings of the 2012 Abstracts IEEE International Conference on Plasma Science, Edinburgh, UK, 8–13 July 2012; IEEE: Piscataway, NJ, USA, 2012. [Google Scholar]
- Mizuno, A.; Rajanikanth, B.S.; Shimizu, K.; Kinoshita, K.; Yanagihara, K.; Okumoto, M.; Katsura, S. Non-Thermal Plasma Applications at Very Low Temperature. Combust. Sci. Technol. 1998, 133, 49–63. [Google Scholar] [CrossRef]
- Takaki, K.; Jani, M.A.; Fujiwara, T. Removal of nitric oxide in flue gases by multi-point to plane dielectric barrier discharge. IEEE Trans. Plasma Sci. 1999, 27, 1137–1145. [Google Scholar] [CrossRef]
- Bai, M.; Zhang, Z.; Bai, M.; Bai, X.; Gao, H. Synthesis of Ammonia Using CH/N Plasmas Based on Micro-Gap Discharge under Environmentally Friendly Condition. Plasma Chem. Plasma Process. 2008, 28, 405–414. [Google Scholar] [CrossRef]
- Blin-Simiand, N.; Jorand, F.; Magne, L.; Pasquiers, S.; Postel, C.; Vacher, J.-R. Plasma Reactivity and Plasma-Surface Interactions during Treatment of Toluene by a Dielectric Barrier Discharge. Plasma Chem. Plasma Process. 2008, 28, 429–466. [Google Scholar] [CrossRef]
- Rubio, S.J.; Rodero, A.; Quintero, M.C. Application of a Microwave Helium Plasma Torch Operating at Atmospheric Pressure to Destroy Trichloroethylene. Plasma Chem. Plasma Process. 2008, 28, 415–428. [Google Scholar] [CrossRef]
- Hagelaar, G.J.M.; Pitchford, L.C. Solving the Boltzmann equation to obtain electron transport coefficients and rate coefficients for fluid models. Plasma Sour. Sci. Technol. 2005, 14, 722–733. [Google Scholar] [CrossRef]
- Abdollahzadeh, M.; Pascoa, J.C.; Oliveira, P.J. Implementation of the classical plasma–fluid model for simulation of dielectric barrier discharge (DBD) actuators in Open FOAM. Comput. Fluids 2016, 128, 77–90. [Google Scholar] [CrossRef]
- Cristofolini, A.; Borghi, C.A.; Neretti, G. Charge distribution on the surface of a dielectric barrier discharge actuator for the fluid-dynamic control. J. Appl. Phys. 2012, 113, 370–410. [Google Scholar] [CrossRef]
- Sato, S.; Ohnishi, N. Theoretical Modeling of Pulse Discharge Cycle in DBD Plasma Actuator. Jpn. J. Appl. Phys. 2016, 55. [Google Scholar] [CrossRef]
- Geng, S.F.; Qiu, X.M.; Cheng, C.M.; Chu, P.K.; Tang, D.L. Three-dimensional particle-in-cell simulation of discharge characteristics in cylindrical anode layer hall plasma accelerator. Phys. Plasmas 2012, 19, 043507. [Google Scholar] [CrossRef]
- Atkinson, R.; Baulch, D.L.; Cox, R.A.; Hampson, R.F., Jr.; Kerr, J.A.; Rossi, M.J.; Troe, J. Evaluated Kinetic, Photochemical and Heterogeneous Data for Atmospheric Chemistry: Supplement V. IUPAC Subcommittee on Gas Kinetic Data Evaluation for Atmospheric Chemistry. J. Phys. Chem. Ref. Data 1997, 26, 521–1011. [Google Scholar] [CrossRef]
- Hemker, R.G. Particle-in-Cell Modeling of Plasma-Based Accelerators in Two and Three Dimensions; University of California: Los Angeles, CA, USA, 2015; p. 338. [Google Scholar]
- Singh, H.; Graves, D.B. Measurements of the electron energy distribution function in molecular gases in an inductively coupled plasma. J. Appl. Phys. 2000, 87, 4098–4106. [Google Scholar] [CrossRef]
- Okabe, H. Photochemistry of Small Molecules; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 1978. [Google Scholar]

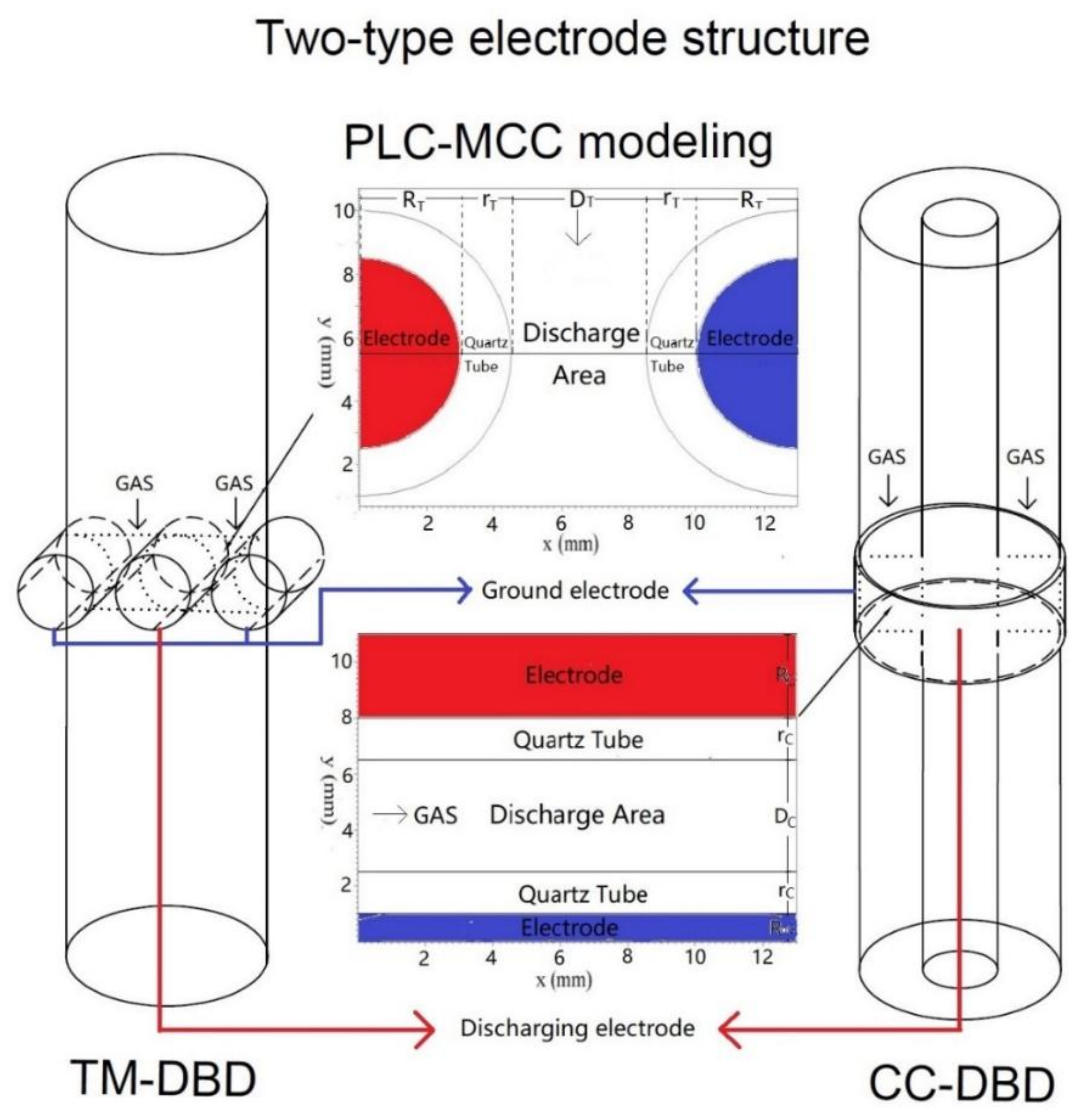

| Model Parameters of DBD in VSim | ||||
|---|---|---|---|---|
| Grid numbers | X ∗ Y ∗ Z = 100 ∗ 100 ∗ 200 | |||
| Numbers of gyrating circles | 50 | |||
| Numbers of microparticle | 50,000 | |||
| Geometry Parameters of CC-DBD and TM-DBD in VSim | ||||
| TM-DBD | RT/mm | rT/mm | DT/mm | |
| 3 | 1.5 | 4 | ||
| CC-DBD | RC/mm | rC/mm | DC/mm | RoC/mm |
| 3 | 1.5 | 4 | 1 | |
| CC-DBD | Low O2 | e+NO→e+NO* NO*+NO*→N2O+O NO*+NO*→N2+O2 |
| High O2 | e+NO→e+NO* e+O2→e+2O NO*+O→NO2 NO*+NO*→N2O+O O+O2→ +M→ O3+NO→NO2+O2 | |
| TM-DBD | Low O2 | e+N2→e+2N e+NO→e+N+O NO+N→N2+O |
| High O2 | e+N2→e+2N e+O2→e+2O e+NO→e+N+O NO+O→NO2 N+O→NO N+O2→NO+O N+NO2→N2O+O |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, W.; Wang, F.; Liu, Y.; Zhang, R.; Hou, H. Effects of Electrode Structure and Electron Energy on Abatement of NO in Dielectric Barrier Discharge Reactor. Appl. Sci. 2018, 8, 618. https://doi.org/10.3390/app8040618
Zhao W, Wang F, Liu Y, Zhang R, Hou H. Effects of Electrode Structure and Electron Energy on Abatement of NO in Dielectric Barrier Discharge Reactor. Applied Sciences. 2018; 8(4):618. https://doi.org/10.3390/app8040618
Chicago/Turabian StyleZhao, Weixuan, Feng Wang, Yanghaichao Liu, Renxi Zhang, and Huiqi Hou. 2018. "Effects of Electrode Structure and Electron Energy on Abatement of NO in Dielectric Barrier Discharge Reactor" Applied Sciences 8, no. 4: 618. https://doi.org/10.3390/app8040618
APA StyleZhao, W., Wang, F., Liu, Y., Zhang, R., & Hou, H. (2018). Effects of Electrode Structure and Electron Energy on Abatement of NO in Dielectric Barrier Discharge Reactor. Applied Sciences, 8(4), 618. https://doi.org/10.3390/app8040618