Treatment of Mineral Oil Refinery Wastewater in Microbial Fuel Cells Using Ionic Liquid Based Separators
Abstract
:1. Introduction
2. Materials and Methods
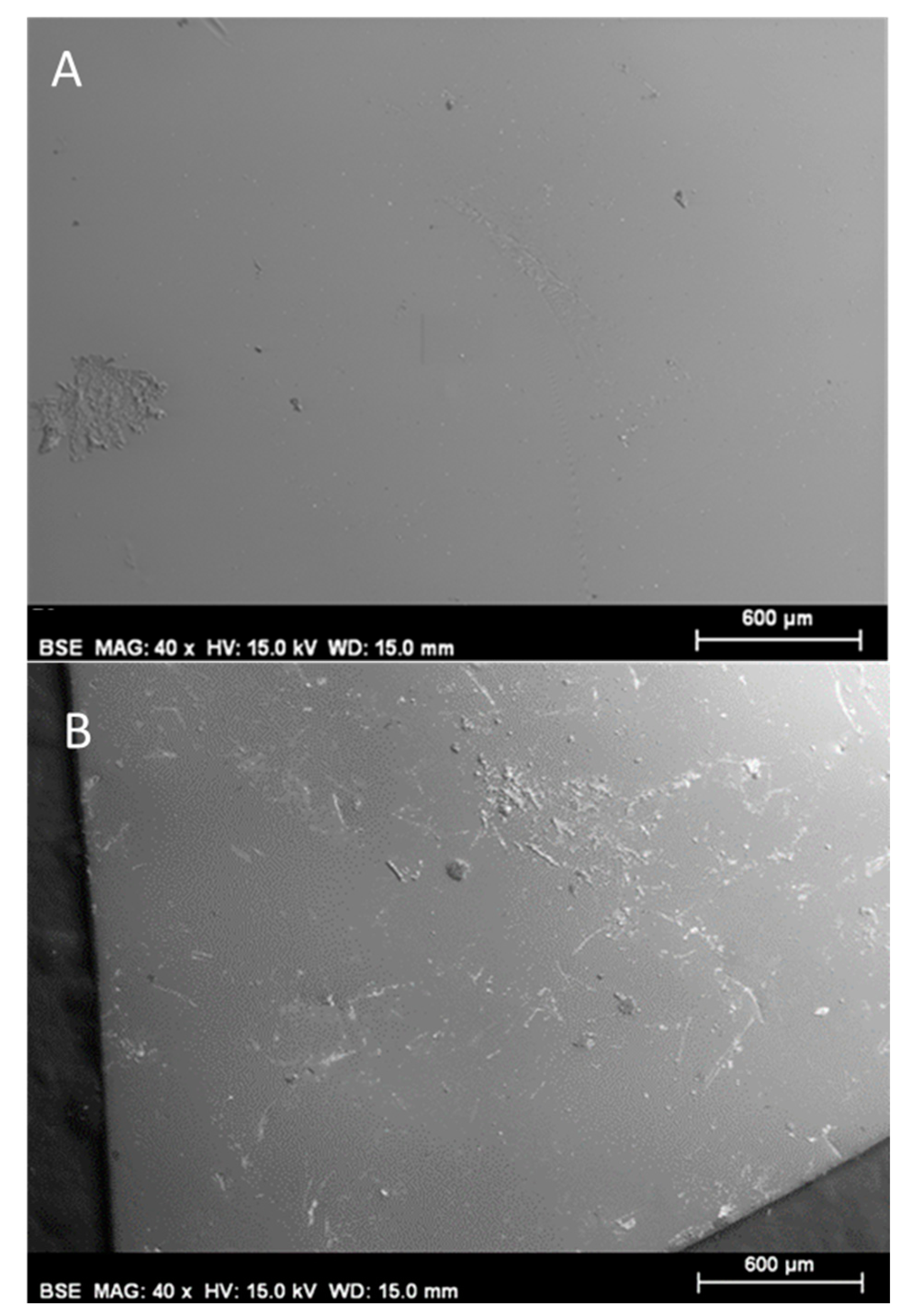
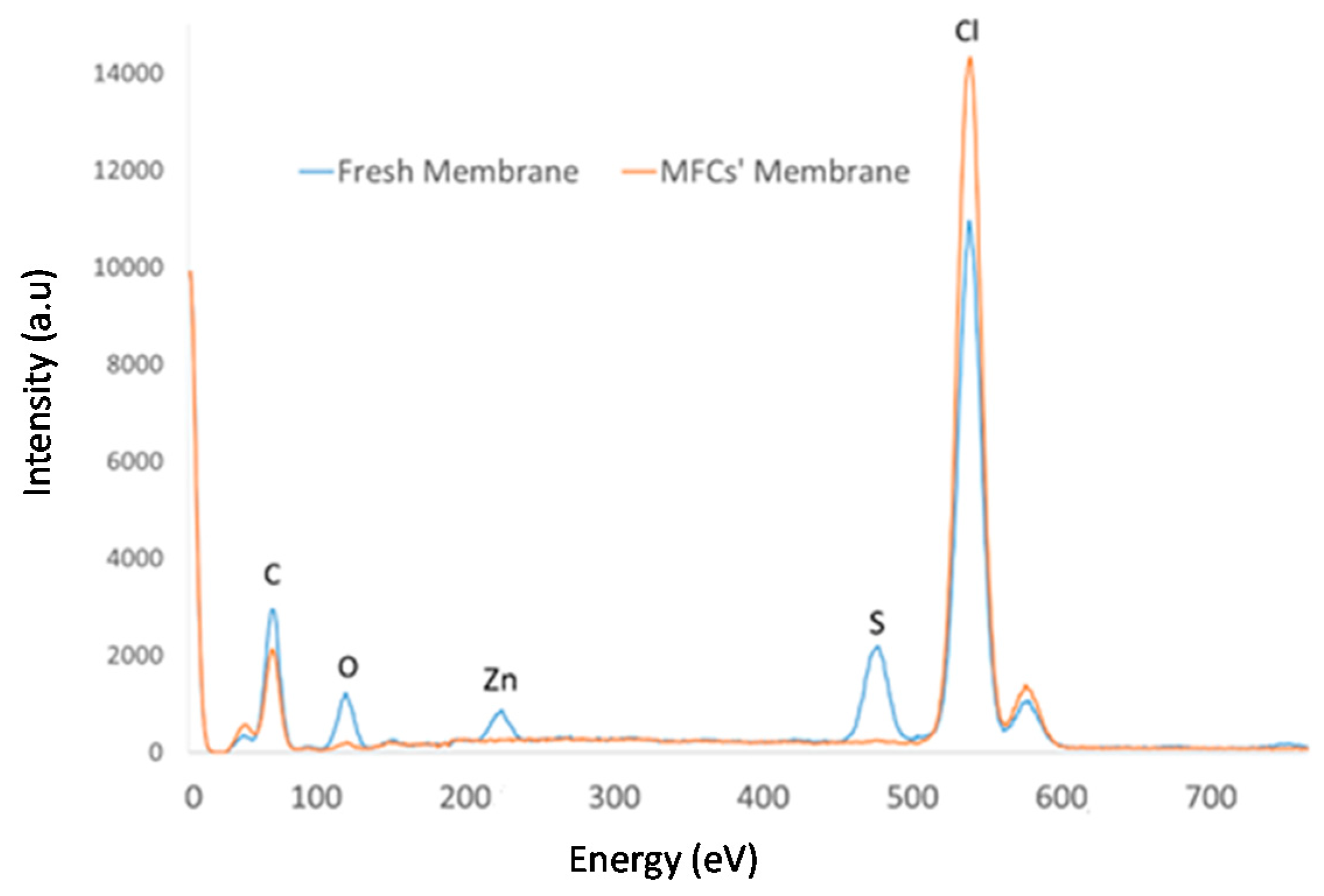
2.1. Preparation and SEM-EDX Characterization of Polymer Ionic Liquid Inclusion Membrane
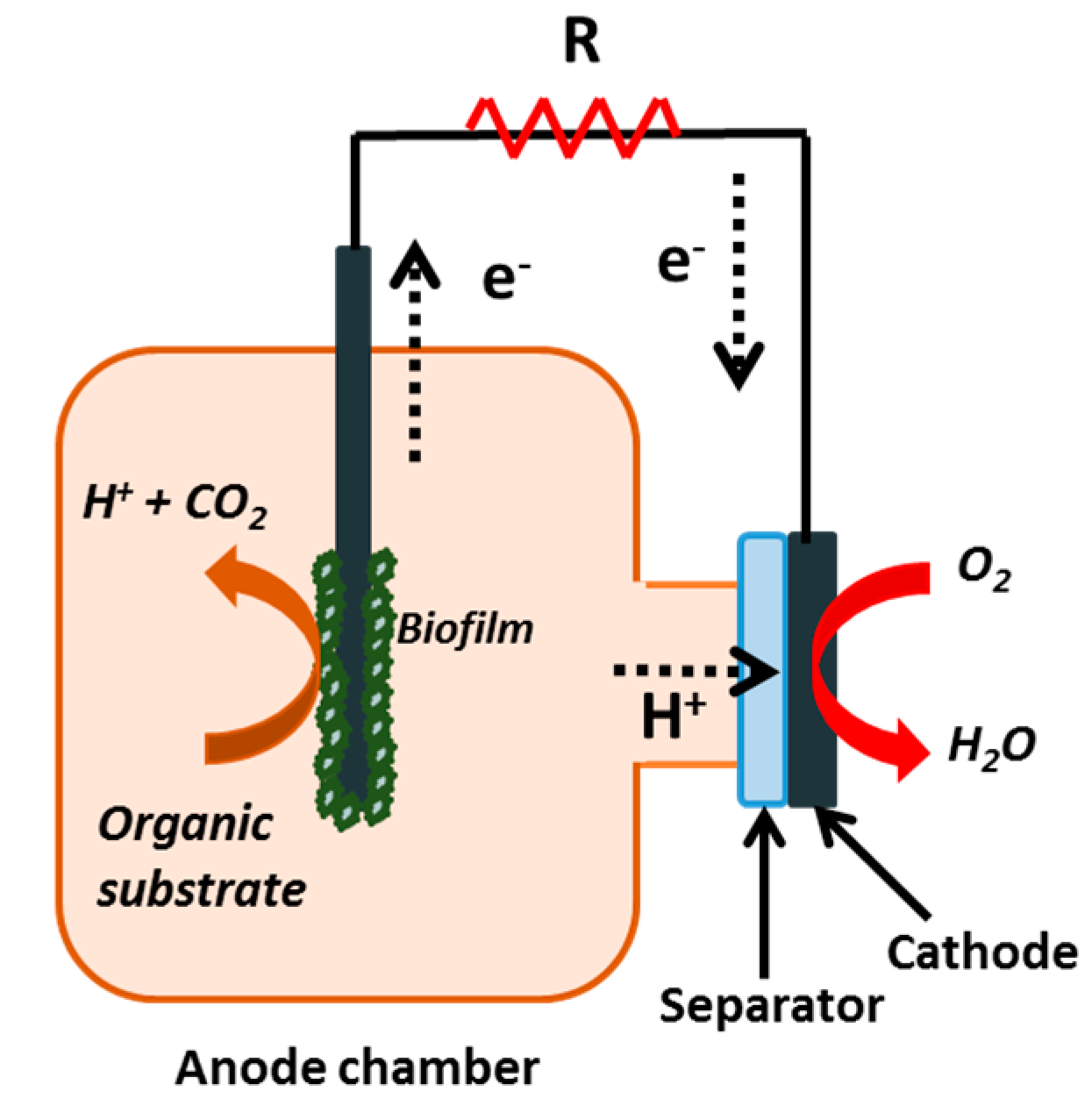
2.2. Microbial Fuel Cell Set-Up
2.3. Substrate
2.4. Wastewater Characterization
2.5. Polarization Method
2.6. Coulombic Efficiency
3. Results and Discussion
3.1. Industrial Wastewater Treatment by Microbial Fuel Cells
3.2. Ionic Liquid-Based Membranes Applied to Wastewater Treatment
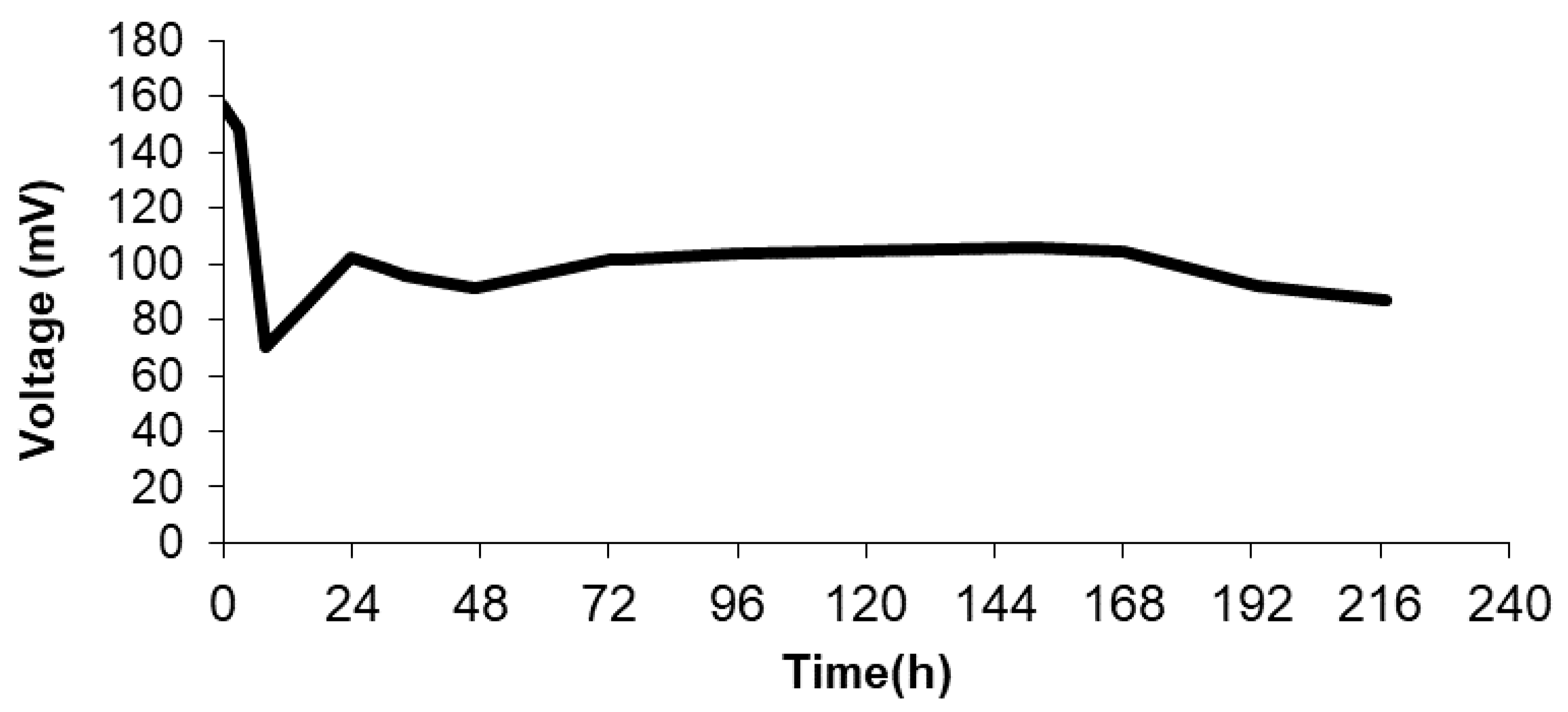
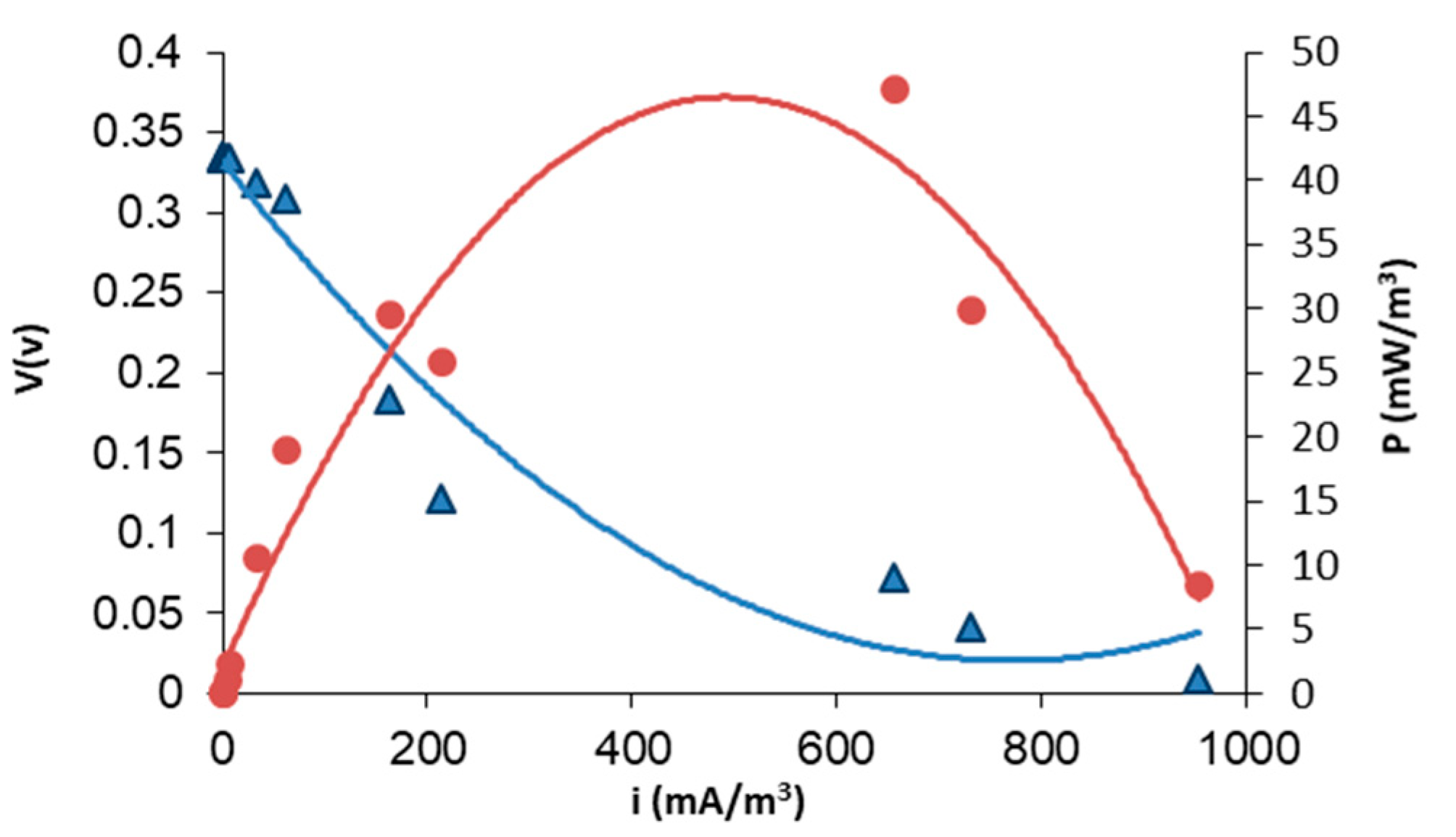
3.3. Electric Performance
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dannys, E.; Green, T.; Wettlaufer, A.; Mouli, C.; Madhurnathakam, R.; Elkamel, A. Wastewater Treatment with Microbial Fuel Cells: A Design and Feasibility Study for Scale-up in Microbreweries. J. Bioprocess Biotech. 2016, 6, 1–6. [Google Scholar] [CrossRef]
- Mohan, S.V.; Velvizhi, G.; Modestra, J.A.; Srikanth, S. Microbial fuel cell: Critical factors regulating bio-catalyzed electrochemical process and recent advancements. Renew. Sustain. Energy Rev. 2014, 40, 779–797. [Google Scholar] [CrossRef]
- Asensio, Y.; Fernández-Marchante, C.M.; Lobato, J.; Cañizares, P.; Rodrigo, M.A. Influence of the fuel and dosage on the performance of double-compartment microbial fuel cells. Water Res. 2016, 99, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Vassallo, P.; Paoli, C.; Fabiano, M. Energy required for the complete treatment of municipal wastewater. Ecol. Eng. 2009, 35, 687–694. [Google Scholar] [CrossRef]
- Gude, V.G. Wastewater treatment in microbial fuel cells—An overview. J. Clean. Prod. 2016, 122, 287–307. [Google Scholar] [CrossRef]
- Rabaey, K.; Verstraete, W. Microbial fuel cells: Novel biotechnology for energy generation. Trends Biotechnol. 2005, 23, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Prestigiacomo, C.; Fernandez-Marchante, C.M.; Fernández-Morales, F.J.; Cañizares, P.; Scialdone, O.; Rodrigo, M.A. New prototypes for the isolation of the anodic chambers in microbial fuel cells. Fuel 2016, 181, 704–710. [Google Scholar] [CrossRef]
- Asensio, Y.; Fernandez-Marchante, C.M.; Lobato, J.; Cañizares, P.; Rodrigo, M.A. Influence of the ion-exchange membrane on the performance of double-compartment microbial fuel cells. J. Electroanal. Chem. 2018, 808, 427–432. [Google Scholar] [CrossRef]
- Hernández-Fernández, F.J.; de los Ríos, A.P.; Mateo-Ramírez, F.; Juarez, M.D.; Lozano-Blanco, L.J.; Godínez, C. New application of polymer inclusion membrane based on ionic liquids as proton exchange membrane in microbial fuel cell. Sep. Purif. Technol. 2015, 160, 51–58. [Google Scholar] [CrossRef]
- Koók, L.; Nemestóthy, N.; Bakonyi, P.; Zhen, G.; Kumar, G.; Lu, X.; Su, L.; Saratale, G.D.; Kim, S.H.; Gubicza, L. Performance evaluation of microbial electrochemical systems operated with Nafion and supported ionic liquid membranes. Chemosphere 2017, 175, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Salar-García, M.J.; Ortiz-Martínez, V.M.; de los Ríos, A.P.; Hernández-Fernández, F.J. A method based on impedance spectroscopy for predicting the behavior of novel ionic liquid-polymer inclusion membranes in microbial fuel cells. Energy 2015, 89, 648–654. [Google Scholar] [CrossRef]
- Greaves, T.L.; Drummond, C.J. Protic Ionic Liquids: Properties and Applications. Chem. Rev. 2008, 108, 206–237. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.; Logan, B.E. Effectiveness of domestic wastewater treatment using microbial fuel cells at ambient and mesophilic temperatures. Bioresour. Technol. 2010, 101, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Min, B.; Kim, J.; Oh, A.S.; Regan, J.M.; Logan, B.E. Electricity generation from swine wastewater using microbial fuel cells. Water Res. 2005, 39, 4961–4968. [Google Scholar] [CrossRef] [PubMed]
- Mansoorian, H.J.; Mahvi, A.H.; Jafari, A.J.; Amin, M.M.; Rajabizadeh, A.; Khanjani, N. Bioelectricity generation using two chamber microbial fuel cell treating wastewater from food processing. Enzym. Microb. Technol. 2013, 10, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Asensio, Y.; Fernandez-Marchante, C.; Villaseñor, J.; Lobato, J.; Cañizares, P.; Rodrigo, M.A. Algal biomass as fuel for stacked-MFCs for profitable, sustainable and carbon neutral bioenergy generation. J. Chem. Technol. Biotechnol. 2018, 93, 287–293. [Google Scholar] [CrossRef]
- Raschitor, A.; Soreanu, G.; Fernandez-Marchante, C.M.; Lobato, J.; Canizares, P.; Cretescu, I.; Rodrigo, M.A. Bioelectro-Claus processes using MFC technology: Influence of co-substrate. Bioresour. Technol. 2015, 189, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Penteado, E.D.; Fernandez-Marchante, C.M.; Zaiat, M.; Cañizares, P.; Gonzalez, E.R.; Rodrigo, M.A.R. Energy recovery from winery wastewater using a dual chamber microbial fuel cell. J. Chem. Technol. Biotechnol. 2016, 91, 1802–1808. [Google Scholar] [CrossRef]
- Angosto, J.M.; Fernández-López, J.A.; Godínez, C. Brewery and liquid manure wastewaters as potential feedstocks for microbial fuel cells: A performance study. Environ. Technol. 2015, 36, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Mansoorian, H.J.; Mahvi, A.H.; Jafaric, A.J.; Khanjanid, N. Evaluation of dairy industry wastewater treatment and simultaneous bioelectricity generation in a catalyst-less and mediator-less membrane microbial fuel cell. J. Saudi Chem. Soc. 2016, 20, 88–100. [Google Scholar] [CrossRef]
- Pant, D.; Bogaert, G.V.; Diels, L.; Vanbroekhoven, K. A review of the substrates used in microbial fuel cells (MFCs) for sustainable energy production. Bioresour. Technol. 2010, 101, 1533–1543. [Google Scholar] [CrossRef] [PubMed]
- Rodier, J. L’Analyse de l’Eau:Eaux Naturelles, Eaux Résiduaires, Eau de Mer, 7th ed.; Dunod Edition: Paris, France, 1984. [Google Scholar]
- Qualité de l'eau-Détermination de la Demande Chimique en Oxygène (DCO); NF T90-101; AFNOR: Paris, France, 2001.
- Logan, B.E. Microbial Fuel Cells; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Mateo-Ramírez, F.; Hasna, A.; Hernández-Fernández, F.J.; Godínez, C.; de los Ríos, A.P.; Lotfi, E.M.; Mahi, M.E.; Lozano Blanco, L.J. Air breathing cathode-microbial fuel cell with separator based on ionic liquid applied to slaughterhouse wastewater treatment and bio-energy production. J. Chem. Technol. Biotechnol. 2016, 92, 642–648. [Google Scholar] [CrossRef]
- Köroğlu, E.O.; Özkaya, B.; Çetinkaya, A.Y. Microbial fuel cells for energy recovery from waste. Int. J. Energy Sci. 2014, 4, 28–30. [Google Scholar] [CrossRef]
- Cirik, K. Optimization of bioelectricity generation in fed-batch microbial fuel cell: Effect of electrode material, initial substrate concentration, and cycle time applied. Biochem. Biotechnol. 2014, 173, 205–214. [Google Scholar] [CrossRef] [PubMed]
| Water Parameter | Method | Initial | Final Values | ||
|---|---|---|---|---|---|
| MFC | Baseline | Control | |||
| pH | [-] | 8.19 | 6.78 | 6.99 | 6.99 |
| Alkalinity (mg/L) | RODIER [22] | 67.60 | 19.60 | 23.60 | 31.60 |
| TSS (mg/L) | AFNOR 90-105 [23] | 0.18 | 1.40 | 0.98 | 0.58 |
| COD (mg/L) | AFNOR 90-101 [23] | 1760.00 | 334.00 | 1200.00 | 1092.00 |
| Total Phosphate (mg/L) | AFNOR 90-022 [23] | 0.51 | 16.44 | 19.27 | 1.58 |
| Kheldahl nitrogen (mg/L) | AFNOR 90-110 [23] | 11.20 | 5.60 | 8.40 | 7.00 |
| Nitrite (mg/L) | AFNOR 90-013 [23] | 0.48 | 0.38 | 0.42 | 0.39 |
| Ammonium (mg/L) | AFNOR 90-015 [23] | 2.67 | 1.40 | 1.48 | 2.19 |
| Sulphate (mg/L) | RODIER [22] | 263.50 | 128.50 | 148.50 | 142.67 |
| Chloride (mg/L) | AFNOR 90-014 | 553.80 | 837.80 | 552.80 | 569.60 |
| Hardness (mg/L) | RODIER [22] | 876.00 | 1076.00 | 836.00 | 816.00 |
| Element | 70% w/w [MTOA+][Cl−] + poly(vinylchloride) (PVC) | |
|---|---|---|
| Fresh | After Opreation | |
| C. Norm (% wt) | C. Norm (% wt) | |
| C | 36.05 | 22.16 |
| N | 13.76 | 4.91 |
| O | 9.27 | 19.97 |
| Cl | 40.91 | 37.63 |
| P | - | - |
| S | - | 5.88 |
| Ca | - | 0.59 |
| Fe | - | 0.63 |
| Zn | - | 5.88 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Addi, H.; Mateo-Ramírez, F.; Ortiz-Martínez, V.M.; Salar-García, M.J.; Hernández-Fernández, F.J.; Pérez de los Ríos, A.; Godínez, C.; Lotfi, E.M.; El Mahi, M.; Blanco, L.J.L. Treatment of Mineral Oil Refinery Wastewater in Microbial Fuel Cells Using Ionic Liquid Based Separators. Appl. Sci. 2018, 8, 438. https://doi.org/10.3390/app8030438
Addi H, Mateo-Ramírez F, Ortiz-Martínez VM, Salar-García MJ, Hernández-Fernández FJ, Pérez de los Ríos A, Godínez C, Lotfi EM, El Mahi M, Blanco LJL. Treatment of Mineral Oil Refinery Wastewater in Microbial Fuel Cells Using Ionic Liquid Based Separators. Applied Sciences. 2018; 8(3):438. https://doi.org/10.3390/app8030438
Chicago/Turabian StyleAddi, Hasna, Francisco Mateo-Ramírez, Víctor Manuel Ortiz-Martínez, María José Salar-García, Francisco José Hernández-Fernández, Antonia Pérez de los Ríos, Carlos Godínez, El Mostapha Lotfi, Mohammed El Mahi, and Luis Javier Lozano Blanco. 2018. "Treatment of Mineral Oil Refinery Wastewater in Microbial Fuel Cells Using Ionic Liquid Based Separators" Applied Sciences 8, no. 3: 438. https://doi.org/10.3390/app8030438
APA StyleAddi, H., Mateo-Ramírez, F., Ortiz-Martínez, V. M., Salar-García, M. J., Hernández-Fernández, F. J., Pérez de los Ríos, A., Godínez, C., Lotfi, E. M., El Mahi, M., & Blanco, L. J. L. (2018). Treatment of Mineral Oil Refinery Wastewater in Microbial Fuel Cells Using Ionic Liquid Based Separators. Applied Sciences, 8(3), 438. https://doi.org/10.3390/app8030438






