Temperature Dependence on Density, Viscosity, and Electrical Conductivity of Ionic Liquid 1-Ethyl-3-Methylimidazolium Fluoride
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
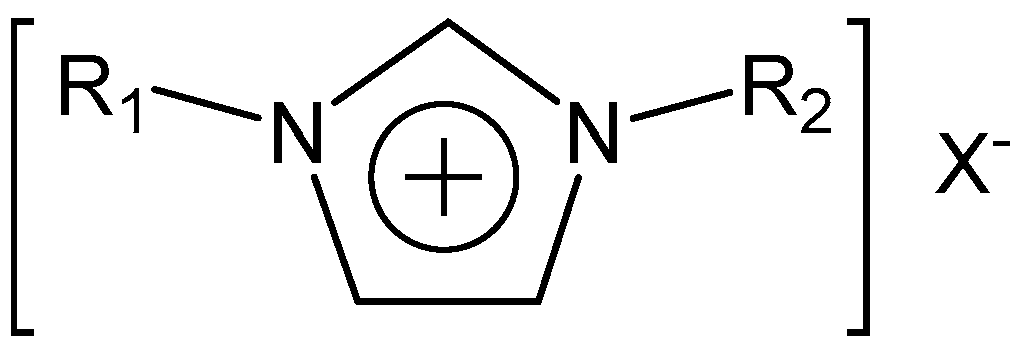
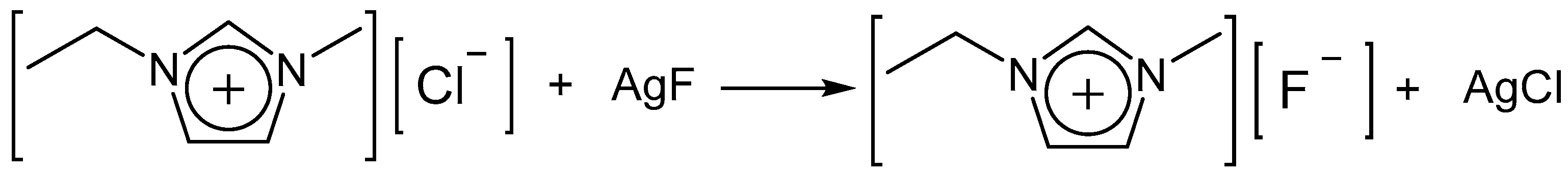
2.2. Synthesis of [EMIm]F
2.3. Physical Properties of [EMIm]F
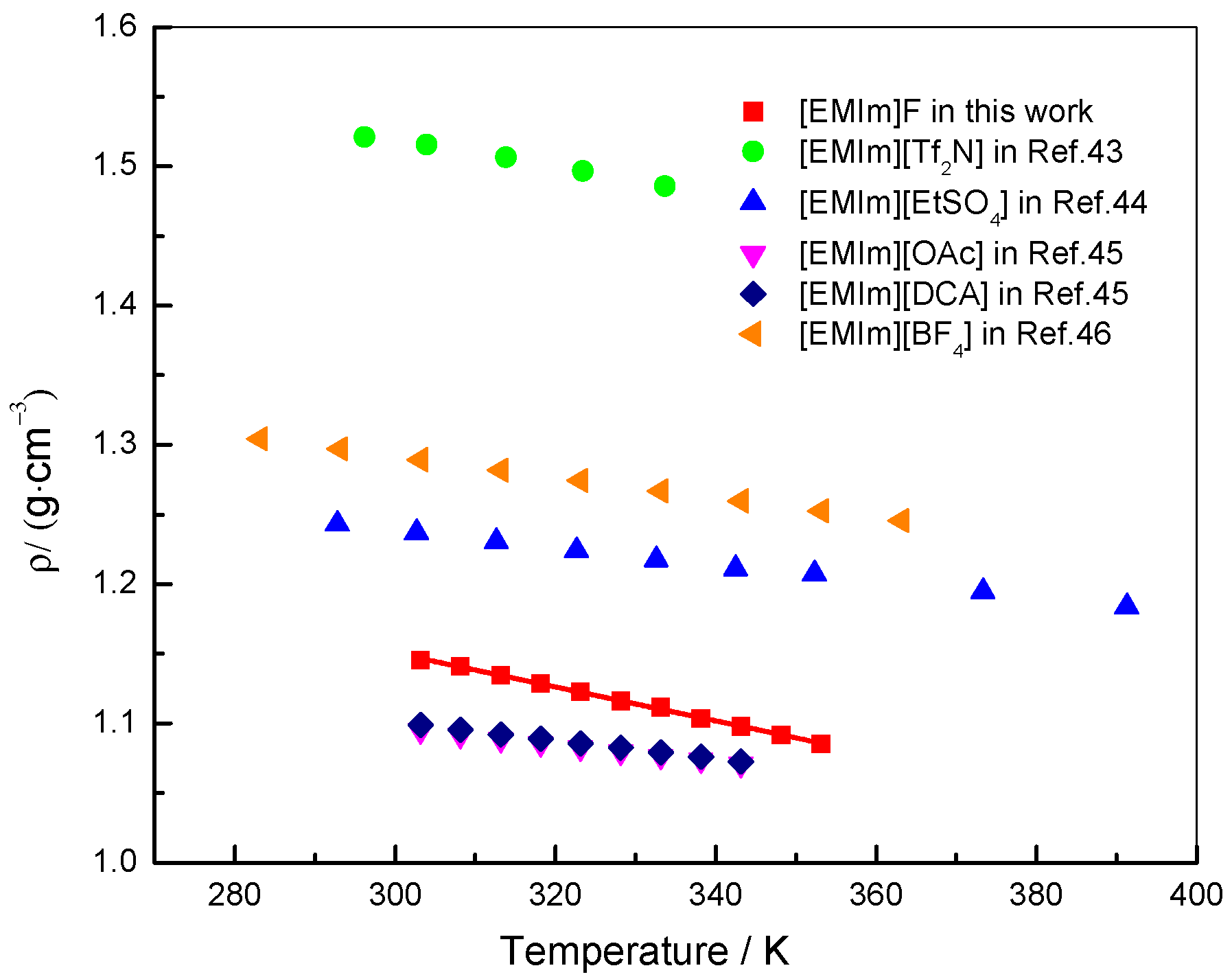
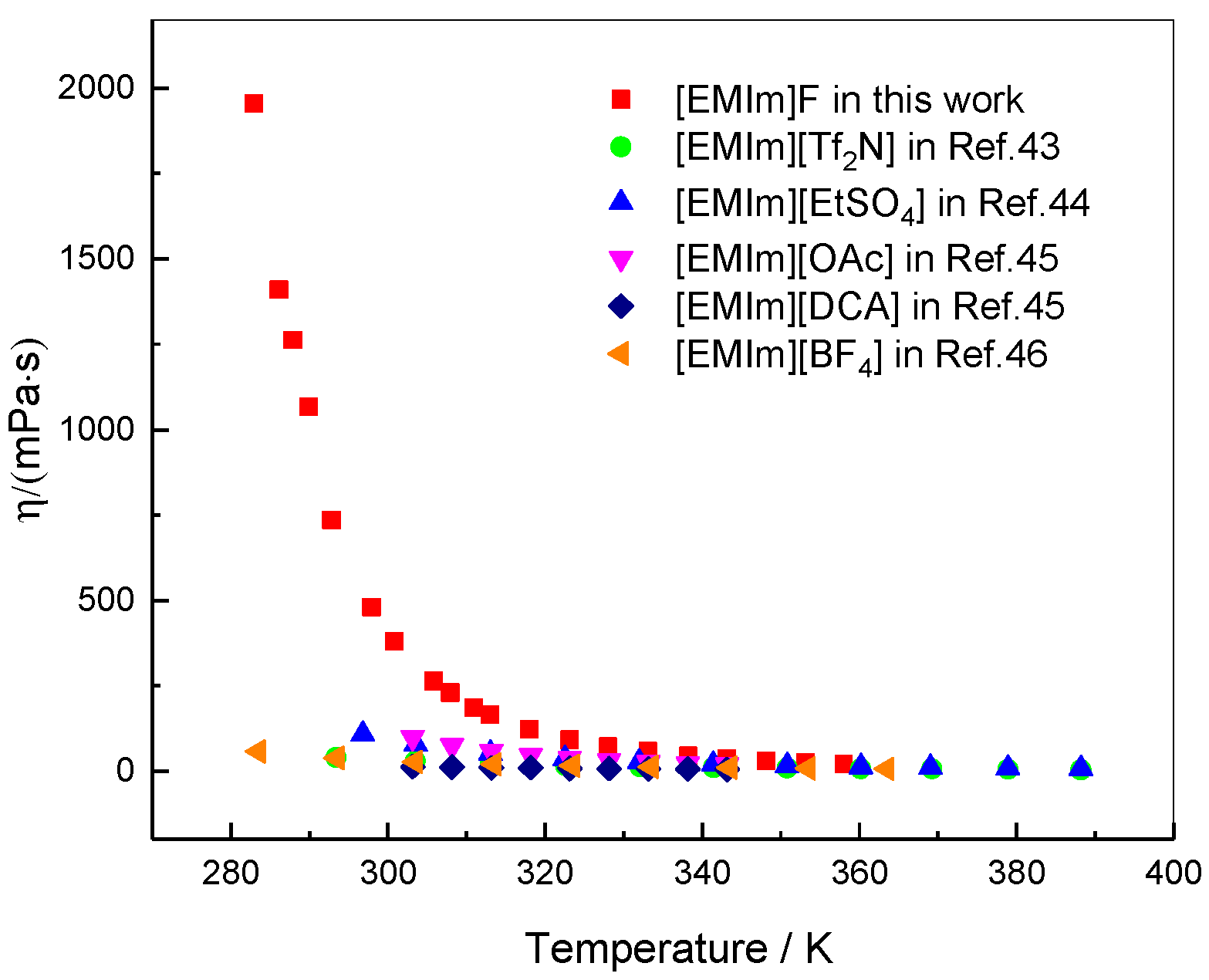
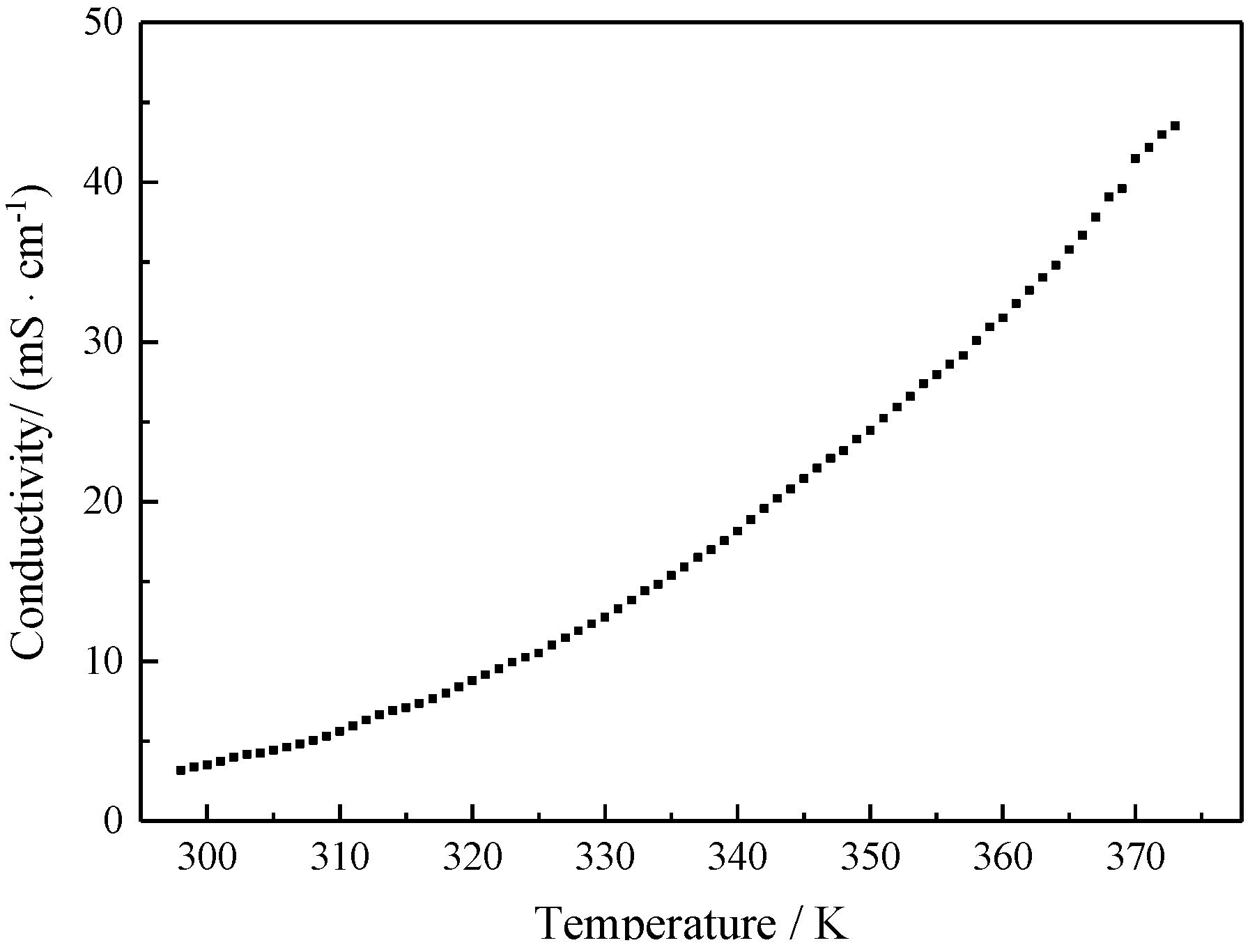
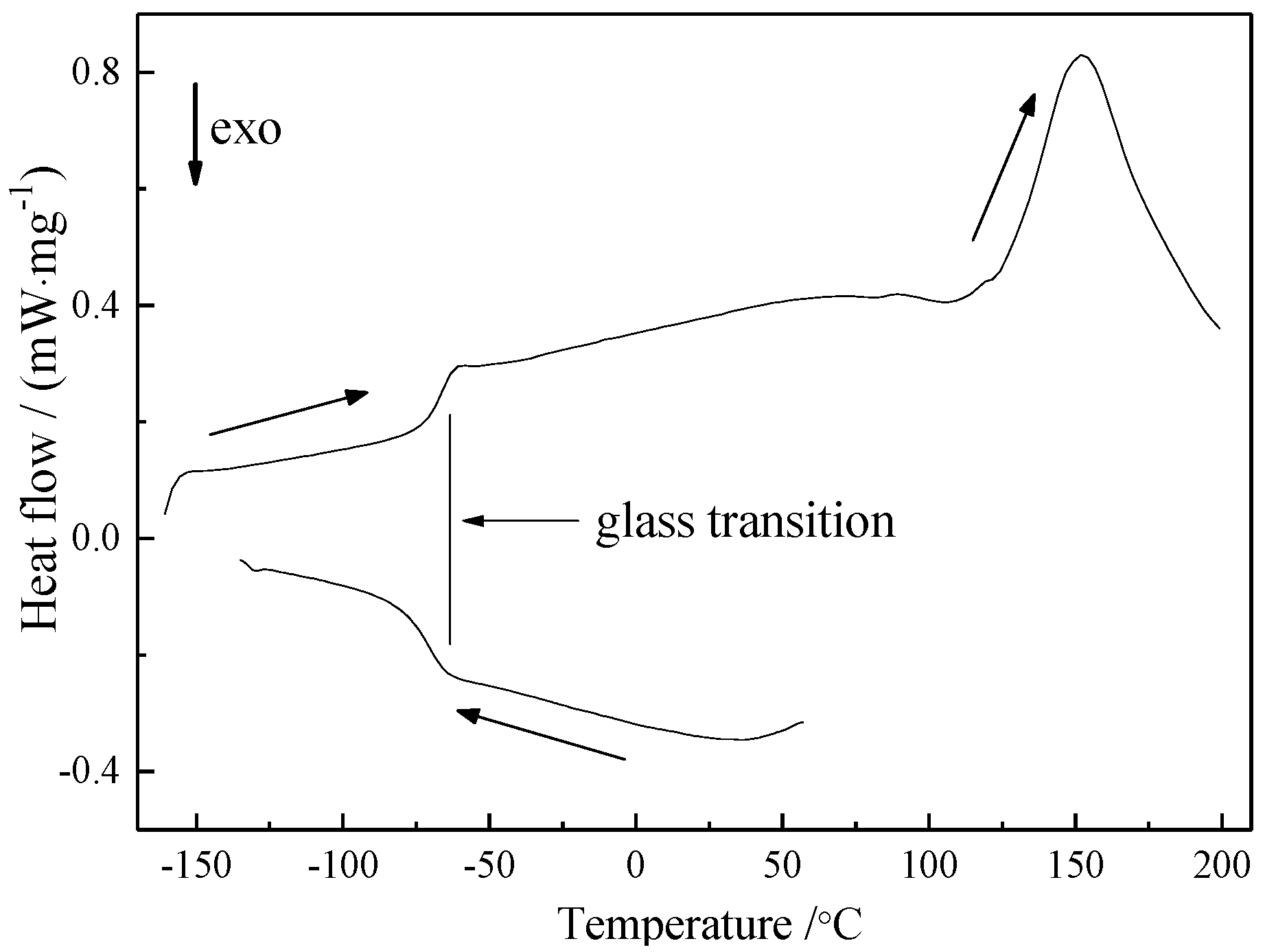
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hurley, F.H.; Weir, T.P. The Electrodeposition of Aluminum from Nonaqueous Solutions at Room Temperature. J. Electrochem. Soc. 1951, 98, 207–212. [Google Scholar] [CrossRef]
- Chum, H.L.; Koch, V.R.; Miller, L.L.; Osteryoung, R.A. Electrochemical scrutiny of organometallic iron complexes and hexamethylbenzene in a room temperature molten salt. J. Am. Chem. Soc. 1975, 97, 3264–3265. [Google Scholar] [CrossRef]
- Wilkes, J.S.; Levisky, J.A.; Wilson, R.A.; Hussey, C.L. Dialkylimidazolium chloroaluminate melts: A new class of room-temperature ionic liquids for electrochemistry, spectroscopy and synthesis. Inorg. Chem. 1982, 21, 1263–1264. [Google Scholar] [CrossRef]
- Wasserscheid, P.; Keim, W. Ionic liquids-new solution for transition metal catalysis. Angew. Chem. Int. Ed. 2000, 39, 3772–3789. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Q.; Zhang, S.; Lu, X.; Zhang, X. Electrodeposition in Ionic Liquids. Chem. Phys. Chem. 2016, 17, 335–351. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, J.; Tu, J.; Dreisinger, D. Ionic liquid electrodeposition of reactive metals. Miner. Process. Extr. Metall. 2008, 117, 113–117. [Google Scholar] [CrossRef]
- Xu, C.; Hua, Y.; Zhang, Q.; Li, J.; Lei, Z.; Lu, D. Electrodeposition of Al-Ti alloy on mild steel from AlCl3-BMIC ionic liquid. J. Solid State Electr. 2017, 21, 1349–1356. [Google Scholar] [CrossRef]
- Zhang, M.; Kamavarum, V.; Reddy, R.G. New electrolytes for aluminum production: Ionic liquids. JOM 2003, 55, 54–57. [Google Scholar] [CrossRef]
- Liu, A.; Shi, Z.; Reddy, R. Electrodeposition of Pb from PbO in urea and 1-butyl-3-methylimidazolium chloride deep eutectic solutions. Electrochim. Acta 2017, 251, 176–186. [Google Scholar] [CrossRef]
- Li, M.; Gao, B.; Liu, C. AlCl3/amide ionic liquids for electrodeposition of aluminium. J. Solid State Electr. 2016, 21, 1–8. [Google Scholar]
- Zou, F.; Yu, X.; Zhang, J.; Cheng, N.; Huang, X. Electropolymerization in a novel proton functionalized room temperature ionic liquid anilinium acetate. Synth. Met. 2015, 204, 76–83. [Google Scholar] [CrossRef]
- Huddleston, J.G.; Willauer, H.D.; Swatloski, R.P.; Visser, A.E.; Rogers, R.D. Room temperature ionic liquids as novel media for ‘clean’ liquid–liquid extraction. Chem. Commun. 1998, 16, 1765–1766. [Google Scholar] [CrossRef]
- Björling, M.; Bair, S.; Mu, L.; Zhu, J.; Shi, Y. Elastohydrodynamic Performance of a Bio-Based, Non-Corrosive Ionic Liquid. Appl. Sci. 2017, 7, 996. [Google Scholar] [CrossRef]
- Zheng, Y.; Zheng, Y.; Wang, Q.; Wang, Z.; Tian, D. Density and Viscosity of Binary Mixtures of 1-Ethyl-3-methylimidazolium Heptachlorodialuminate and Tetrachloroaluminate Ionic Liquids. J. Chem. Eng. Data 2017, 62, 4006–4014. [Google Scholar] [CrossRef]
- Jacquemin, J.; Husson, P.; Mayer, V.; Cibulka, I. High-Pressure Volumetric Properties of Imidazolium-Based Ionic Liquids: Effect of the Anion. J. Chem. Eng. Data 2007, 52, 2204–2211. [Google Scholar] [CrossRef]
- Anuar, N.K.; Subban, R.H.Y.; Mohamed, N.S. Properties of PEMA-NH4CF3SO3 Added to BMATSFI Ionic Liquid. Materials 2012, 5, 2609–2620. [Google Scholar] [CrossRef]
- Grothe, D.C.; Meyer, W.; Janietz, S. Acrylate Functionalized Tetraalkylammonium Salts with Ionic Liquid Properties. Molecules 2012, 17, 6593–6604. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.H.; Chen, Y.P.; Su, C.S. Density and Viscosity Measurements for Binary Mixtures of 1-Ethyl-3-methylimidazolium Tetrafluoroborate ([Emim][BF4]) with Dimethylacetamide, Dimethylformamide, and Dimethyl Sulfoxide. J. Chem. Eng. Data 2016, 61, 920–927. [Google Scholar] [CrossRef]
- Ghatee, M.H.; Zare, M.; Moosavi, F.; Zolghadr, A.R. Temperature-Dependent Density and Viscosity of the Ionic Liquids 1-Alkyl-3-methylimidazolium Iodides: Experiment and Molecular Dynamics Simulation. J. Chem. Eng. Data 2010, 55, 3084–3088. [Google Scholar] [CrossRef]
- Jacquemin, J.; Nancarrow, P.; Rooney, D.W.; Costa Gomes, M.F.; Husson, P.; Majer, V.; Pádua, A.A.; Hardacre, C. Prediction of Ionic Liquid Properties. II. Volumetric Properties as a Function of Temperature and Pressure. J. Chem. Eng. Data 2008, 53, 716–726. [Google Scholar] [CrossRef]
- Tokuda, H.; Hayamizu, K.; Ishii, K.; Susan, M.A.B.H.; Watanabe, M. Physicochemical Properties and Structures of Room Temperature Ionic Liquids. 1. Variation of Anionic Species. J. Phys. Chem. B 2004, 108, 16593–16600. [Google Scholar] [CrossRef]
- Tokuda, H.; Hayamizu, K.; Ishii, K.; Susan, M.A.B.H.; Watanabe, M. Physicochemical Properties and Structures of Room Temperature Ionic Liquids. 2. Variation of Alkyl Chain Length in Imidazolium Cation. J. Phys. Chem. B 2005, 109, 6103–6110. [Google Scholar] [CrossRef] [PubMed]
- Tokuda, H.; Ishii, K.; Susan, M.A.B.H.; Tsuzuki, S.; Hayamizu, K.; Watanabe, M. Physicochemical Properties and Structures of Room-Temperature Ionic Liquids. 3. Variation of Cationic Structures. J. Phys. Chem. B 2006, 110, 2833–2839. [Google Scholar] [CrossRef] [PubMed]
- Yadav, J.S.; Reddy, B.V.S.; Basak, A.K.; Narsaiah, A.V. [Bmim]BF4 Ionic Liquid: A Novel Reaction Medium for the Synthesis of β-Amino Alcohols. Tetrahedron Lett. 2003, 44, 1047–1050. [Google Scholar] [CrossRef]
- Chang, H.C.; Wang, T.H.; Burba, C.M. Probing Structures of Interfacial 1-Butyl-3-Methylimidazolium Trifluoromethanesulfonate Ionic Liquid on Nano-Aluminum Oxide Surfaces Using High-Pressure Infrared Spectroscopy. Appl. Sci. 2017, 7, 855. [Google Scholar] [CrossRef]
- Zhou, Z.; He, D.L.; Yang, R.H.; Guo, Y.N.; Zhong, J.F.; Li, G.X. Electropolymerization of benzotriazole in room temperature ionic liquid [bmim]PF6. J. Appl. Electrochem. 2008, 38, 1757–1761. [Google Scholar] [CrossRef]
- Harris, K.R.; Kanakubo, M. Self-diffusion, velocity cross-correlation, distinct diffusion and resistance coefficients of the ionic liquid [BMIM][Tf2N] at high pressure. Phys. Chem. Chem. Phys. 2015, 17, 23977. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; An, M.; Chen, Q.; Liu, A.; Jiang, X.; Ji, S.; Lian, Y.; Wen, X. Electrochemical Study of the Diffusion and Nucleation of Gallium(III) in [Bmim][TfO] Ionic Liquid. Electrochim. Acta 2016, 190, 1066–1077. [Google Scholar] [CrossRef]
- Larriba, M.; Navarro, P.; García, J.; Rodríguez, F. Liquid–Liquid Extraction of Toluene from Heptane Using [emim][DCA], [bmim][DCA], and [emim][TCM] Ionic Liquids. Ind. Eng. Chem. Res. 2013, 52, 2714–2720. [Google Scholar] [CrossRef]
- Fernández, A.; García, J.; Torrecilla, J.S.; Oliet, M.; Rodríguez, F. Volumetric, Transport and Surface Properties of [bmim][MeSO4] and [emim][EtSO4] Ionic Liquids As a Function of Temperature. J. Chem. Eng. Data 2008, 53, 1518–1522. [Google Scholar] [CrossRef]
- Tagiuri, A.; Sumon, K.Z.; Henni, A. Solubility of carbon dioxide in three [Tf2N] ionic liquids. Fluid Phase Equilib. 2014, 380, 39–47. [Google Scholar] [CrossRef]
- Zhao, X.; Hu, L.; Geng, Y.; Wang, Y. The structure of acidified ionic liquid [emim]BF4 and its catalytic performance in the reaction for 4,4′-MDC synthesis. J. Mol. Catal. A Chem. 2007, 276, 168–173. [Google Scholar] [CrossRef]
- Lin, Y.F.; Sun, I.W. Electrodeposition of zinc from a Lewis acidic zinc chloride-1-ethyl-3-methylimidazolium chloride molten salt. Electrochim. Acta 1999, 46, 1169–1177. [Google Scholar] [CrossRef]
- Horikawa, Y.; Tokushima, T.; Takahashi, O.; Hoke, H.; Takamuku, T. Correlation between Soft X-ray Absorption and Emission Spectra of the Nitrogen Atoms within Imidazolium-Based Ionic Liquids. J. Phys. Chem. B 2016, 120, 7480–7487. [Google Scholar] [CrossRef] [PubMed]
- Alavi, S.; Thompson, D.L. Molecular dynamics studies of melting and some liquid-state properties of 1-ethyl-3-methylimidazoliumhexafluorophosphate [emim][PF6]. J. Chem. Phys. 2005, 122, 154704–154715. [Google Scholar] [CrossRef] [PubMed]
- Afsharpour, A.; Kheiri, A. Implementation of CPA EoS for simultaneous solubility modeling of CO2 and H2S in ionic liquid [OMIM][Tf2N]. Pet. Sci. Technol. 2017, 36, 280–286. [Google Scholar] [CrossRef]
- Hagiwara, R.; Hirashige, T.; Tsuda, T.; Ito, Y. Acidic 1-ethyl-3-methylimidazolium fluoride: A new room temperature ionic liquid. J. Fluor. Chem. 1999, 99, 1–3. [Google Scholar] [CrossRef]
- Pagoria, P.F.; Maiti, A.; Gash, A.; Han, T.Y.; Orme, C.; Fried, L. Ionic Liquids as Solvents. US 20090012297, 8 January 2009. [Google Scholar]
- Zhu, Z.Q.; Jiang, M.Y.; Zheng, C.G.; Xiao, J.C. Efficient synthesis of 1-alkyl-3-methylimidazolium fluorides and possibility of the existence of hydrogen bonding between fluoride anion and C(sp3)–H. J. Fluor. Chem. 2012, 133, 160–162. [Google Scholar] [CrossRef]
- Nuñez, N.O.; Ocaña, M. An ionic liquid based synthesis method for uniform luminescent lanthanide fluoride nanoparticles. Nanotechnology 2007, 18, 455606. [Google Scholar] [CrossRef]
- Olchowka, J.; Suta, M.; Wickleder, C. Green synthesis of small nanoparticles of A2SiF6 (A = Li-Cs) using Ionic Liquids as Solvent and Fluorine Source: A novel simple approach without HF. Chem. Eur. J. 2017, 23, 12092–12095. [Google Scholar] [CrossRef] [PubMed]
- Brennecke, J.F.; Maginn, E.J. Ionic liquids: Innovative fluids for chemical processing. Aiche J. 2001, 47, 2384–2389. [Google Scholar] [CrossRef]
- Fredlake, C.P.; Crosthwaite, J.M.; Hert, D.G.; Aki, S.N. Thermophysical Properties of Imidazolium-Based Ionic Liquids. J. Chem. Eng. Data 2004, 49, 954–964. [Google Scholar] [CrossRef]
- Jacquemin, J.; Husson, P.; Padua, A.A.H.; Majer, V. Density and viscosity of several pure and water-saturated ionic liquids. Green Chem. 2006, 8, 172–180. [Google Scholar] [CrossRef]
- Quijada-Maldonado, E.; Boogaart, S.V.D.; Lijbers, J.H.; Meindersma, G.W.; De Haan, A.B. Experimental densities, dynamic viscosities and surface tensions of the ionic liquids series 1-ethyl-3-methylimidazolium acetate and dicyanamide and their binary and ternary mixtures with water and ethanol at T = (298.15 to 343.15 K). J. Chem. Thermodyn. 2012, 51, 51–58. [Google Scholar] [CrossRef]
- Shamsipur, M.; Beigi, A.A.M.; Teymouri, M.; Pourmortazavi, S.M.; Irandoust, M. Physical and electrochemical properties of ionic liquids 1-ethyl-3-methylimidazolium tetrafluoroborate, 1-butyl-3-methylimidazolium trifluoromethanesulfonate and 1-butyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl)imide. J. Mol. Liq. 2010, 157, 43–50. [Google Scholar] [CrossRef]
- Yang, J.Z.; Lu, X.M.; Gui, J.S.; Xu, W.G. A new theory for ionic liquids-the Interstice Model Part 1. The density and surface tension of ionic liquid EMISE. Green Chem. 2004, 6, 541–543. [Google Scholar] [CrossRef]
- Gu, Z.; Brennecke, J.F. Volume Expansivities and Isothermal Compressibilities of Imidazolium and Pyridinium-Based Ionic Liquids. J. Chem. Eng. Data 2002, 47, 339–345. [Google Scholar] [CrossRef]
- Nishikawa, K.; Wang, S.; Katayanagi, H.; Hayashi, S.; Hamaguchi, H.; Koga, Y.; Tozaki, K. Melting and Freezing Behaviors of Prototype Ionic Liquids, 1-Butyl-3-methylimidazolium Bromide and Its Chloride, Studied by Using a Nano-Watt Differential Scanning Calorimeter. J. Phys. Chem. B 2007, 111, 4894–4900. [Google Scholar] [CrossRef] [PubMed]
- Huddleston, J.G.; Visser, A.E.; Reichert, W.M.; Willauer, H.D.; Broker, G.A.; Rogers, R.D. Characterization and comparison of hydrophilic and hydrophobic room temperature ionic liquids incorporating the imidazoliumcation. Green Chem. 2001, 3, 156–164. [Google Scholar] [CrossRef]
- Imanari, M.; Fujii, K.; Endo, T.; Seki, H.; Tozaki, K.; Nishikawa, K. Ultraslow Dynamics at Crystallization of a Room Temperature Ionic Liquid, 1-Butyl-3-methylimidazolium Bromide. J. Phys. Chem. B 2012, 116, 3991–3997. [Google Scholar] [CrossRef] [PubMed]
- Ngo, H.L.; Lecompte, K.; Hargens, L.; Mcewen, A.B. Thermal properties of imidazolium ionic liquids. Thermochim. Acta 2000, 357, 97–102. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Zhong, X.; Xu, J.; Kamali, A.; Shi, Z. Temperature Dependence on Density, Viscosity, and Electrical Conductivity of Ionic Liquid 1-Ethyl-3-Methylimidazolium Fluoride. Appl. Sci. 2018, 8, 356. https://doi.org/10.3390/app8030356
Liu F, Zhong X, Xu J, Kamali A, Shi Z. Temperature Dependence on Density, Viscosity, and Electrical Conductivity of Ionic Liquid 1-Ethyl-3-Methylimidazolium Fluoride. Applied Sciences. 2018; 8(3):356. https://doi.org/10.3390/app8030356
Chicago/Turabian StyleLiu, Fengguo, Xiongwei Zhong, Junli Xu, Ali Kamali, and Zhongning Shi. 2018. "Temperature Dependence on Density, Viscosity, and Electrical Conductivity of Ionic Liquid 1-Ethyl-3-Methylimidazolium Fluoride" Applied Sciences 8, no. 3: 356. https://doi.org/10.3390/app8030356
APA StyleLiu, F., Zhong, X., Xu, J., Kamali, A., & Shi, Z. (2018). Temperature Dependence on Density, Viscosity, and Electrical Conductivity of Ionic Liquid 1-Ethyl-3-Methylimidazolium Fluoride. Applied Sciences, 8(3), 356. https://doi.org/10.3390/app8030356





