Excitation of Wet Perovskite Films by Ultrasonic Vibration Improves the Device Performance
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- National Renewable Energy Laboratory. Efficiency Chart. Available online: https://www.nrel.gov/pv/assets/images/efficiency-chart.png (accessed on 8 February 2018).
- Saliba, M.; Matsui, T.; Domanski, K.; Seo, J.-Y.; Ummadisingu, A.; Zakeeruddin, S.M.; Correa-Baena, J.-P.; Tress, W.R.; Abate, A.; Hagfeldt, A.; et al. Incorporation of rubidium cations into perovskite solar cells improves photovoltaic performance. Science 2016, 354, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Saliba, M.; Matsui, T.; Seo, J.-Y.; Domanski, K.; Correa-Baena, J.-P.; Nazeeruddin, M.K.; Zakeeruddin, S.M.; Tress, W.; Abate, A.; Hagfeldt, A.; et al. Cesium-containing triple cation perovskite solar cells: Improved stability, reproducibility and high efficiency. Energy Environ. Sci. 2016, 9, 1989–1997. [Google Scholar] [CrossRef] [PubMed]
- Habibi, M.; Zabihi, F.; Ahmadian-Yazdi, M.R.; Eslamian, M. Progress in emerging solution-processed thin film solar cells – part II: Perovskite solar cells. Renew. Sustain. Energy Rev. 2016, 62, 1012–1031. [Google Scholar] [CrossRef]
- Pellet, N.; Gao, P.; Gregori, G.; Yang, T.-Y.; Nazeeruddin, M.K.; Maier, J.; Grätzel, M. Mixed-organic-cation perovskite photovoltaics for enhanced solar-light harvesting. Angew. Chem. Int. Ed. 2014, 53, 3151–3157. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Peng, J.; Chen, Y.; Yao, Y.; Liang, Z. Triple-cation mixed-halide perovskites: Towards efficient, annealing-free and air-stable solar cells enabled by Pb(SCN)2 additive. Sci. Rep. 2017, 7, 46193. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.S.; Park, N.-G. Perovskite solar cells: From materials to devices. Small 2015, 11, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Nejand, B.A.; Gharibzadeh, S.; Ahmadi, V.; Shahverdi, H.R. Novel solvent-free perovskite deposition in fabrication of normal and inverted architectures of perovskite solar cells. Sci. Rep. 2016, 6, 33649. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chueh, C.-C.; Eslamian, M.; Jen, A.K.Y. Modulation of PEDOT: PSS pH for efficient inverted perovskite solar cells with reduced potential loss and enhanced stability. ACS Appl. Mater. Interfaces 2016, 8, 32068–32076. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; McLeod, J.A.; Yang, Y.; Wang, Y.; Wu, Z.; Bai, S.; Yuan, Z.; Song, T.; Wang, Y.; Si, J.; et al. Iodomethane-mediated organometal halide perovskite with record photoluminescence lifetime. ACS Appl. Mater. Interfaces 2016, 8, 23181–23189. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Jin, Y.; Gao, F. Organometal halide perovskites for photovoltaic applications. In Advanced Functional Materials; Tiwari, A., Uzun, L., Eds.; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 535–566. [Google Scholar] [CrossRef]
- Eperon, G.E.; Stranks, S.D.; Menelaou, C.; Johnston, M.B.; Herz, L.M.; Snaith, H.J. Formamidinium lead trihalide: A broadly tunable perovskite for efficient planar heterojunction solar cells. Energy Environ. Sci. 2014, 7, 982–988. [Google Scholar] [CrossRef]
- Heo, J.H.; Song, D.H.; Im, S.H. Planar CH3NH3PbBr3 hybrid solar cells with 10.4% power conversion efficiency, fabricated by controlled crystallization in the spin-coating process. Adv. Mater. 2014, 26, 8179–8183. [Google Scholar] [CrossRef] [PubMed]
- Docampo, P.; Hanusch, F.C.; Stranks, S.D.; Döblinger, M.; Feckl, J.M.; Ehrensperger, M.; Minar, N.K.; Johnston, M.B.; Snaith, H.J.; Bein, T. Solution deposition-conversion for planar heterojunction mixed halide perovskite solar cells. Adv. Energy Mater. 2014, 4. [Google Scholar] [CrossRef]
- Zuo, C.; Ding, L. An 80.11% FF record achieved for perovskite solar cells by using the NH4Cl additive. Nanoscale 2014, 6, 9935–9938. [Google Scholar] [CrossRef] [PubMed]
- Colella, S.; Mosconi, E.; Fedeli, P.; Listorti, A.; Gazza, F.; Orlandi, F.; Ferro, P.; Besagni, T.; Rizzo, A.; Calestani, G.; et al. MAPbI3−xClx mixed halide perovskite for hybrid solar cells: The role of chloride as dopant on the transport and structural properties. Chem. Mater. 2013, 25, 4613–4618. [Google Scholar] [CrossRef]
- Liang, P.-W.; Liao, C.-Y.; Chueh, C.-C.; Zuo, F.; Williams, S.T.; Xin, X.-K.; Lin, J.; Jen Alex, K.-Y. Additive enhanced crystallization of solution-processed perovskite for highly efficient planar-heterojunction solar cells. Adv. Mater. 2014, 26, 3748–3754. [Google Scholar] [CrossRef] [PubMed]
- Jeon, N.J.; Noh, J.H.; Kim, Y.C.; Yang, W.S.; Ryu, S.; II Seok, S. Solvent engineering for high-performance inorganic-organic hybrid perovskite solar cells. Nat. Mater. 2014, 13, 897. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Wu, Z.; Dong, H.; Xi, J.; Wu, W.; Lei, T.; Xi, K.; Yuan, F.; Jiao, B.; Xiao, L.; et al. Formation of ultrasmooth perovskite films toward highly efficient inverted planar heterojunction solar cells by micro-flowing anti-solvent deposition in air. J. Mater. Chem. A 2016, 4, 6295–6303. [Google Scholar] [CrossRef]
- Xie, Y.; Zabihi, F.; Eslamian, M. Fabrication of highly reproducible polymer solar cells using ultrasonic substrate vibration posttreatment. J. Photonics Energy 2016, 6, 045502. [Google Scholar] [CrossRef]
- Zabihi, F.; Chen, Q.; Xie, Y.; Eslamian, M. Fabrication of efficient graphene-doped polymer/fullerene bilayer organic solar cells in air using spin coating followed by ultrasonic vibration post treatment. Superlattices Microstruct. 2016, 100, 1177–1192. [Google Scholar] [CrossRef]
- Zabihi, F.; Ahmadian-Yazdi, M.-R.; Eslamian, M. Fundamental study on the fabrication of inverted planar perovskite solar cells using two-step sequential substrate vibration-assisted spray coating (2S-SVASC). Nanoscale Res. Lett. 2016, 11, 71. [Google Scholar] [CrossRef] [PubMed]
- Rahimzadeh, A.; Eslamian, M. On evaporation of thin liquid films subjected to ultrasonic substrate vibration. Int. Commun. Heat Mass Transf. 2017, 83, 15–22. [Google Scholar] [CrossRef]
- Rahimzadeh, A.; Eslamian, M. Stability of thin liquid films subjected to ultrasonic vibration and characteristics of the resulting thin solid films. Chem. Eng. Sci. 2017, 158, 587–598. [Google Scholar] [CrossRef]
- Eslamian, M. Excitation by acoustic vibration as an effective tool for improving the characteristics of the solution-processed coatings and thin films. Prog. Org. Coat. 2017, 113, 60–73. [Google Scholar] [CrossRef]
- Habibi, M.; Rahimzadeh, A.; Eslamian, M. On dewetting of thin films due to crystallization (crystallization dewetting). Eur. Phys. J. E 2016, 39, 30. [Google Scholar] [CrossRef] [PubMed]
- Carnie, M.J.; Charbonneau, C.; Davies, M.L.; Troughton, J.; Watson, T.M.; Wojciechowski, K.; Snaith, H.; Worsley, D.A. A one-step low temperature processing route for organolead halide perovskite solar cells. Chem. Commun. 2013, 49, 7893–7895. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Zou, X.; Qi, X.; Teng, G.; Li, Q.; Guo, D.; Zeng, S. Effect of perovskite film preparation on performance of solar cells. J. Chem. 2016, 2016, 10. [Google Scholar] [CrossRef]
- Xu, Q.-Y.; Yuan, D.-X.; Mu, H.-R.; Igbari, F.; Bao, Q.; Liao, L.-S. Efficiency enhancement of perovskite solar cells by pumping away the solvent of precursor film before annealing. Nanoscale Res. Lett. 2016, 11, 248. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian-Yazdi, M.R.; Zabihi, F.; Habibi, M.; Eslamian, M. Effects of process parameters on the characteristics of mixed-halide perovskite solar cells fabricated by one-step and two-step sequential coating. Nanoscale Res. Lett. 2016, 11, 408. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Zhang, B.; Chen, Y.; Zhang, A.; Ke, X. Improving the photoluminescence properties of perovskite CH3NH3PbBr3−xClx films by modulating organic cation and chlorine concentrations. ACS Appl. Mater. Interfaces 2016, 8, 12756–12763. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Saliba, M.; Moore, D.T.; Pathak, S.K.; Hörantner, M.T.; Stergiopoulos, T.; Stranks, S.D.; Eperon, G.E.; Alexander-Webber, J.A.; Abate, A.; et al. Ultrasmooth organic–inorganic perovskite thin-film formation and crystallization for efficient planar heterojunction solar cells. Nat. Commun. 2015, 6, 6142. [Google Scholar] [CrossRef] [PubMed]
- Patterson, A.L. The scherrer formula for x-ray particle size determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Zhou, Y.; Vasiliev, A.L.; Wu, W.; Yang, M.; Pang, S.; Zhu, K.; Padture, N.P. Crystal morphologies of organolead trihalide in mesoscopic/planar perovskite solar cells. J. Phys. Chem. Lett. 2015, 6, 2292–2297. [Google Scholar] [CrossRef] [PubMed]
- Zabihi, F.; Eslamian, M. Effect of the ultrasonic substrate vibration on nucleation and crystallization of PbI2 crystals and thin films. Crystals 2018, 8, 60. [Google Scholar] [CrossRef]
- Ko, H.-S.; Lee, J.-W.; Park, N.-G. 15.76% efficiency perovskite solar cells prepared under high relative humidity: Importance of PbI2 morphology in two-step deposition of CH3NH3PbI3. J. Mater. Chem. A 2015, 3, 8808–8815. [Google Scholar] [CrossRef]
- Wehrenfennig, C.; Liu, M.; Snaith, H.J.; Johnston, M.B.; Herz, L.M. Charge carrier recombination channels in the low-temperature phase of organic-inorganic lead halide perovskite thin films. APL Mater. 2014, 2, 081513. [Google Scholar] [CrossRef]
- Li, D.; Wang, G.; Cheng, H.-C.; Chen, C.-Y.; Wu, H.; Liu, Y.; Huang, Y.; Duan, X. Size-dependent phase transition in methylammonium lead iodide perovskite microplate crystals. Nat. Commun. 2016, 7, 11330. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Kranz, L.; Yoon, S.; Löckinger, J.; Jäger, T.; Perrenoud, J.; Feurer, T.; Gretener, C.; Buecheler, S.; Tiwari, A.N. Controlled growth of PbI2 nanoplates for rapid preparation of CH3NH3PbI3 in planar perovskite solar cells. Phys. Status Solidi A 2015, 212, 2708–2717. [Google Scholar] [CrossRef]
- Kong, W.; Ye, Z.; Qi, Z.; Zhang, B.; Wang, M.; Rahimi-Iman, A.; Wu, H. Characterization of an abnormal photoluminescence behavior upon crystal-phase transition of perovskite CH3NH3PbI3. Phys. Chem. Chem. Phys. 2015, 17, 16405–16411. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Park, N.-G. Parameters affecting I–V hysteresis of CH3NH3PbI3 perovskite solar cells: Effects of perovskite crystal size and mesoporous TiO2 layer. J. Phys. Chem. Lett. 2014, 5, 2927–2934. [Google Scholar] [CrossRef] [PubMed]
- Nie, W.; Tsai, H.; Asadpour, R.; Blancon, J.-C.; Neukirch, A.J.; Gupta, G.; Crochet, J.J.; Chhowalla, M.; Tretiak, S.; Alam, M.A.; et al. High-efficiency solution-processed perovskite solar cells with millimeter-scale grains. Science 2015, 347, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Raga, S.R.; Jung, M.-C.; Lee, M.V.; Leyden, M.R.; Kato, Y.; Qi, Y. Influence of air annealing on high efficiency planar structure perovskite solar cells. Chem. Mater. 2015, 27, 1597–1603. [Google Scholar] [CrossRef]
- Xiao, Z.; Dong, Q.; Bi, C.; Shao, Y.; Yuan, Y.; Huang, J. Solvent annealing of perovskite-induced crystal growth for photovoltaic-device efficiency enhancement. Adv. Mater. 2014, 26, 6503–6509. [Google Scholar]
- Im, J.-H.; Kim, H.-S.; Park, N.-G. Morphology-photovoltaic property correlation in perovskite solar cells: One-step versus two-step deposition of CH3NH3PbI3. APL Mater. 2014, 2, 081510. [Google Scholar] [CrossRef]
- Shi, Y.; Xing, Y.; Li, Y.; Dong, Q.; Wang, K.; Du, Y.; Bai, X.; Wang, S.; Chen, Z.; Ma, T. CH3NH3PbI3 and CH3NH3PbI3−xClx in planar or mesoporous perovskite solar cells: Comprehensive insight into the dependence of performance on architecture. J. Phys. Chem. C 2015, 119, 15868–15873. [Google Scholar]
- Wu, C.G.; Chiang, C.H.; Tseng, Z.L.; Zazeeruddin, M.K.; Hagfeldt, A.; Gratzel, M. High efficiency stable inverted perovskite solar cells without current hysteresis. Energy Environ. Sci. 2015, 8, 2725–2733. [Google Scholar]
- Jiang, L.; Zheng, J.; Chen, W.; Huang, Y.; Hu, L.; Hayat, T.; Alsaedi, A.; Zhang, C.; Dai, S. High-performance perovskite solar cells with a weak covalent TiO2: Eu3+ mesoporous structure. ACS Appl. Energy Mater. 2017, 1, 93–102. [Google Scholar] [CrossRef]
- Ahmadian-Yazdi, M.-R.; Eslamian, M. Toward scale-up of perovskite solar cells: Annealing-free perovskite layer by low-cost ultrasonic substrate vibration of wet films. Mater. Today Commun. 2018, 14, 151–159. [Google Scholar] [CrossRef]
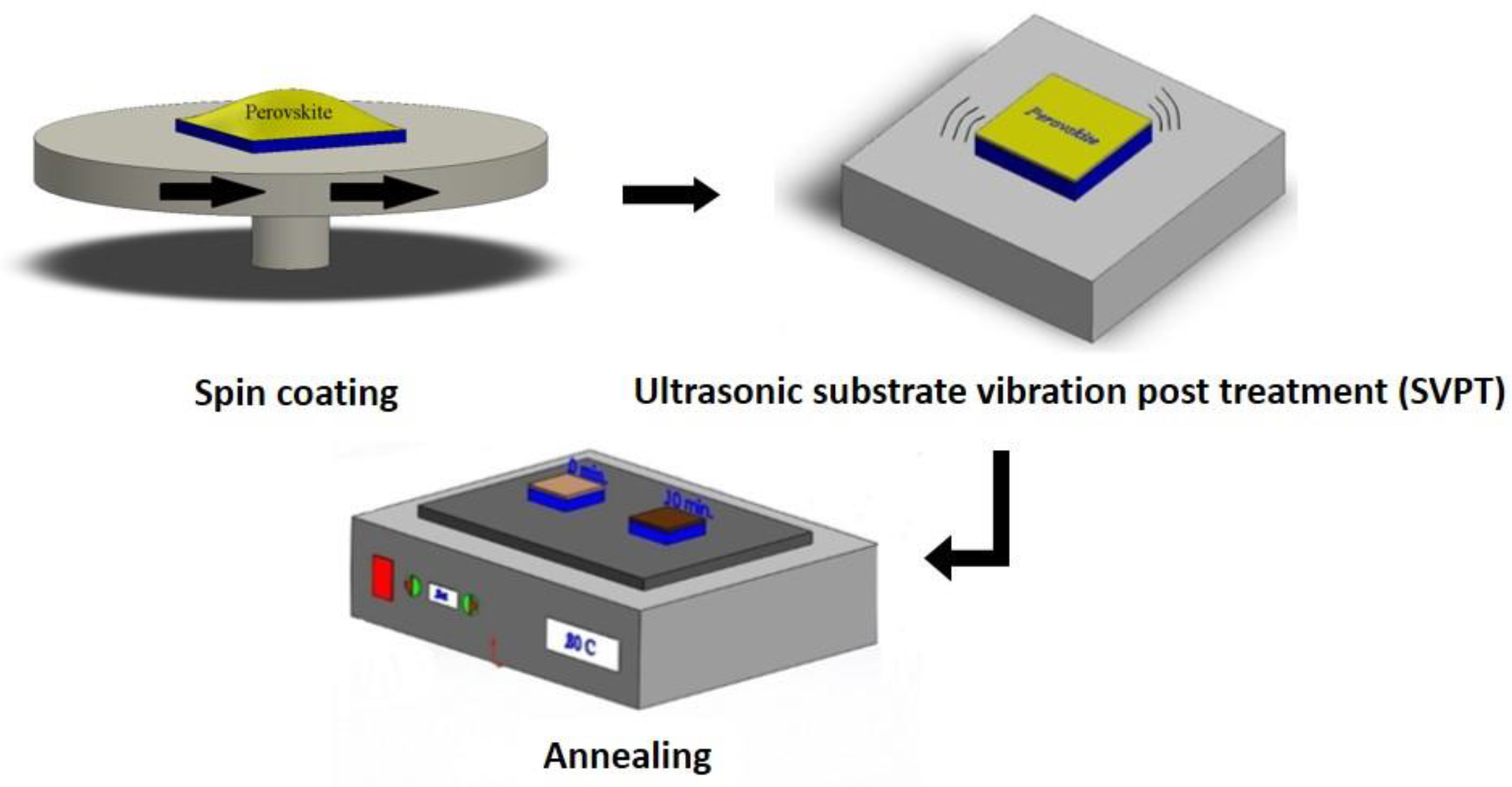
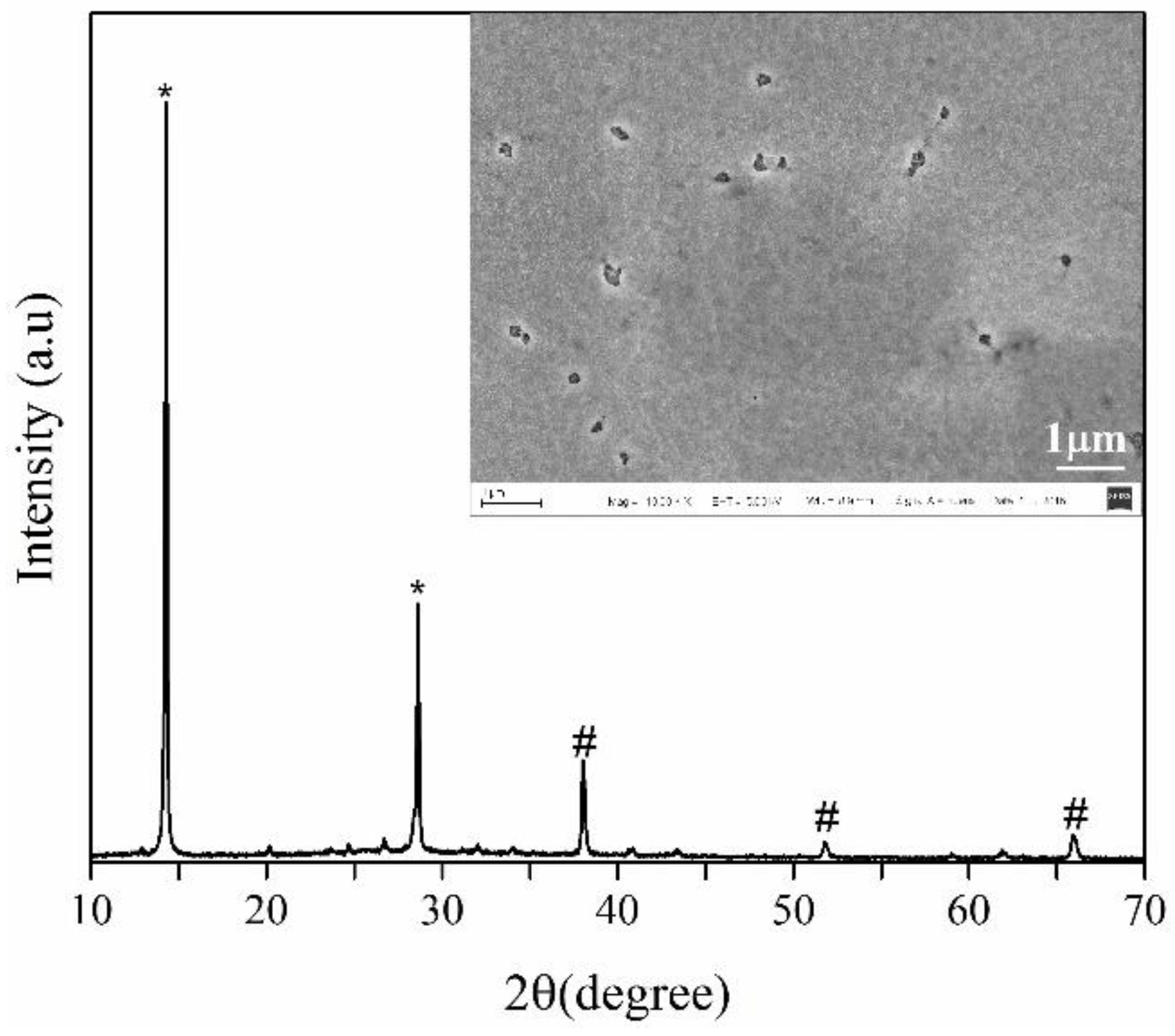
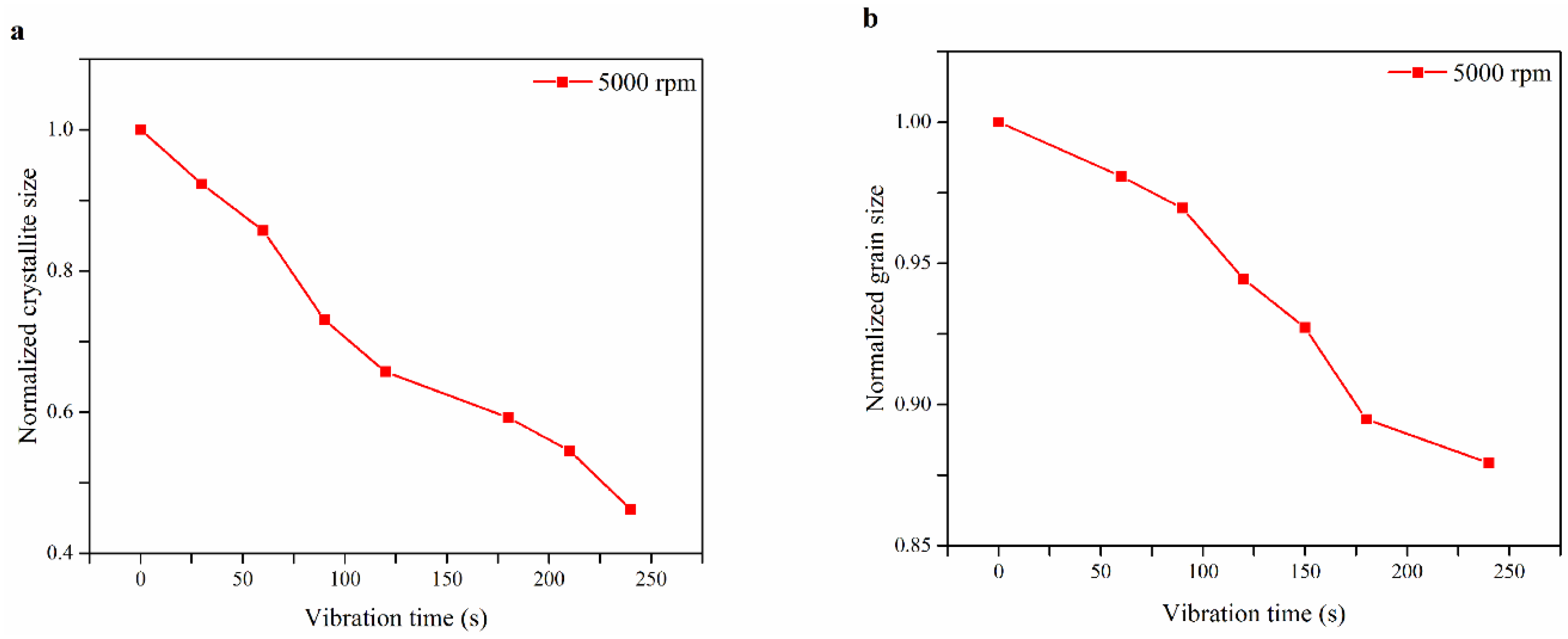
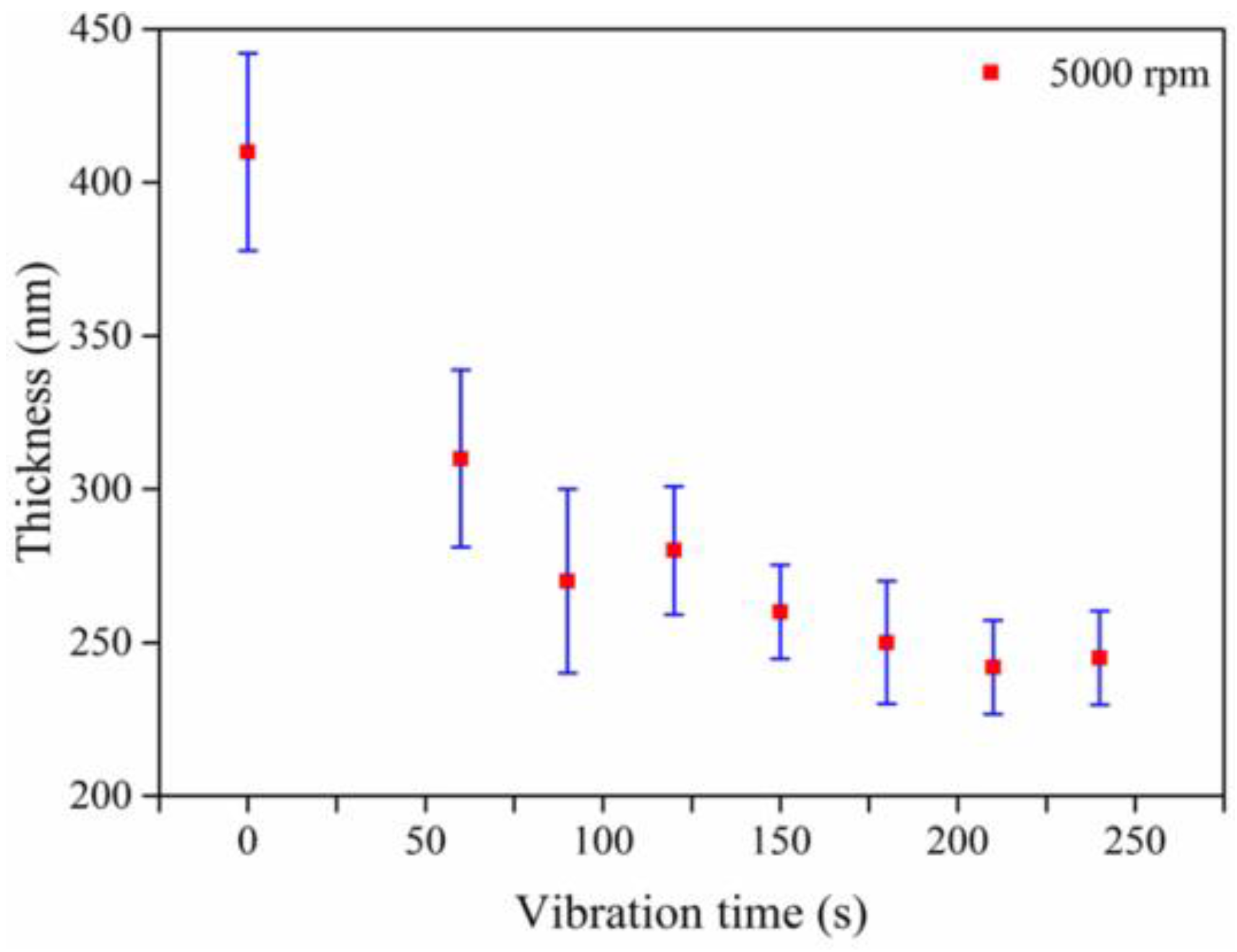
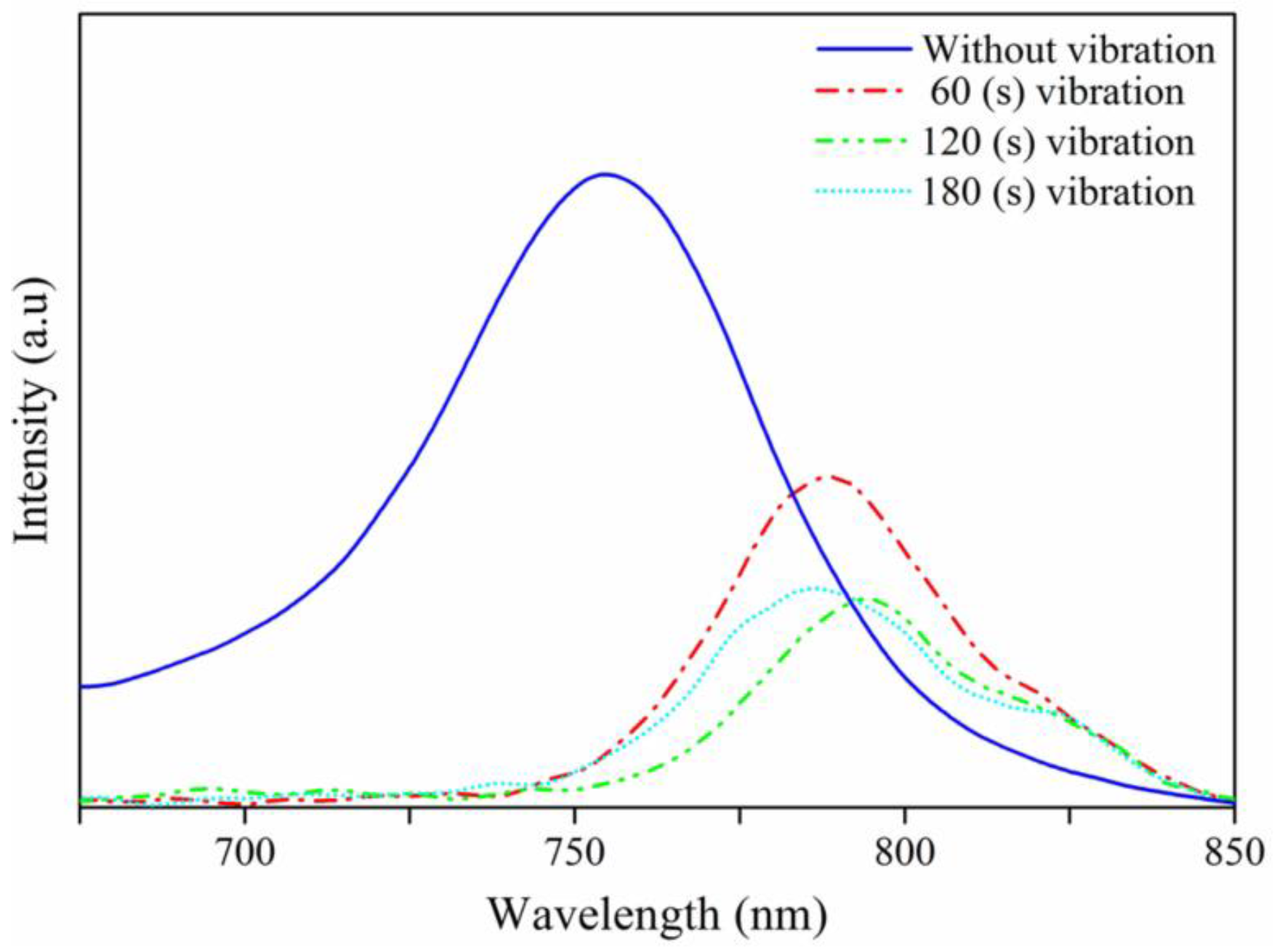
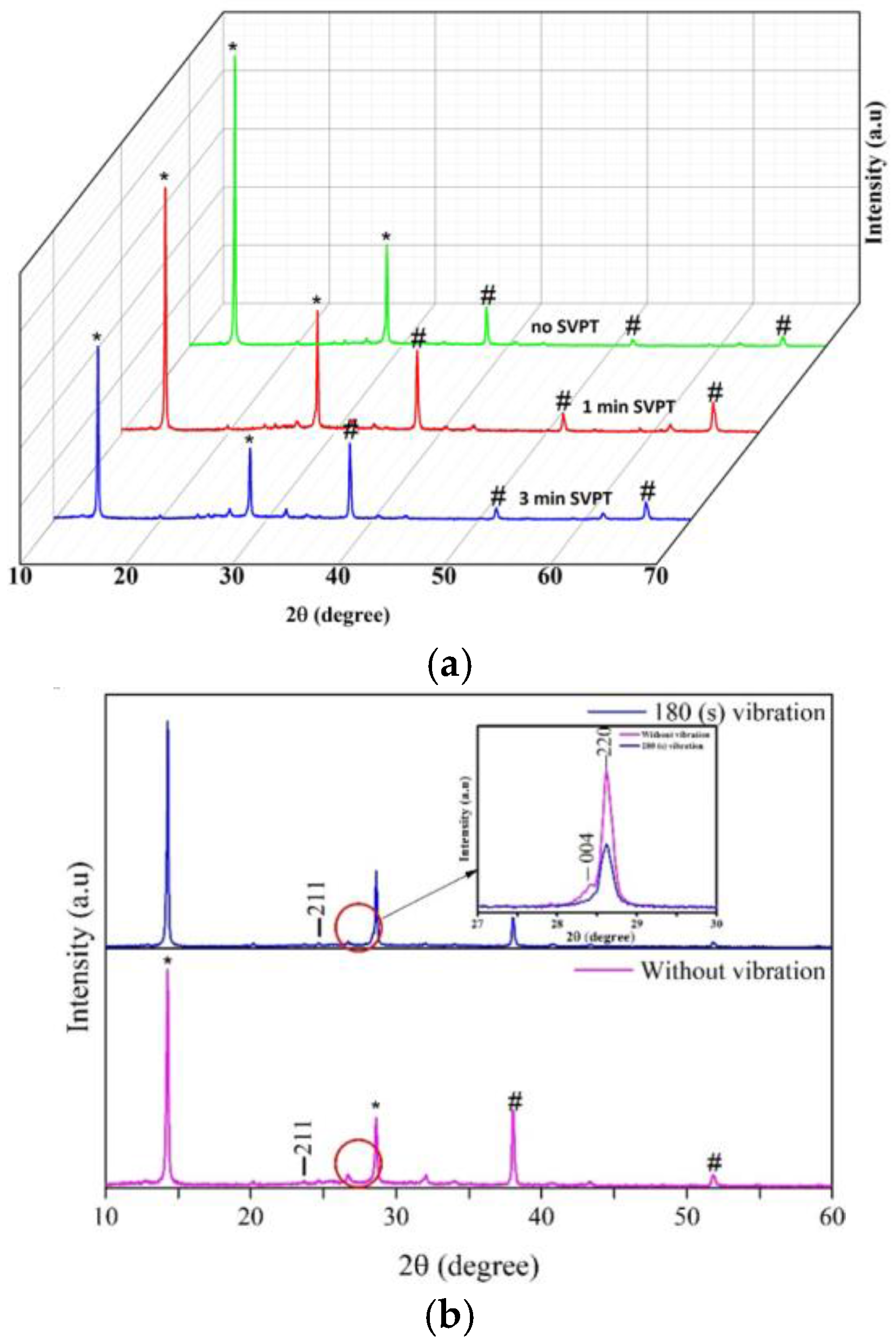
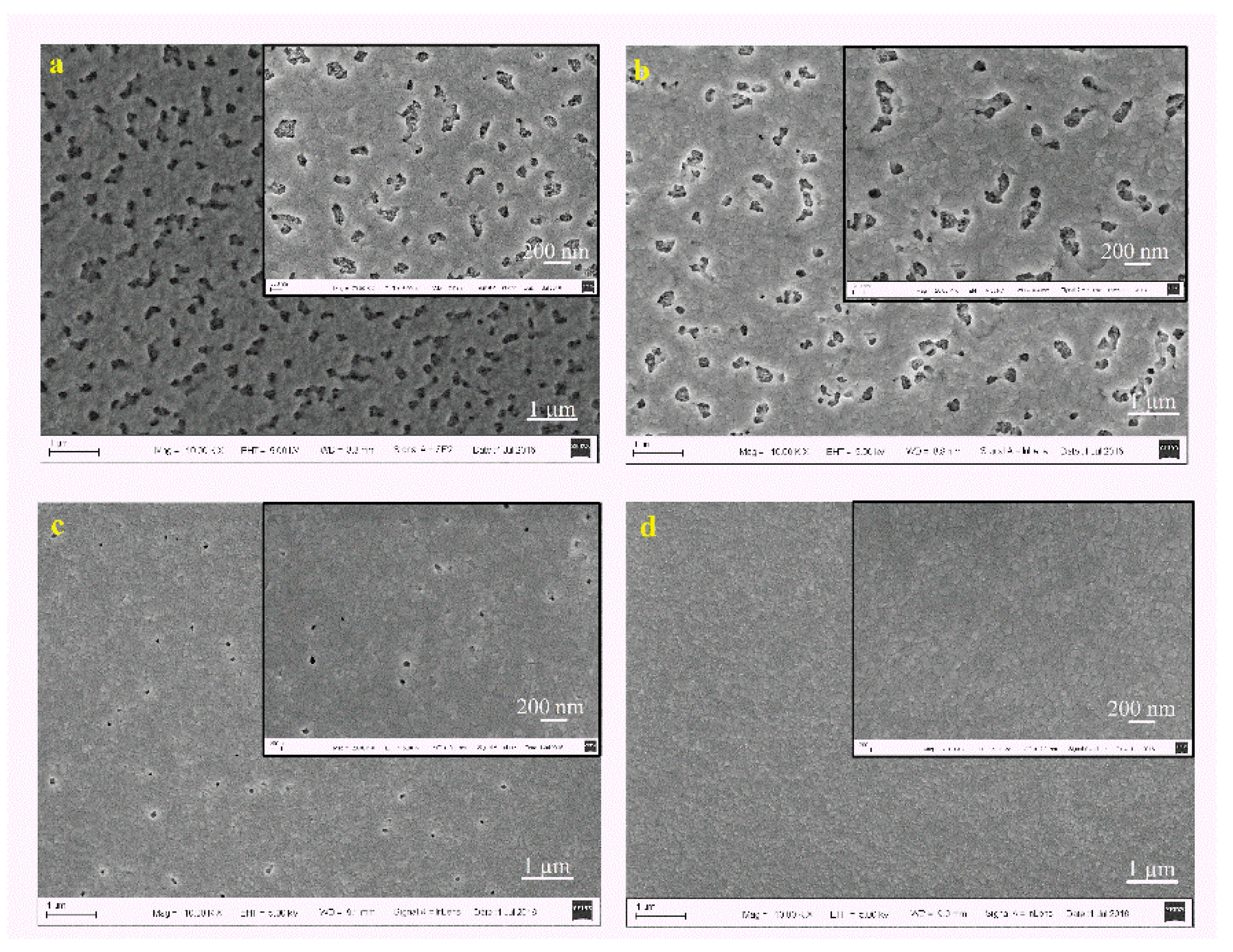
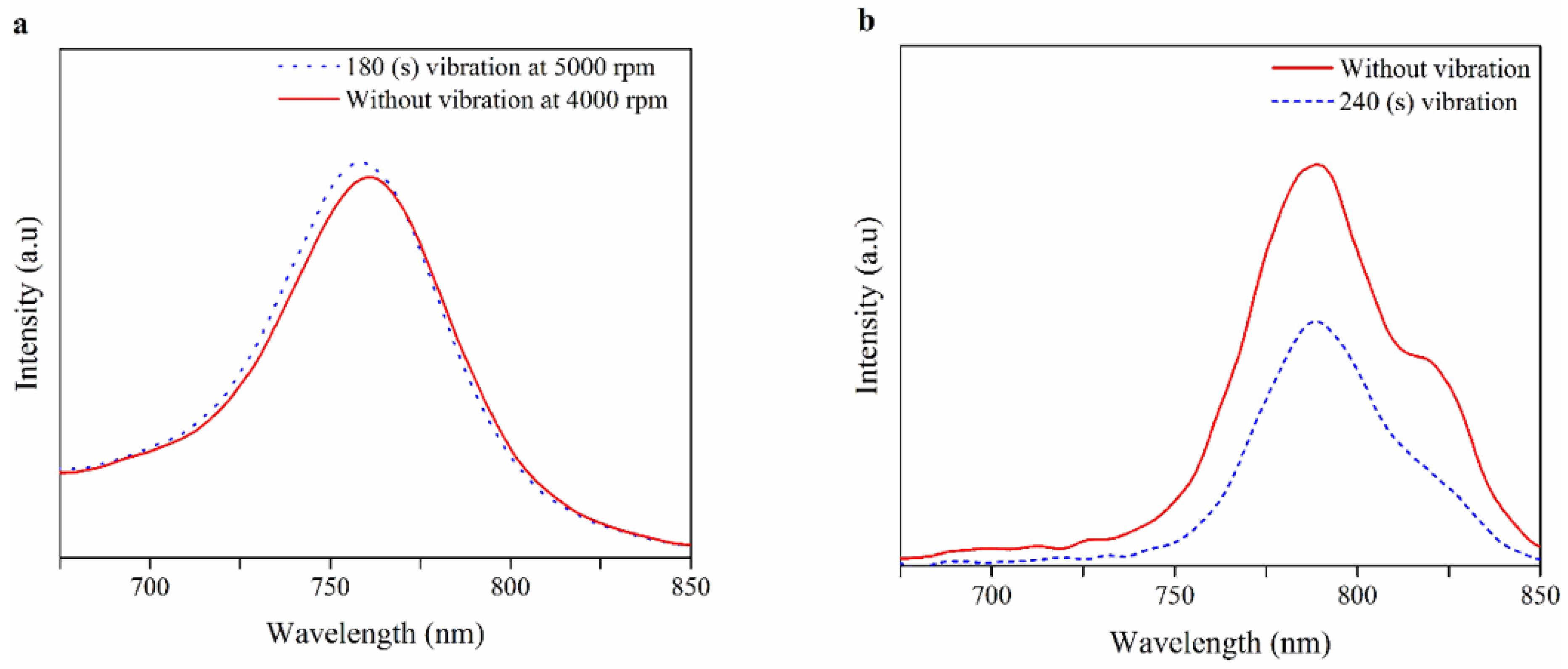
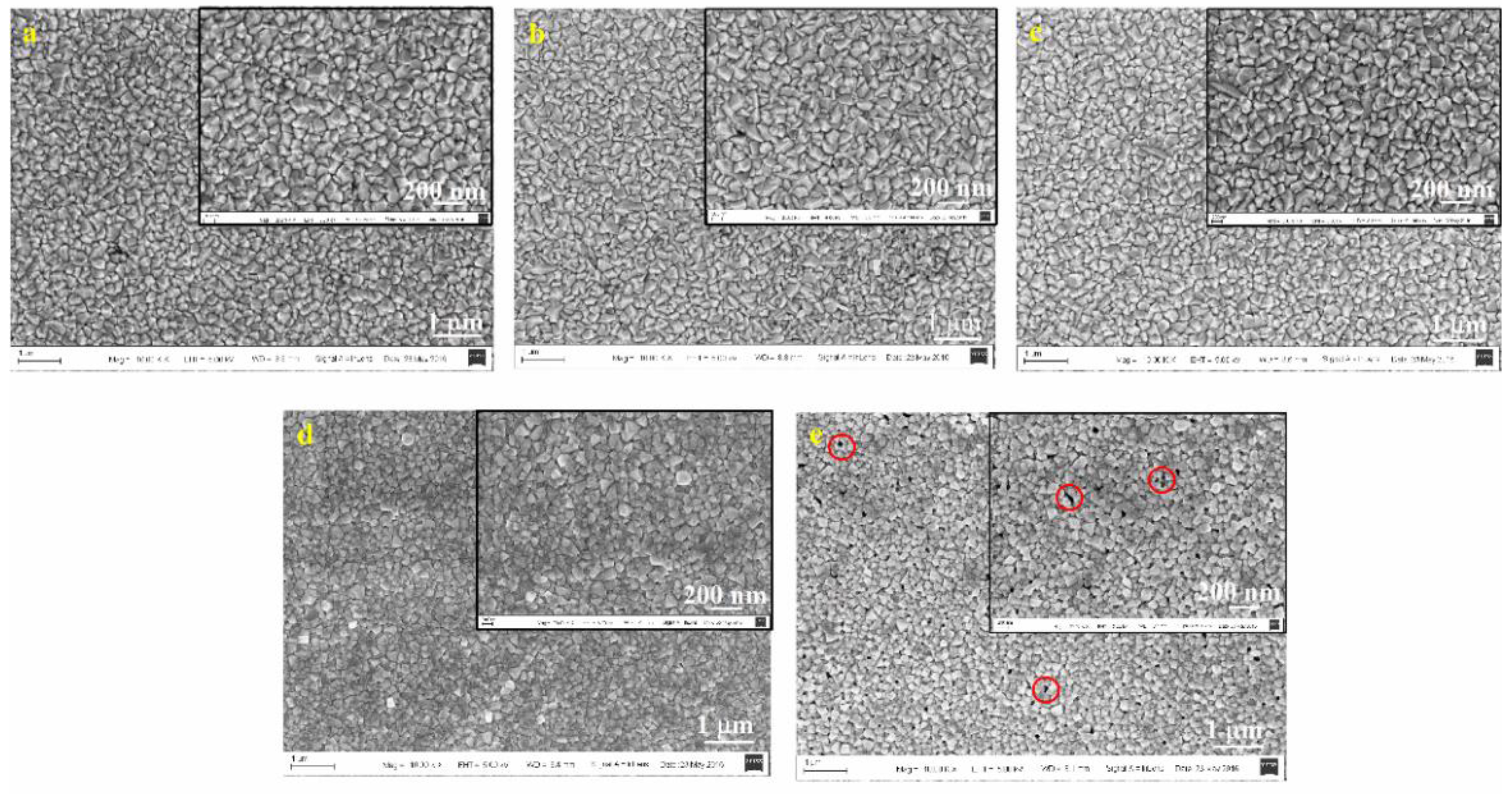
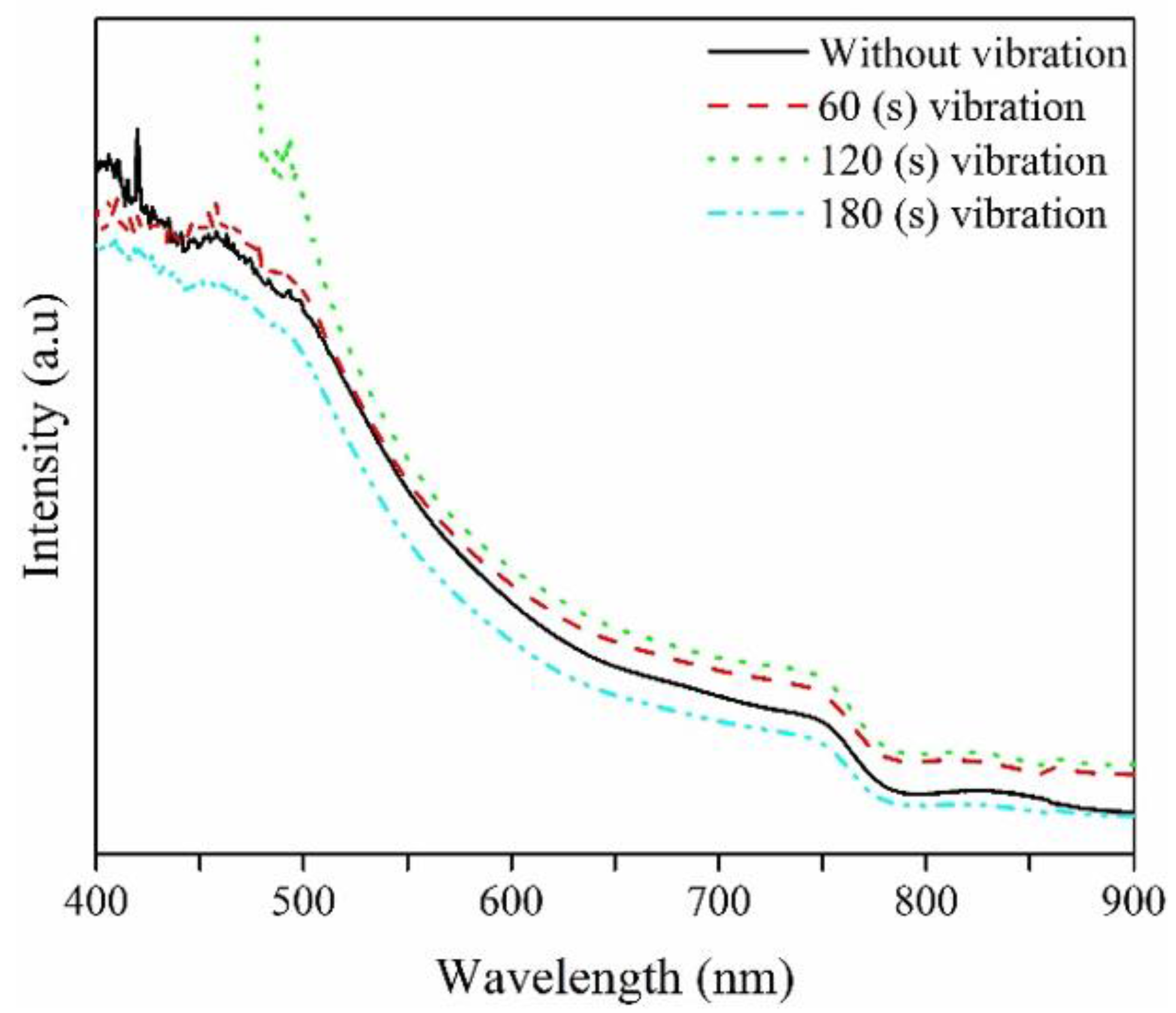
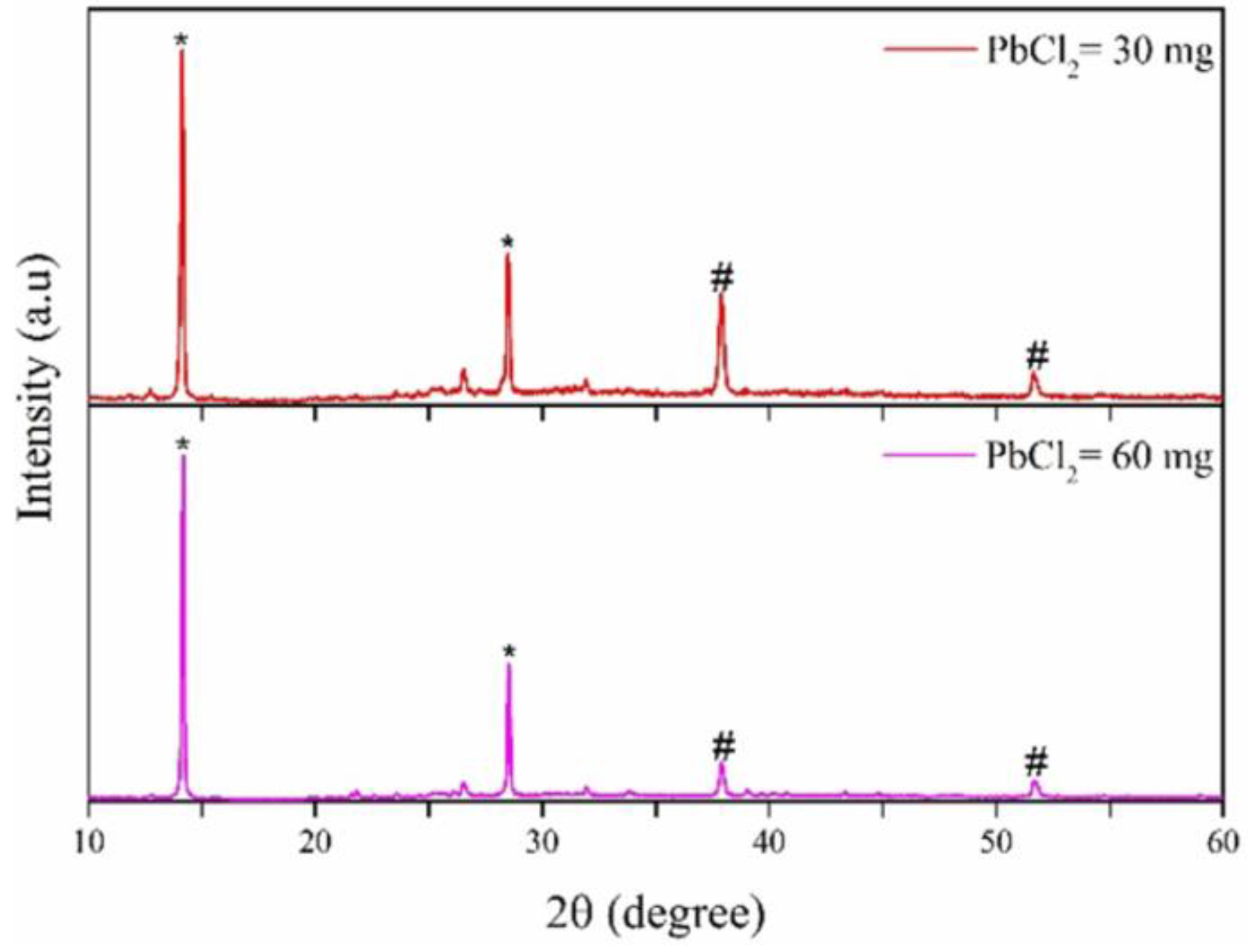
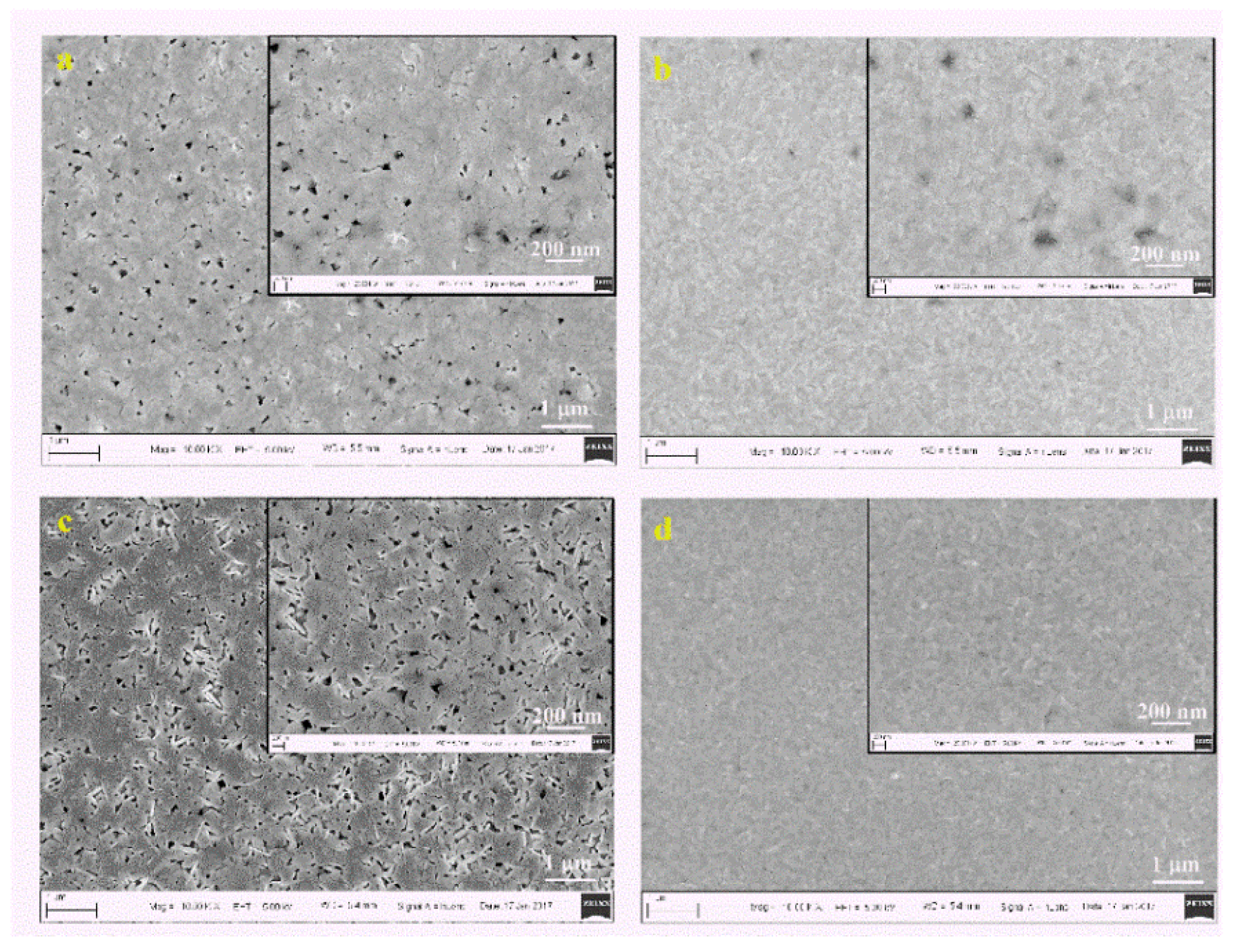
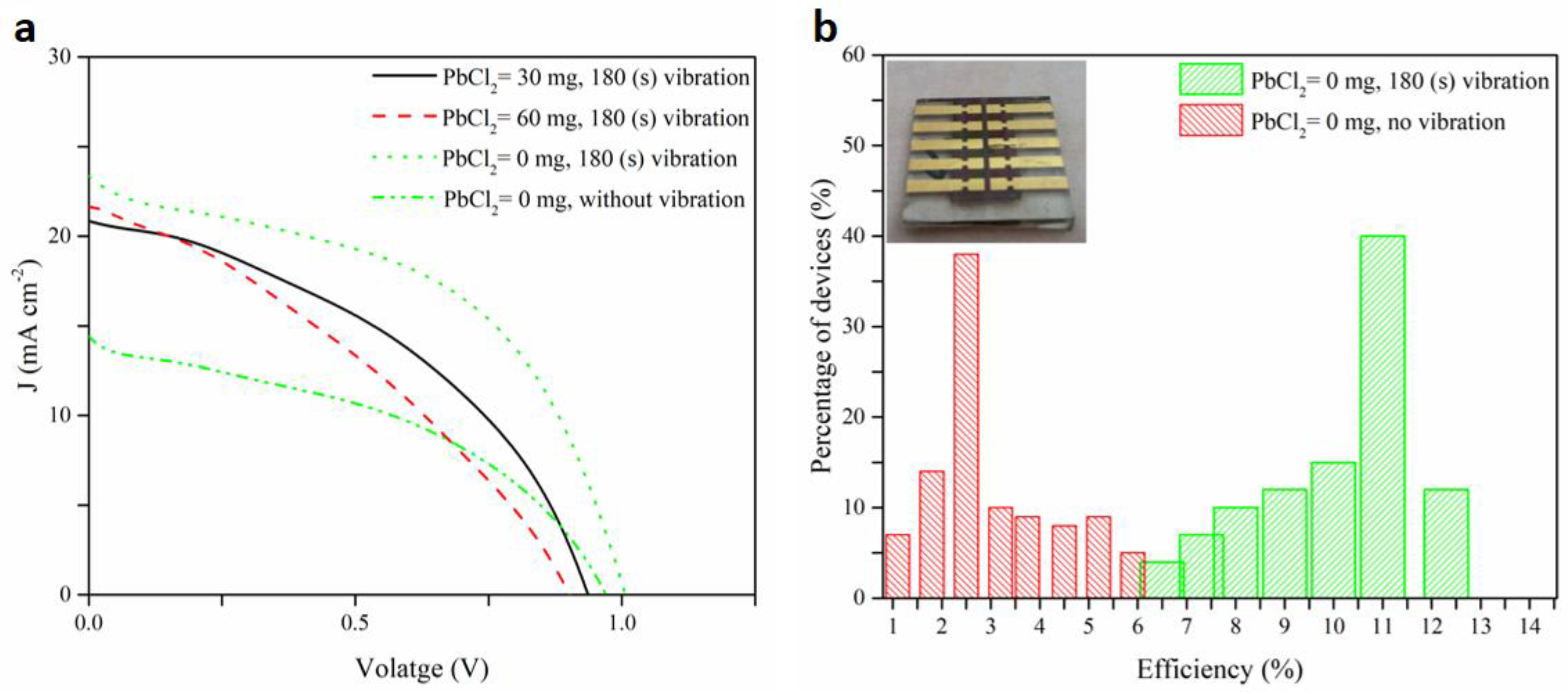
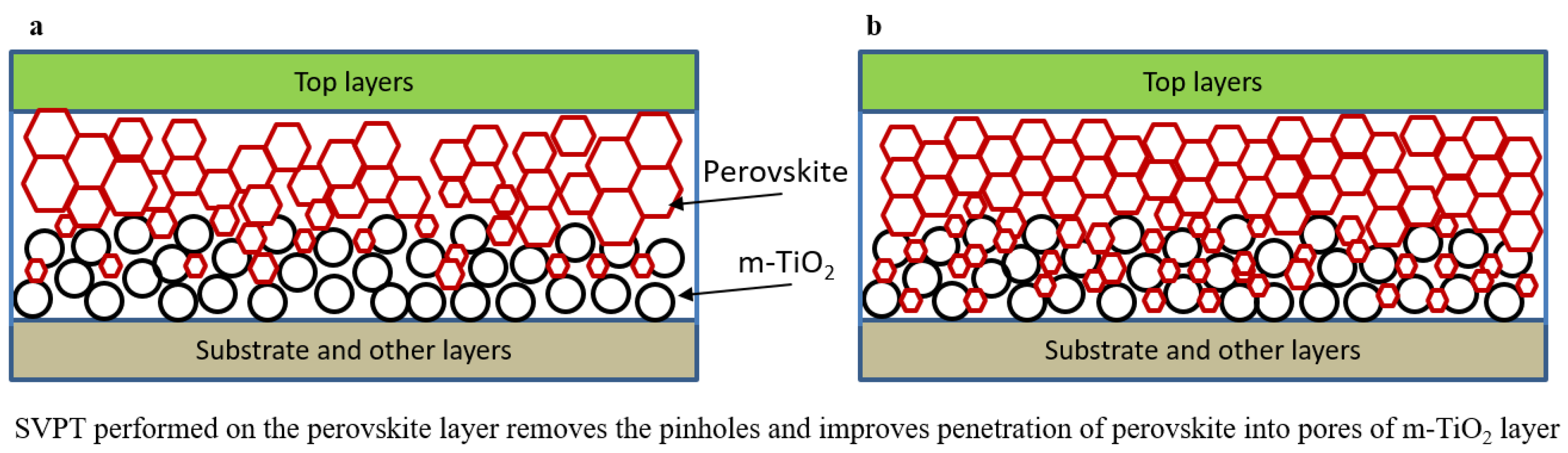
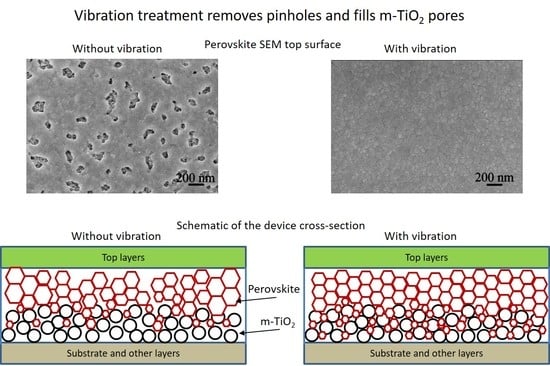
| Spin Speed (rpm) | Vibration Time (s) | PL Intensity (a.u) | Film Thickness (nm) | FWHM at 14° (degree) |
|---|---|---|---|---|
| 4000 | 0 | 0.44 | 450 | 0.12 |
| 4000 | 60 | 0.36 | 400 | 0.19 |
| 4000 | 120 | 0.34 | 400 | 0.20 |
| 4000 | 180 | 0.33 | 390 | 0.22 |
| 4000 | 240 | 0.27 | 388 | 0.26 |
| 5000 | 0 | 1.6 | 410 | 0.13 |
| 5000 | 60 | 0.73 | 310 | 0.14 |
| 5000 | 120 | 0.48 | 280 | 0.18 |
| 5000 | 180 | 0.46 | 245 | 0.20 |
| Sample | SVPT Time | RShunt (Ω·cm2) | RSeries (Ω·cm2) | Jsc (mA·cm−2) | Voc (V) | FF | PCE (%) |
|---|---|---|---|---|---|---|---|
| MAPbI3 | 180 s | 347 | 36.0 | 22.5 | 1.02 | 0.54 | 12.51 |
| MAPbI3 | Pristine | 74.0 | 41.0 | 14.5 | 0.98 | 0.42 | 6.00 |
| MAPbI3−xClx 1 | 180 s | 200 | 45.9 | 20.83 | 0.94 | 0.43 | 8.31 |
| MAPbI3−xClx 2 | 180 s | 127 | 21.6 | 21.77 | 0.89 | 0.36 | 6.97 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmadian-Yazdi, M.-R.; Habibi, M.; Eslamian, M. Excitation of Wet Perovskite Films by Ultrasonic Vibration Improves the Device Performance. Appl. Sci. 2018, 8, 308. https://doi.org/10.3390/app8020308
Ahmadian-Yazdi M-R, Habibi M, Eslamian M. Excitation of Wet Perovskite Films by Ultrasonic Vibration Improves the Device Performance. Applied Sciences. 2018; 8(2):308. https://doi.org/10.3390/app8020308
Chicago/Turabian StyleAhmadian-Yazdi, Mohammad-Reza, Mehran Habibi, and Morteza Eslamian. 2018. "Excitation of Wet Perovskite Films by Ultrasonic Vibration Improves the Device Performance" Applied Sciences 8, no. 2: 308. https://doi.org/10.3390/app8020308
APA StyleAhmadian-Yazdi, M.-R., Habibi, M., & Eslamian, M. (2018). Excitation of Wet Perovskite Films by Ultrasonic Vibration Improves the Device Performance. Applied Sciences, 8(2), 308. https://doi.org/10.3390/app8020308