Management of Waste Streams from Dairy Manufacturing Operations Using Membrane Filtration and Dissolved Air Flotation
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
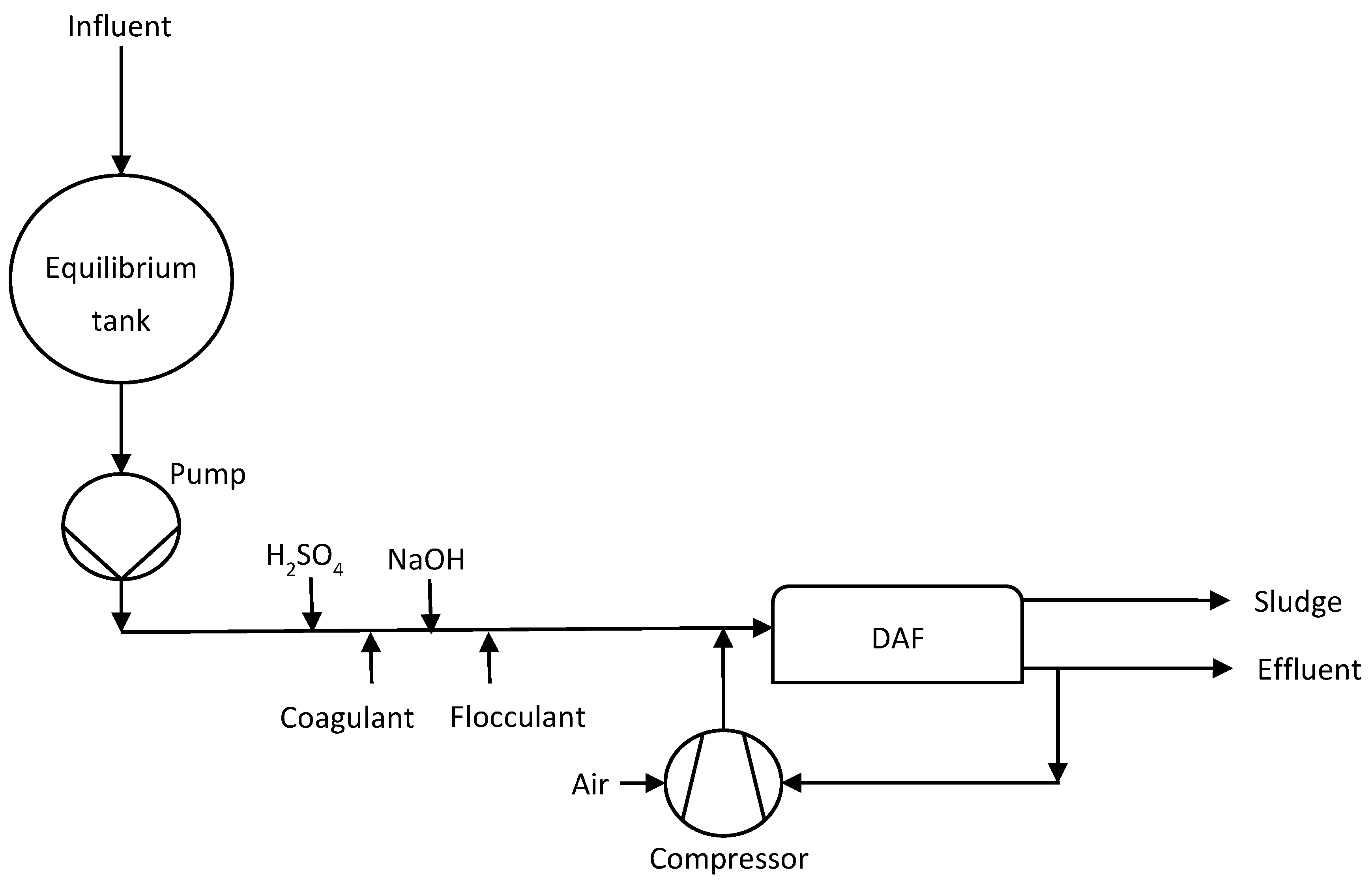
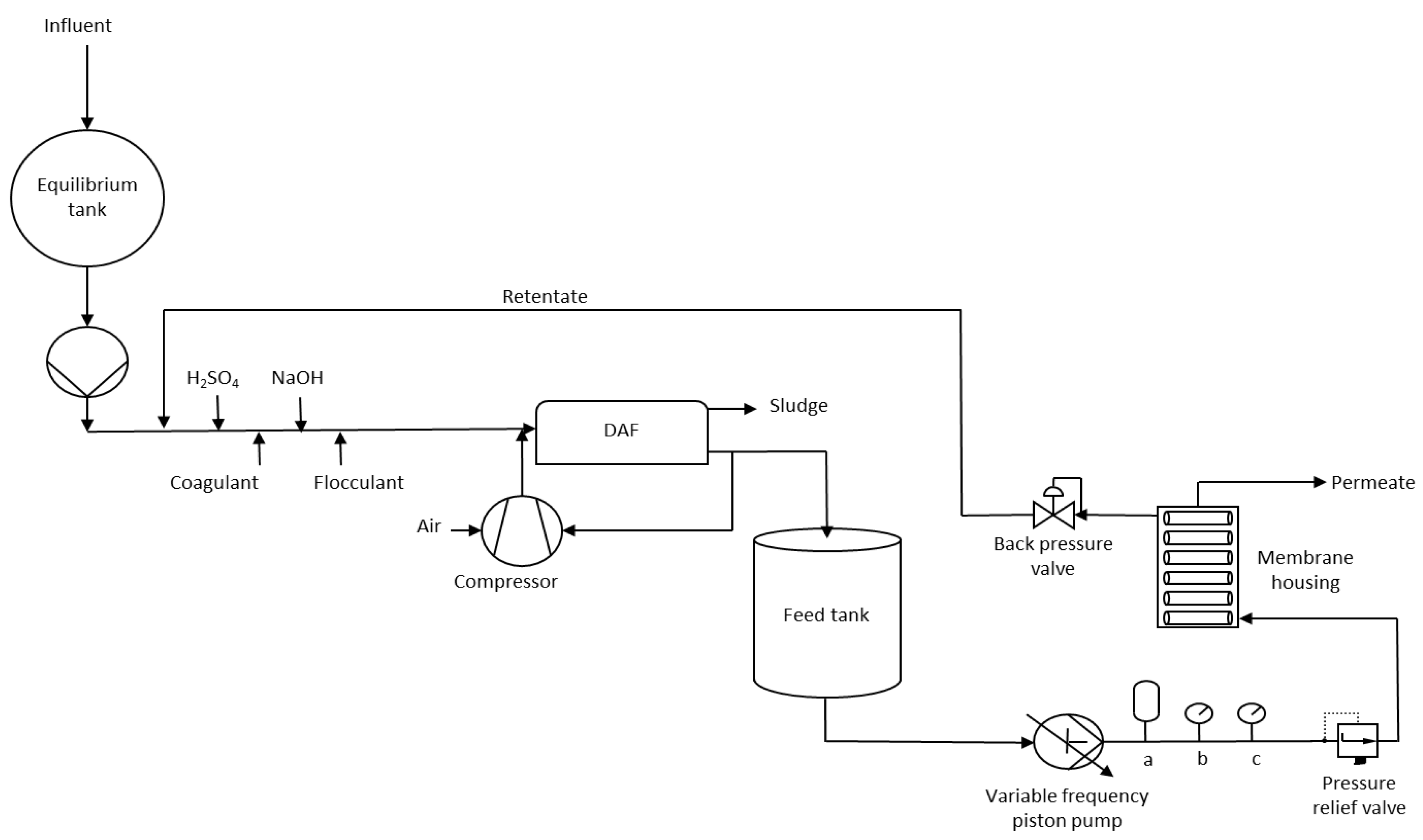
2.1. Current Wastewater Treatment
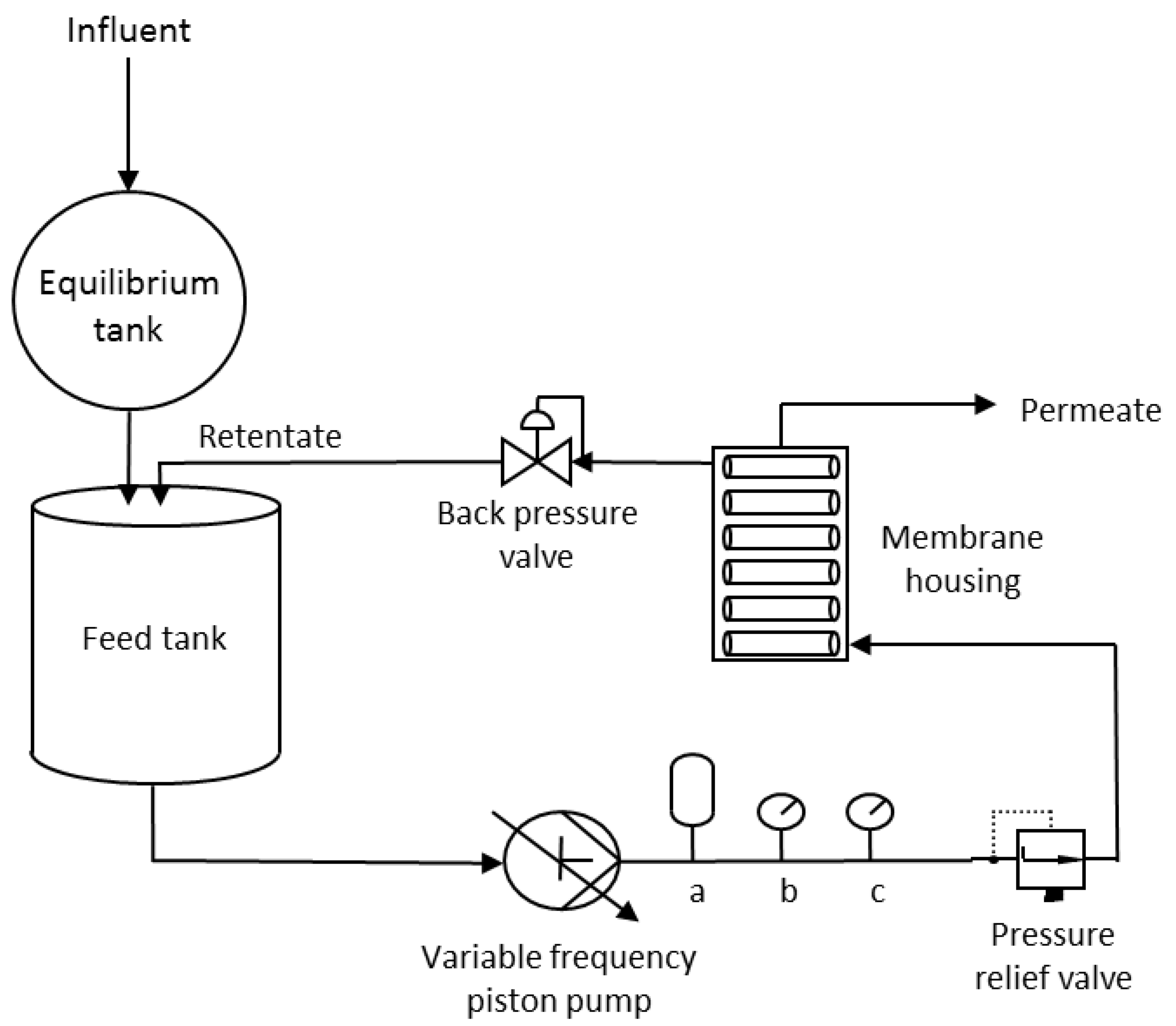
2.2. Membranes
2.3. Filtration Experiment
2.4. Analytical Methods
2.5. Operating Conditions
2.5.1. Membrane Selection
2.5.2. Extended Fouling Study
3. Results and Discussion
3.1. Feed Source Characterization
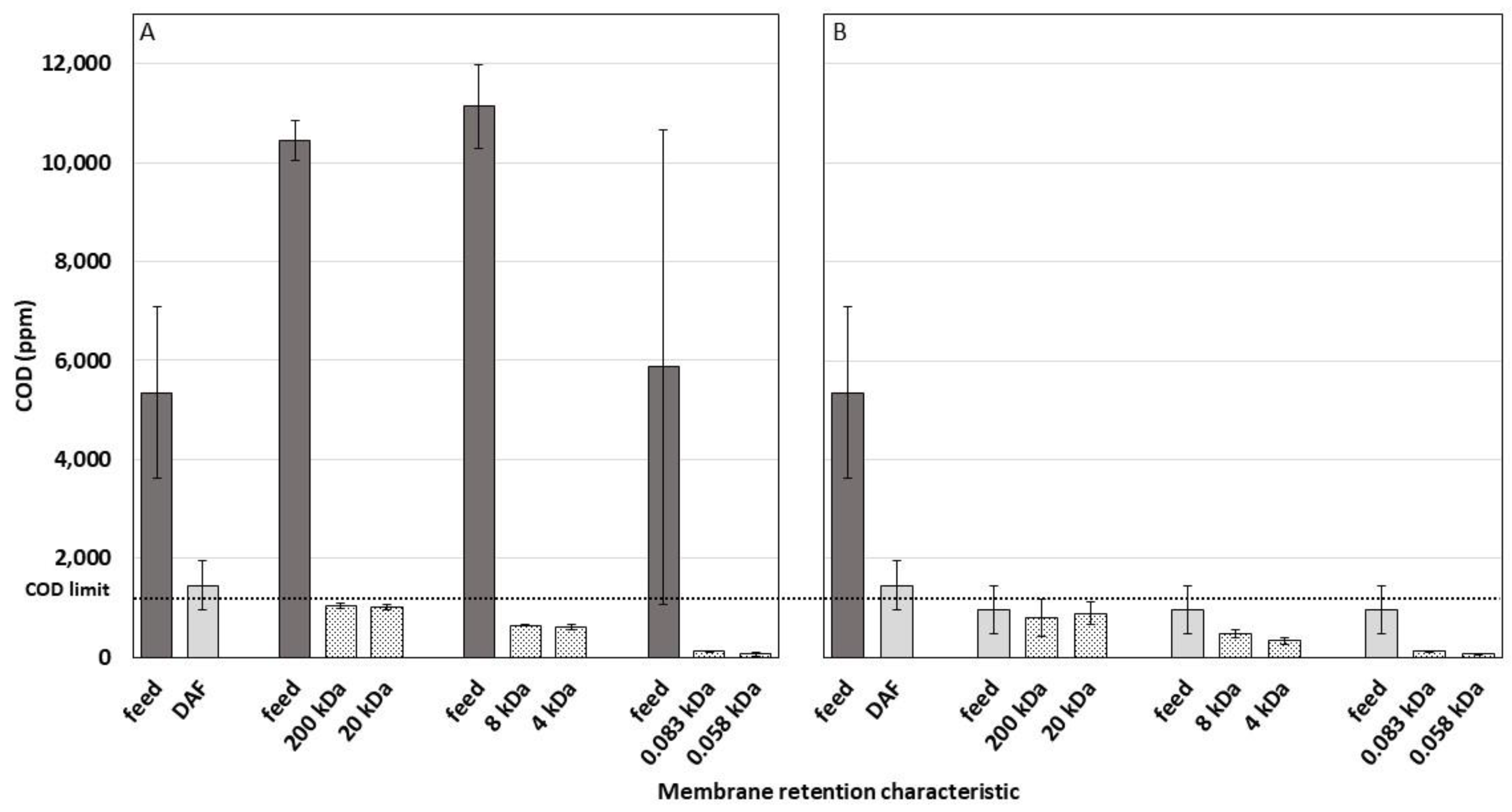
3.2. Chemical Oxygen Demand
3.2.1. Comparison of Input and Output COD in Various Treatments
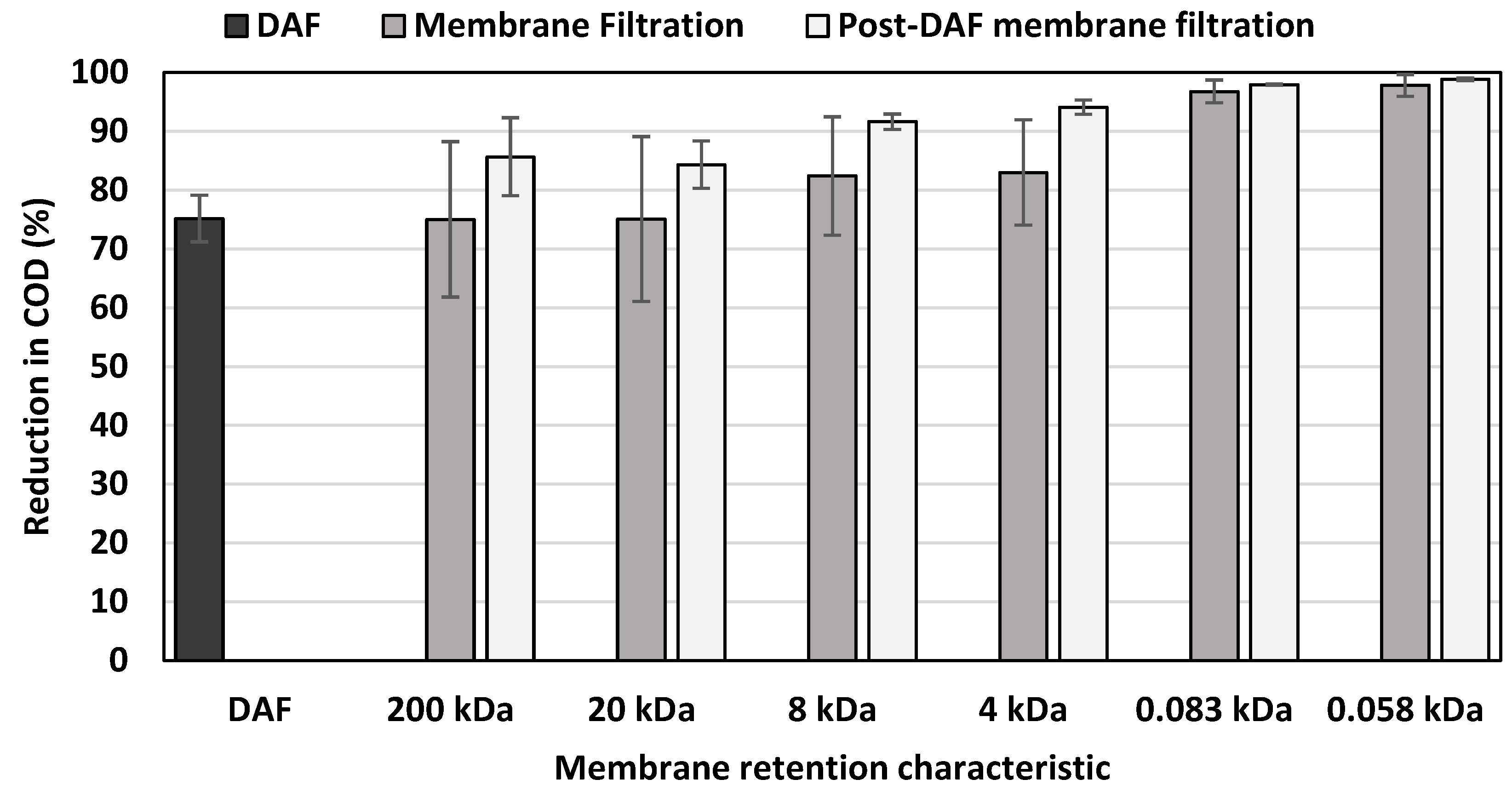
3.2.2. Reduction in COD
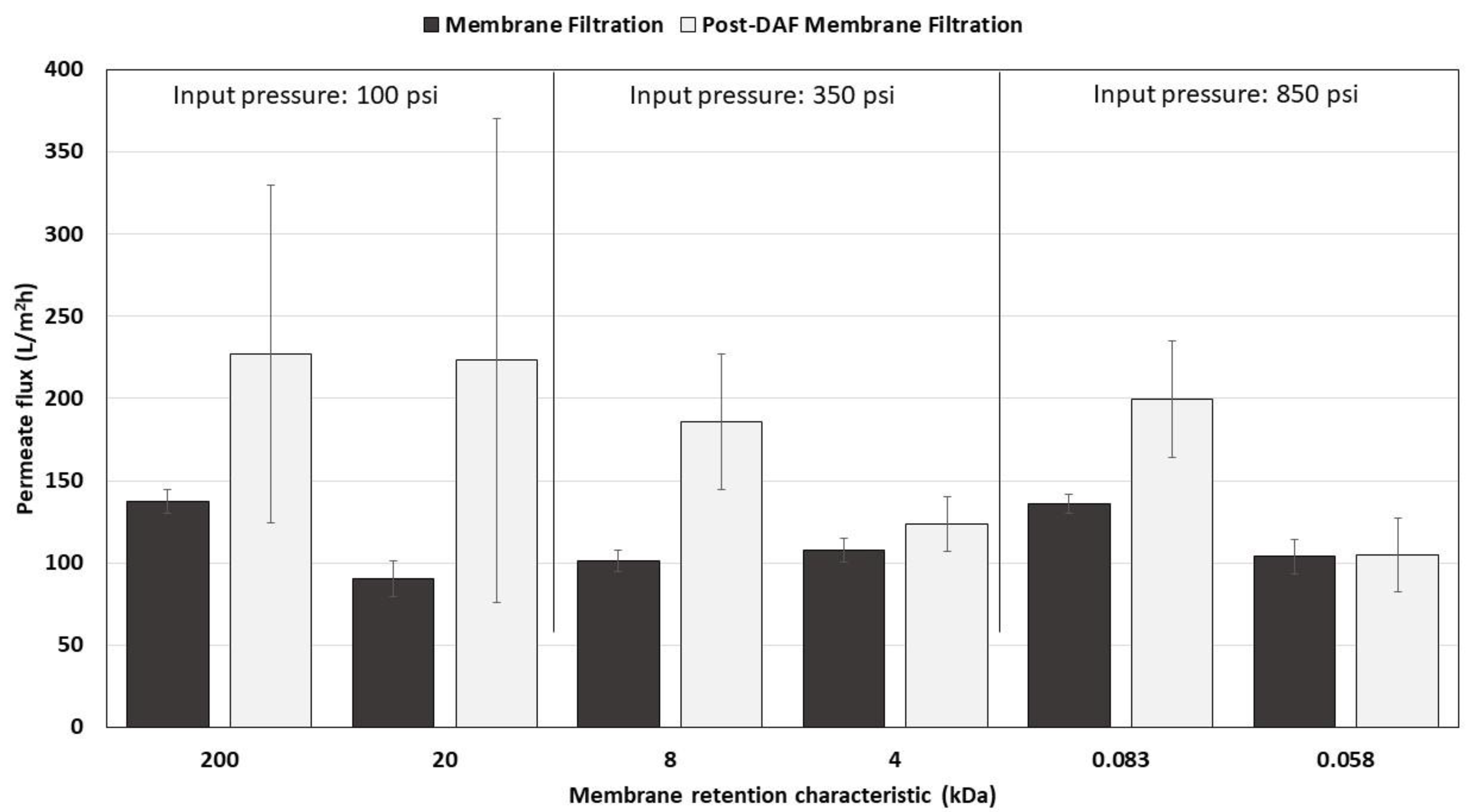
3.2.3. Permeate Flux
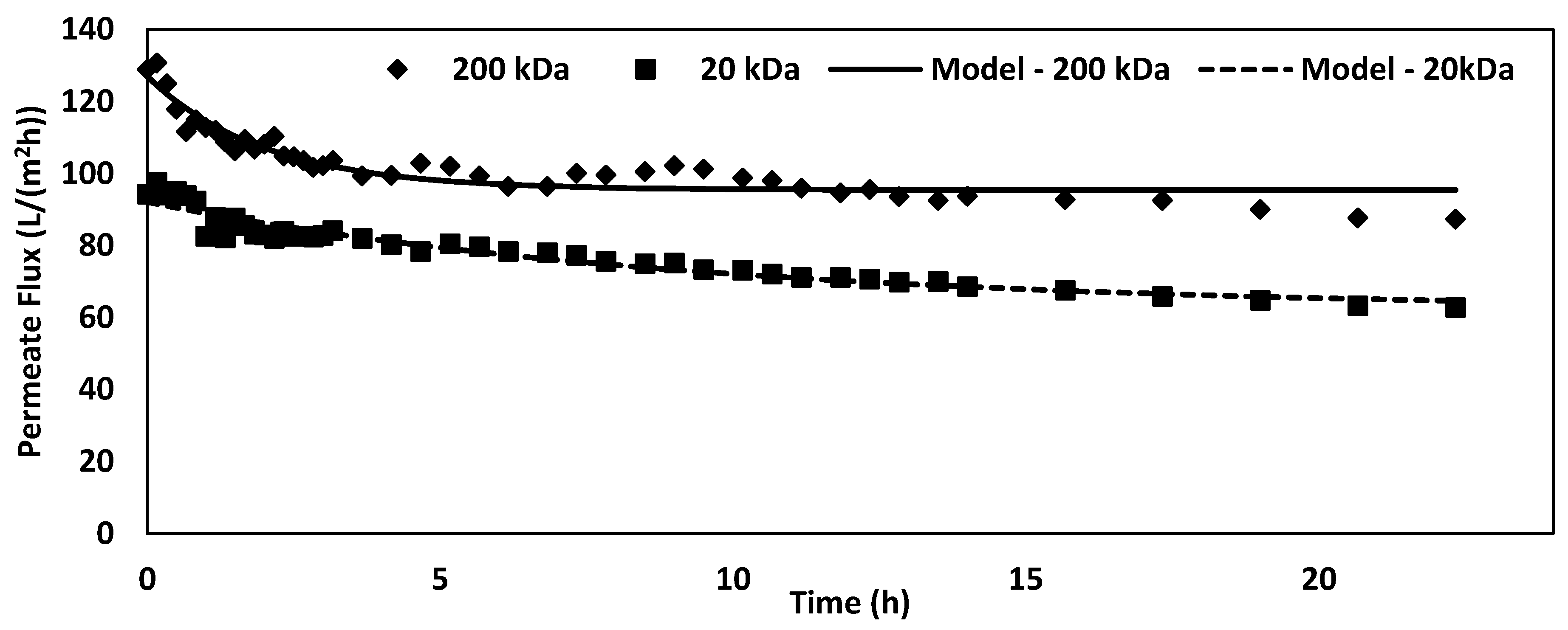
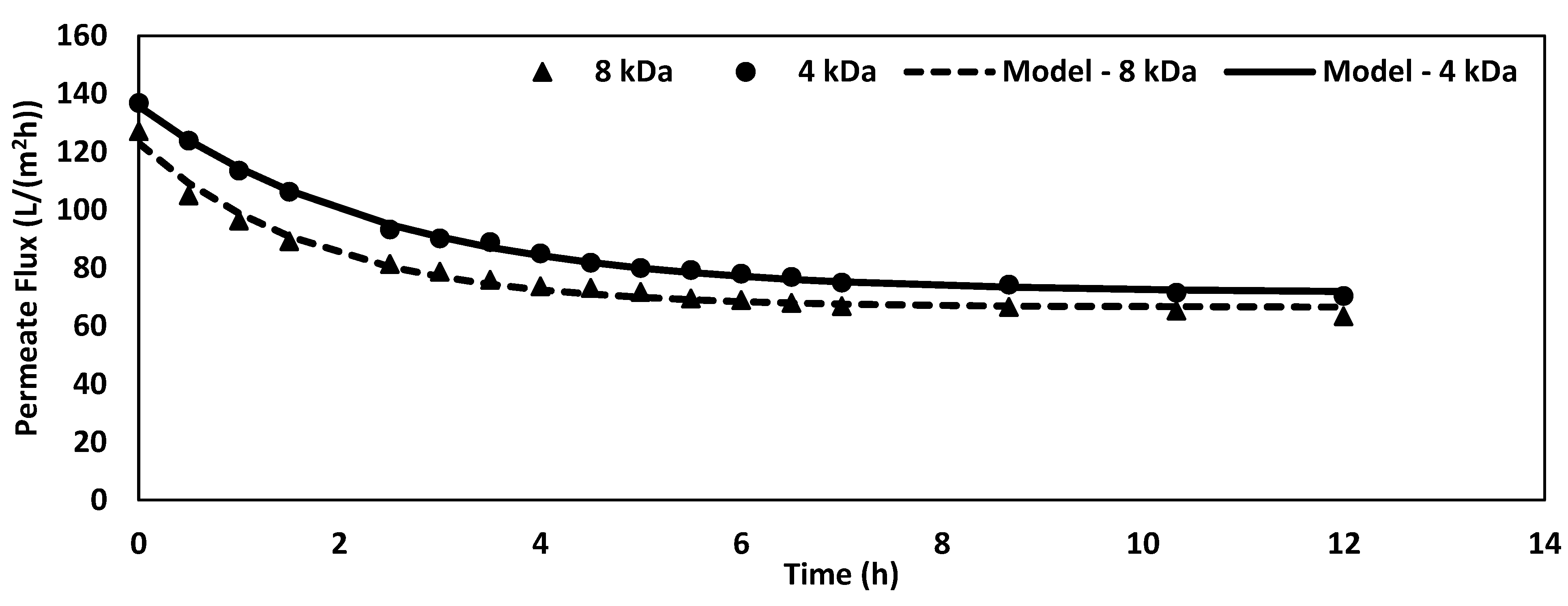
3.3. Extended Fouling Study
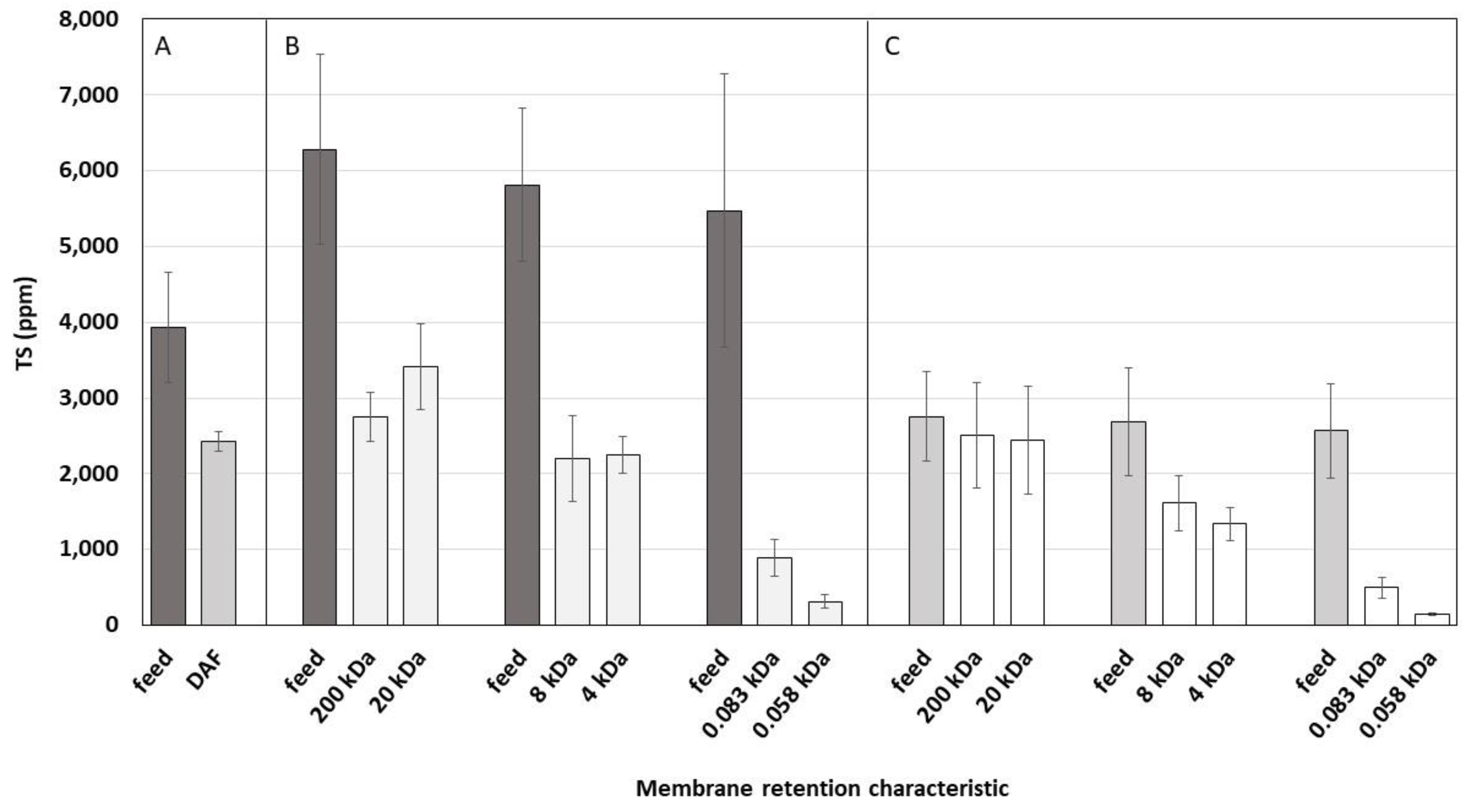
3.4. Solids Content
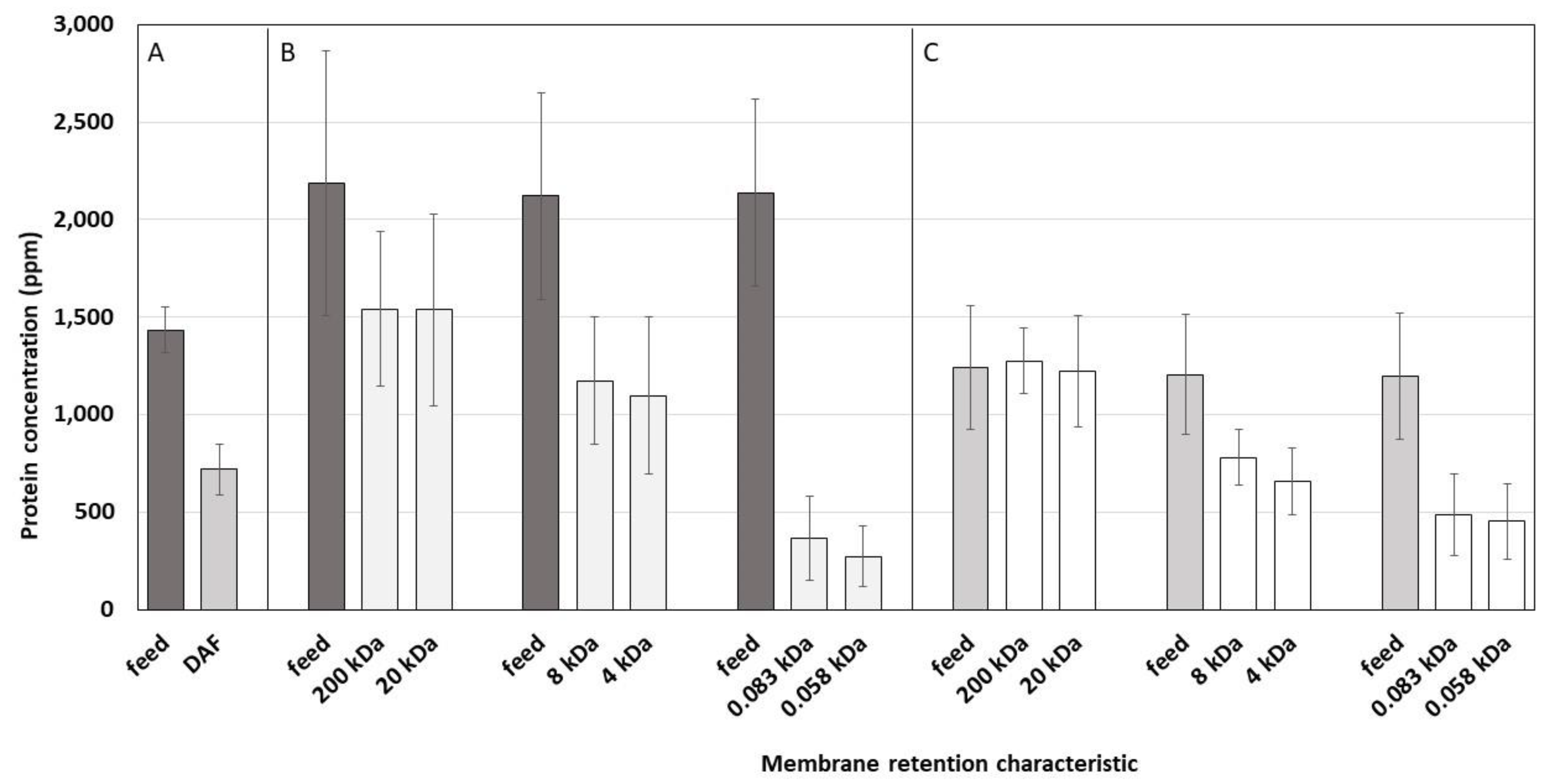
3.5. Protein Concentration
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| Abbreviations | |
| EQ | Equilibrium tank |
| DAF | Dissolved air floatation |
| PVDF | Polyvinylidene fluoride |
| RO | Reverse osmosis |
| COD | Chemical oxygen demand |
| BCA | Bicinchoninic acid assay |
| CIP | Clean in place |
| FOG | Fats, oils and grease |
| TS | Total solids |
| TSS | Total suspended solids |
| kDa | Kilo Dalton |
| MWWT | Municipal waste water treatment |
| Symbols | |
| Cp | Permeate concentration (ppm) |
| Cf | Feed concentration (ppm) |
| J | Permeate flux (L/(m2h)) |
| a | Asymptote (L/(m2h)) |
| b | Scale (L/(m2h)) |
| c | Fouling rate h−1 |
References
- Wojdalski, J.; Drózdz, B.; Piechocki, J.; Gaworski, M.; Zander, Z.; Marjanowski, J. Determinants of Water Consumption in the Dairy Industry. Pol. J. Chem. Technol. 2013, 15, 61–72. [Google Scholar] [CrossRef]
- Carawan, R.E.; Jones, V.A.; Hansen, A.P. Water Use in a Multiproduct Dairy. J. Dairy Sci. 1979, 62, 1238–1242. [Google Scholar] [CrossRef]
- Balannec, B.; Vourch, M.; Rabiller-Baudry, M.; Chaufer, B. Comparative Study of Different Nanofiltration and Reverse Osmosis Membranes for Dairy Effluent Treatment by Dead-End Filtration. Sep. Purif. Technol. 2005, 42, 195–200. [Google Scholar] [CrossRef]
- Demirel, B.; Yenigun, O.; Onay, T.T. Anaerobic Treatment of Dairy Wastewaters: A Review. Process Biochem. 2005, 40, 2583–2595. [Google Scholar] [CrossRef]
- Perle, M.; Kimchie, S.; Shelef, G. Some Biochemical Aspects of the Anaerobic Degradation of Dairy Wastewater. Water Res. 1995, 29, 1549–1554. [Google Scholar] [CrossRef]
- Wang, L.K.; Hung, Y.; Lo, H.H.; Yapijakis, C. Handbook of industrial and hazardous wastes treatment; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Chimenos, J.M.; Fernández, A.I.; Hernández, A.; Haurie, L.; Espiell, F.; Ayora, C. Optimization of Phosphate Removal in Anodizing Aluminium Wastewater. Water Res. 2006, 40, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Tchamango, S.; Nanseu-Njiki, C.P.; Ngameni, E.; Hadjiev, D.; Darchen, A. Treatment of Dairy Effluents by Electrocoagulation Using Aluminium Electrodes. Sci. Total Environ. 2010, 408, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Mavrov, V.; Bdli, E. Reuse of Vapour Condensate from Milk Processing Using Nanofiltration. Filtr. Sep. 2000, 37, 24–27. [Google Scholar] [CrossRef]
- Buntner, D.; Sánchez, A.; Garrido, J.M. Feasibility of Combined UASB and MBR System in Dairy Wastewater Treatment at Ambient Temperatures. Chem. Eng. J. 2013, 230, 475–481. [Google Scholar] [CrossRef]
- Şengil, I.A.; özacar, M. Treatment of Dairy Wastewaters by Electrocoagulation Using Mild Steel Electrodes. J. Hazard. Mater. 2006, 137, 1197–1205. [Google Scholar] [CrossRef] [PubMed]
- Thompson, G.; Swain, J.; Kay, M.; Forster, C.F. The Treatment of Pulp and Paper Mill Effluent: A Review. Bioresour. Technol. 2001, 77, 275–286. [Google Scholar] [CrossRef]
- Vourch, M.; Balannec, B.; Chaufer, B.; Dorange, G. Treatment of Dairy Industry Wastewater by Reverse Osmosis for Water Reuse. Desalination 2008, 219, 190–202. [Google Scholar] [CrossRef]
- Suárez, A.; Fidalgo, T.; Berdasco, M.A.; Riera, F.A. UHT Condensate Recovery by Reverse Osmosis: A Pilot-Plant Study. Ind. Eng. Chem. Res. 2014, 53, 15237–15244. [Google Scholar] [CrossRef]
- Riera, F.A.; Suárez, A.; Muro, C. Nanofiltration of UHT Flash Cooler Condensates from a Dairy Factory: Characterisation and Water Reuse Potential. Desalination 2013, 309, 52–63. [Google Scholar] [CrossRef]
- Zydney, A.L. Protein Separations Using Membrane Filtration: New Opportunities for Whey Fractionation. Int. Dairy J. 1998, 8, 243–250. [Google Scholar] [CrossRef]
- Trägardh, G.; Johansson, D. Purification of Alkaline Cleaning Solutions from the Dairy Industry Using Membrane Separation Technology. Desalination 1998, 119, 21–29. [Google Scholar] [CrossRef]
- Gésan-Guiziou, G. Removal of Bacteria, Spores and Somatic Cells from Milk by Centrifugation and Microfiltration Techniques. In Improving the Safety and Quality of Milk; Elsevier: Amsterdam, The Netherlands, 2010; pp. 349–372. [Google Scholar] [CrossRef]
- Shi, X.; Tal, G.; Hankins, N.P.; Gitis, V. Fouling and Cleaning of Ultrafiltration Membranes: A Review. J. Water Process Eng. 2014, 1, 121–138. [Google Scholar] [CrossRef]
- PCI Membranes, “PCI Tubular Membranes Product Information,” Product sheet, 2018. Available online: https://www.pcimembranes.com/en-US/download-centre/product-information (accessed on 20 May 2017).
- Huang, X.; Liu, R.; Qian, Y. Behaviour of Soluble Microbial Products in a Membrane Bioreactor. Process Biochem. 2000, 36, 401–406. [Google Scholar] [CrossRef]
- Chan, R.; Chen, V. The Effects of Electrolyte Concentration and PH on Protein Aggregation and Deposition: Critical Flux and Constant Flux Membrane Filtration. J. Memb. Sci. 2001, 185, 177–192. [Google Scholar] [CrossRef]

| Membrane Commercial Name | Retention Characteristic (kDa) | Membrane Material | pH Range | Maximum Pressure (bar) | Maximum Temperature (°C) |
|---|---|---|---|---|---|
| FP200 | 200 | PVDF * | 1.5–12 | 10 | 80 |
| FPA03 | 20 | PVDF * | 1.5–10.5 | 7 | 60 |
| PU608 | 8 | Polysulphone | 1.5–12 | 30 | 80 |
| ES404 | 4 | Polyethersulphone | 1.5–12 | 30 | 80 |
| AFC30 | 0.083 | Polyamide film | 1.5–9.5 | 60 | 60 |
| AFC99 ** | 0.058 | Polyamide film | 1.5–12 | 64 | 80 |
| Membrane Type | Cleaning chemistry | Acid | Temperature (°C) |
|---|---|---|---|
| AFC99 | 0.25% sodium hydroxide | 0.3% nitric acid | 50 |
| AFC30 | 0.5% enzyme | 0.3% nitric acid | 45 |
| Others | 1% chlorinated alkaline detergent | 0.3% nitric acid | 50 |
| Membrane | Retention characteristic (kDa) | Operating Pressure (bar) |
|---|---|---|
| FP200 | 200 | 6.89 |
| FPA03 | 20 | 6.89 |
| PU608 | 8 | 24.13 |
| ES404 | 4 | 24.13 |
| AFC30 | 0.083 | 58.61 |
| AFC99 | 0.058 | 58.61 |
| Parameter | Concentration ± SD |
|---|---|
| pH | 11.32 ± 0.55 |
| Free Cl | 5 ± 1 ppm |
| COD | 8201 ± 3010 ppm |
| Protein | 1970 ± 359 ppm |
| FOG | 98 ± 113 ppm |
| TS | 5857 ± 1261 ppm |
| TSS | 2 ± 1 ppm |
| Membrane | Asymptote (L/(m2h)) | Scale (L/(m2h)) | Fouling Rate (1/h) |
|---|---|---|---|
| 200 kDa | 95.40 ± 0.84 | 31.59 ± 2.02 | 0.50 ± 0.06 |
| 20 kDa | 62.01 ± 2.48 | 30.09 ± 2.18 | 0.11 ± 0.02 |
| 8 kDa | 66.43 ± 1.00 | 56.54 ± 1.94 | 0.56 ± 0.05 |
| 4 kDa | 71.37 ± 0.62 | 64.34 ± 0.88 | 0.40 ± 0.01 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagappan, S.; Phinney, D.M.; Heldman, D.R. Management of Waste Streams from Dairy Manufacturing Operations Using Membrane Filtration and Dissolved Air Flotation. Appl. Sci. 2018, 8, 2694. https://doi.org/10.3390/app8122694
Nagappan S, Phinney DM, Heldman DR. Management of Waste Streams from Dairy Manufacturing Operations Using Membrane Filtration and Dissolved Air Flotation. Applied Sciences. 2018; 8(12):2694. https://doi.org/10.3390/app8122694
Chicago/Turabian StyleNagappan, Subbiah, David M. Phinney, and Dennis R. Heldman. 2018. "Management of Waste Streams from Dairy Manufacturing Operations Using Membrane Filtration and Dissolved Air Flotation" Applied Sciences 8, no. 12: 2694. https://doi.org/10.3390/app8122694
APA StyleNagappan, S., Phinney, D. M., & Heldman, D. R. (2018). Management of Waste Streams from Dairy Manufacturing Operations Using Membrane Filtration and Dissolved Air Flotation. Applied Sciences, 8(12), 2694. https://doi.org/10.3390/app8122694





