Chemical Composition of Lipophilic Bark Extracts from Pinus pinaster and Pinus pinea Cultivated in Portugal
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
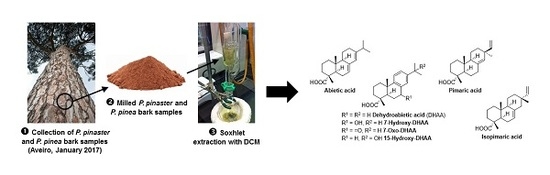
2.2. Samples’ Preparation and Extraction
2.3. The GC-MS Analysis
3. Results and Discussion
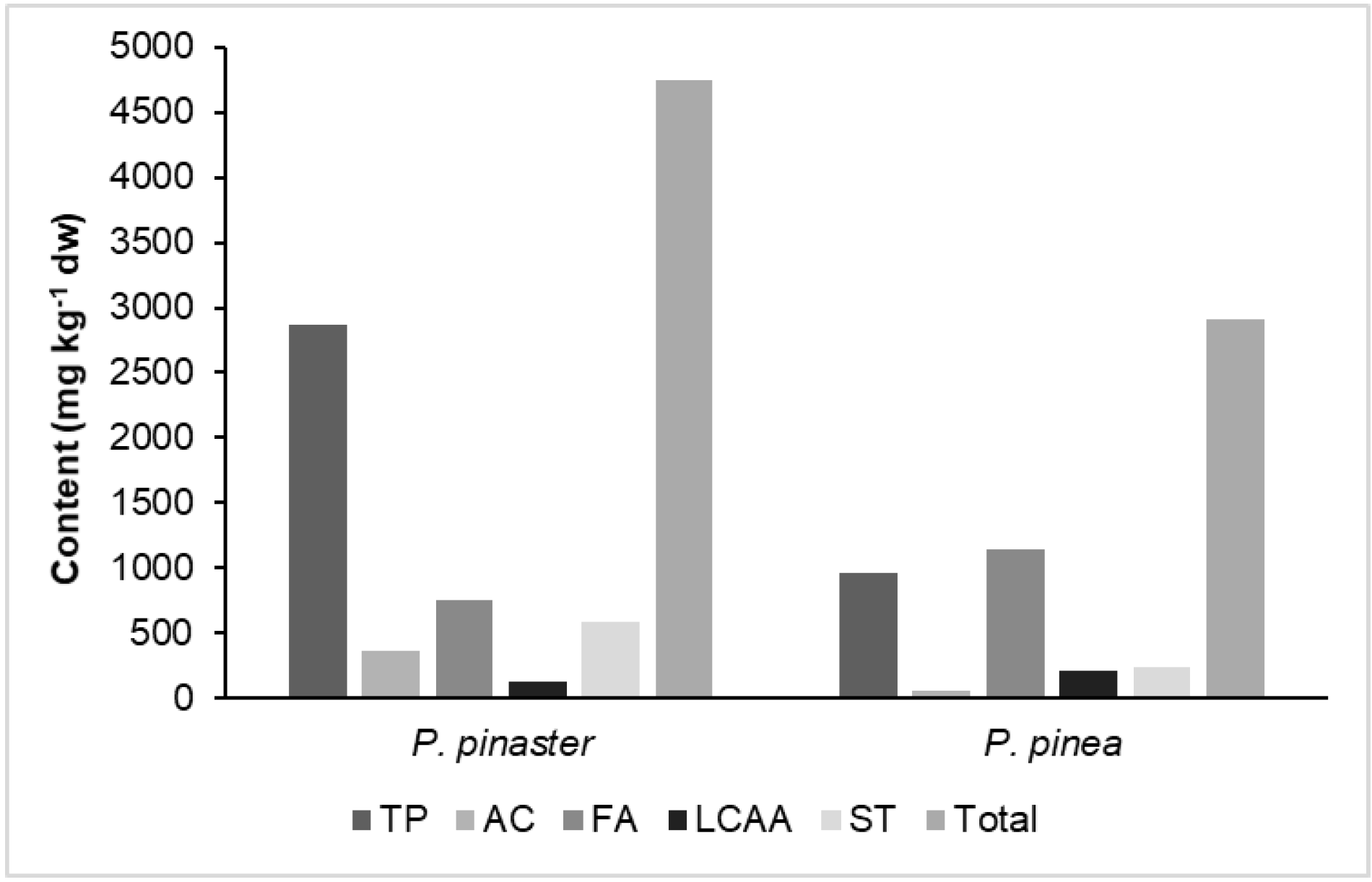
3.1. The Extraction Yield
3.2. The Chemical Characterization of Lipophilic Fractions Derived from the P. pinaster and P. pinea Barks
3.2.1. Terpenic Compounds
3.2.2. Fatty Acids
3.2.3. Sterols
3.2.4. Aromatic Compounds
3.2.5. Long-Chain Aliphatic Alcohols and Minor Compounds
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- ICNF. IFN6—Áreas dos Usos do solo e das Espécies Florestais de Portugal Continental em 1995, 2005 e 2010. Resultados Preliminares; ICNF: Lisboa, Portugal, 2013; pp. 1–34. [Google Scholar]
- Figueiredo, A.C.; Pedro, L.G.; Barroso, J.G.; Trindade, H.; Sanches, J.; Oliveira, C.; Correia, M. Pinus pinaster Aiton e Pinus pinea L. Agrotec 2014, 12, 14–18. [Google Scholar]
- ICNF. IFN5—5º Inventário Florestal Nacional. Relatório Final; p Table 309 (FloreStat App); ICNF: Lisboa, Portugal, 2010. [Google Scholar]
- Clark, J.H.; Deswarte, F.E.I. The Biorefinery Concept—An Integrated Approach. In Introduction to Chemicals from Biomass; Clark, J., Deswarte, F., Eds.; John Wiley & Sons, Ltd.: Hoboken, NK, USA, 2008; pp. 1–20. [Google Scholar]
- Silvestre, A.J.D.; Gandini, A. Rosin: Major Sources, Properties and Applications. In Monomers, Polymers and Composites from Renewable Resources; Belgacem, M.N., Gandini, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 67–88. [Google Scholar]
- Keeling, C.I.; Bohlmann, J. Diterpene resin acids in conifers. Phytochemistry 2006, 67, 2415–2423. [Google Scholar] [CrossRef] [PubMed]
- Feio, S.S.; Gigante, B.; Roseiro, J.C.; Marcelo-Curto, M.J. Antimicrobial activity of diterpene resin acid derivatives. J. Microbiol. Methods 1999, 35, 201–206. [Google Scholar] [CrossRef]
- Feliciano, A.S.; Gordaliza, M.; Salinero, M.A.; del Corral, J.M.M. Abietane Acids: Sources, Biological Activities, and Therapeutic Uses. Planta Med. 1993, 59, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Gazim, Z.; Rodrigues, F.; Amorin, A.; Rezende, C.; Soković, M.; Tešević, V.; Vučković, I.; Krstić, G.; Cortez, L.; Colauto, N.; et al. New Natural Diterpene-Type Abietane from Tetradenia riparia Essential Oil with Cytotoxic and Antioxidant Activities. Molecules 2014, 19, 514–521. [Google Scholar] [CrossRef] [PubMed]
- González, M.A.; Correa-Royero, J.; Agudelo, L.; Mesa, A.; Betancur-Galvis, L. Synthesis and biological evaluation of abietic acid derivatives. Eur. J. Med. Chem. 2009, 44, 2468–2472. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Zhang, Y.; Liu, M.; Yang, S.; Gan, M.; Zi, J.; Song, W.; Fan, X.; Wang, S.; Liu, Y.; et al. Abietane and C20-Norabietane Diterpenes from the Stem Bark of Fraxinus sieboldiana and Their Biological Activities. J. Nat. Prod. 2010, 73, 1914–1921. [Google Scholar] [CrossRef] [PubMed]
- Theoduloz, C.; Pertino, M.W.; Rodríguez, J.A.; Schmeda-Hirschmann, G. Gastroprotective Effect and Cytotoxicity of Carnosic Acid Derivatives. Planta Med. 2011, 77, 882–887. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.; Zhang, D.-W.; Qu, G.-W.; Li, G.-S.; Dai, S.J. New abietane norditerpenoid from Salvia miltiorrhiza with cytotoxic activities. J. Asian Nat. Prod. Res. 2012, 14, 913–917. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.-Y.; Liu, L.; Tao, W.-W.; Duan, J.-A.; Tian, L.-J. Diterpenoids from Pinus massoniana resin and their cytotoxicity against A431 and A549 cells. Phytochemistry 2010, 71, 1528–1533. [Google Scholar] [CrossRef] [PubMed]
- Kolak, U.; Kabouche, A.; Öztürk, M.; Kabouche, Z.; Topçu, G.; Ulubelen, A. Antioxidant diterpenoids from the roots of Salvia barrelieri. Phytochem. Anal. 2009, 20, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.-J.; Li, Y.-H.; Jiang, J.-D.; Yu, S.-S.; Qu, J.; Ma, S.-G.; Liu, Y.-B.; Yu, D.-Q. Anti-Coxsackie virus B diterpenes from the roots of Illicium jiadifengpi. Tetrahedron 2013, 69, 1017–1023. [Google Scholar] [CrossRef]
- Arrabal, C.; García-Vallejo, M.C.; Cadahia, E.; Cortijo, M.; de Simón, B.F. Characterization of two chemotypes of Pinus pinaster by their terpene and acid patterns in needles. Plant Syst. Evol. 2012, 298, 511–522. [Google Scholar] [CrossRef]
- Arrabal, C.; García-Vallejo, M.C.; Cadahia, E.; Cortijo, M.; de Simón, B.F. Seasonal variations of lipophilic compounds in needles of two chemotypes of Pinus pinaster Ait. Plant Syst. Evol. 2014, 300, 359–367. [Google Scholar] [CrossRef]
- Conde, E.; Fang, W.; Hemming, J.; Willför, S.; Domínguez, H.; Parajó, J.C. Recovery of bioactive compounds from Pinus pinaster wood by consecutive extraction stages. Wood Sci. Technol. 2014, 48, 311–323. [Google Scholar] [CrossRef]
- De Simón, B.F.; Vallejo, M.C.G.; Cadahía, E.; Miguel, C.A.; Martinez, M.C. Analysis of lipophilic compounds in needles of Pinus pinea L. Ann. For. Sci. 2001, 58, 449–454. [Google Scholar] [CrossRef]
- Hafizoğlu, H.; Holmbom, B.; Reunanen, M. Chemical Composition of Lipophilic and Phenolic Constituents of Barks from Pinus nigra, Abies bornmülleriana and Castanea sativa. Holzforschung 2002, 56, 257–260. [Google Scholar] [CrossRef]
- Koutsaviti, A.; Ioannou, E.; Couladis, M.; Tzakou, O.; Roussis, V. 1H and 13C NMR spectral assignments of abietane diterpenes from Pinus heldreichii and Pinus nigra subsp. nigra. Magn. Reson. Chem. 2017, 55, 772–778. [Google Scholar] [CrossRef] [PubMed]
- Hafızoğlu, H. Studies on the Wood and Bark Constituents of Pinus pinea L. Holzforschung 1989, 43, 41–43. [Google Scholar] [CrossRef]
- Conde, E.; Hemming, J.; Smeds, A.; Reinoso, B.D.; Moure, A.; Willför, S.; Domínguez, H.; Parajó, J.C. Extraction of low-molar-mass phenolics and lipophilic compounds from Pinus pinaster wood with compressed CO2. J. Supercrit. Fluids 2013, 81, 193–199. [Google Scholar] [CrossRef]
- Willför, S.; Ali, M.; Karonen, M.; Reunanen, M.; Arfan, M.; Harlamow, R. Extractives in bark of different conifer species growing in Pakistan. Holzforschung 2009, 63, 551–558. [Google Scholar] [CrossRef]
- Fradinho, D.M.; Neto, C.P.; Evtuguin, D.; Jorge, F.C.; Irle, M.A.; Gil, M.H.; de Jesus, J.P. Chemical characterisation of bark and of alkaline bark extracts from maritime pine grown in Portugal. Ind. Crop. Prod. 2002, 16, 23–32. [Google Scholar] [CrossRef]
- Nunes, E.; Quilhó, T.; Pereira, H. Anatomy and Chemical Composition of Pinus Pinaster Bark. IAWA J. 1996, 17, 141–150. [Google Scholar] [CrossRef]
- Nunes, E.; Quilhó, T.; Pereira, H. Anatomy and chemical composition of Pinus pinea L. bark. Ann. For. Sci. 1999, 56, 479–484. [Google Scholar] [CrossRef]
- Kettunen, H.; van Eerden, E.; Lipiński, K.; Rinttilä, T.; Valkonen, E.; Vuorenmaa, J. Dietary resin acid composition as a performance enhancer for broiler chickens. J. Appl. Anim. Nutr. 2017, 5, 1–8. [Google Scholar] [CrossRef]
- Kettunen, H.; Vuorenmaa, J.; Rinttilä, T.; Grönberg, H.; Valkonen, E.; Apajalahti, J. Natural resin acid-enriched composition as a modulator of intestinal microbiota and performance enhancer in broiler chicken. J. Appl. Anim. Nutr. 2015, 3, 1–9. [Google Scholar] [CrossRef]
- Roy, K.; Lyhs, U.; Vuorenmaa, J.; Pedersen, K. In vitro inhibition studies of natural resin acids to Clostridium perfringens, Staphylococcus aureus and Escherichia coli O149. J. Appl. Anim. Nutr. 2018, 5, 1–5. [Google Scholar] [CrossRef]
- Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P. Identification of New Hydroxy Fatty Acids and Ferulic Acid Esters in the Wood of Eucalyptus globulus. Holzforschung 2002, 56, 143–149. [Google Scholar] [CrossRef]
- Villaverde, J.J.; Domingues, R.M.A.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P.; Ligero, P.; Vega, A. Miscanthus × giganteus Extractives: A Source of Valuable Phenolic Compounds and Sterols. J. Agric. Food Chem. 2009, 57, 3626–3631. [Google Scholar] [CrossRef] [PubMed]
- Aveling, E.M.; Heron, C. Identification of Birch Bark Tar at the Mesolithic Site of Star Carr. Anc. Biomol. 1998, 2, 69–80. [Google Scholar]
- Birkemeyer, C.; Kopka, J. Design of Metabolite Recovery by Variations of the Metabolite Profiling Protocol. In Concepts in Plant Metabolomics; Nikolau, B.J., Wurtele, E.S., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 45–70. [Google Scholar]
- Brettell, R.C.; Schotsmans, E.M.J.; Walton Rogers, P.; Reifarth, N.; Redfern, R.C.; Stern, B.; Heron, C.P. ‘Choicest unguents’: Molecular evidence for the use of resinous plant exudates in late Roman mortuary rites in Britain. J. Archaeol. Sci. 2015, 53, 639–648. [Google Scholar] [CrossRef]
- Coelho, D.; Marques, G.; Gutiérrez, A.; Silvestre, A.J.D.; del Río, J.C. Chemical characterization of the lipophilic fraction of giant reed (Arundo donax) fibres used for pulp and paper manufacturing. Ind. Crops Prod. 2007, 26, 229–236. [Google Scholar] [CrossRef]
- Rontani, J.-F.; Aubert, C.; Belt, S.T. EIMS Fragmentation Pathways and MRM Quantification of 7α/β-Hydroxy-Dehydroabietic Acid TMS Derivatives. J. Am. Soc. Mass Spectrom. 2015, 26, 1606–1616. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.A.O.; Vilela, C.; Domingues, R.M.A.; Oliveira, C.S.D.; Villaverde, J.J.; Freire, C.S.R.; Neto, C.P.; Silvestre, A.J.D. Secondary metabolites from Eucalyptus grandis wood cultivated in Portugal, Brazil and South Africa. Ind. Crop. Prod. 2017, 95, 357–364. [Google Scholar] [CrossRef]
- Szczepaniak, L.; Isidorov, V.A. Gas chromatographic retention indices of trimethylsilyl derivatives of terpene alcohols. J. Chromatogr. A 2011, 1218, 7061–7064. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, K.J.; Boon, J.J.; Pastorova, I.; Spetter, L.F.M. Mass spectrometric methodology for the analysis of highly oxidized diterpenoid acids in Old Master paintings. J. Mass Spectrom. 2000, 35, 512–533. [Google Scholar] [CrossRef]
- Costa, P.; Grosso, C.; Gonçalves, S.; Andrade, P.B.; Valentão, P.; Gabriela Bernardo-Gil, M.; Romano, A. Supercritical fluid extraction and hydrodistillation for the recovery of bioactive compounds from Lavandula viridis L’Hér. Food Chem. 2012, 135, 112–121. [Google Scholar] [CrossRef]
- Flores, R.M.; Doskey, P.V. Evaluation of multistep derivatization methods for identification and quantification of oxygenated species in organic aerosol. J. Chromatogr. A 2015, 1418, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.S.; Rego, A.; Ramos, V.; Afonso, T.B.; Freitas, S.; Preto, M.; Lopes, V.; Vasconcelos, V.; Magalhães, C.; Leão, P.N. The conifer biomarkers dehydroabietic and abietic acids are widespread in Cyanobacteria. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- González, M.A. Aromatic abietane diterpenoids: Their biological activity and synthesis. Nat. Prod. Rep. 2015, 32, 684–704. [Google Scholar] [CrossRef]
- Silvestre, A.J.D.; Gandini, A. Terpenes: Major Sources, Properties and Applications. In Monomers, Polymers and Composites from Renewable Resources; Belgacem, M.N., Gandini, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 17–38. [Google Scholar]
- Mathe, C.; Culioli, G.; Archier, P.; Vieillescazes, C. Characterization of archaeological frankincense by gas chromatography–mass spectrometry. J. Chromatogr. A 2004, 1023, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Masendra; Ashitani, T.; Takahashi, K.; Lukmandaru, G. Lipophilic extractives of the inner and outer barks from six different Pinus species grown in Indonesia. J. For. Res. 2018, 29, 1329–1336. [Google Scholar] [CrossRef]
- Domingues, R.M.A.; Guerra, A.R.; Duarte, M.; Freire, C.S.R.; Neto, C.P.; Silva, C.M.S.; Silvestre, A.J.D. Bioactive Triterpenic Acids: From Agroforestry Biomass Residues to Promising Therapeutic Tools. Mini-Rev. Org. Chem. 2014, 11, 382–399. [Google Scholar] [CrossRef]
- Braga, M.E.M.; Santos, R.M.S.; Seabra, I.J.; Facanali, R.; Marques, M.O.M.; de Sousa, H.C. Fractioned SFE of antioxidants from maritime pine bark. J. Supercrit. Fluids 2008, 47, 37–48. [Google Scholar] [CrossRef]
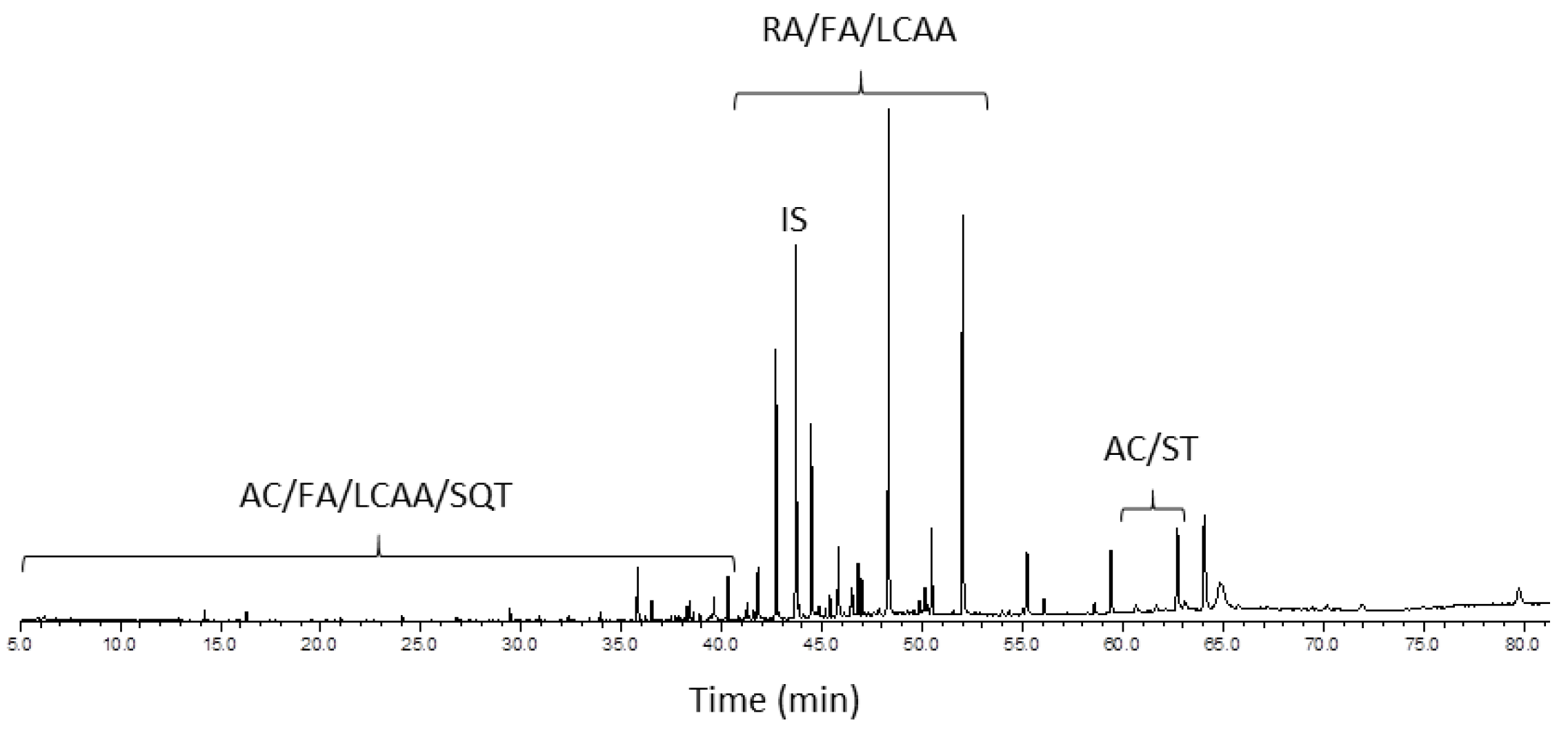
| RT (min) | No. | Compound | P. pinaster | P. pinea |
|---|---|---|---|---|
| Terpenic compounds | 2865 | 965 | ||
| Diterpenic resin acids | 2353 | 960 | ||
| Pimarane-type resin acids | 399 | 166 | ||
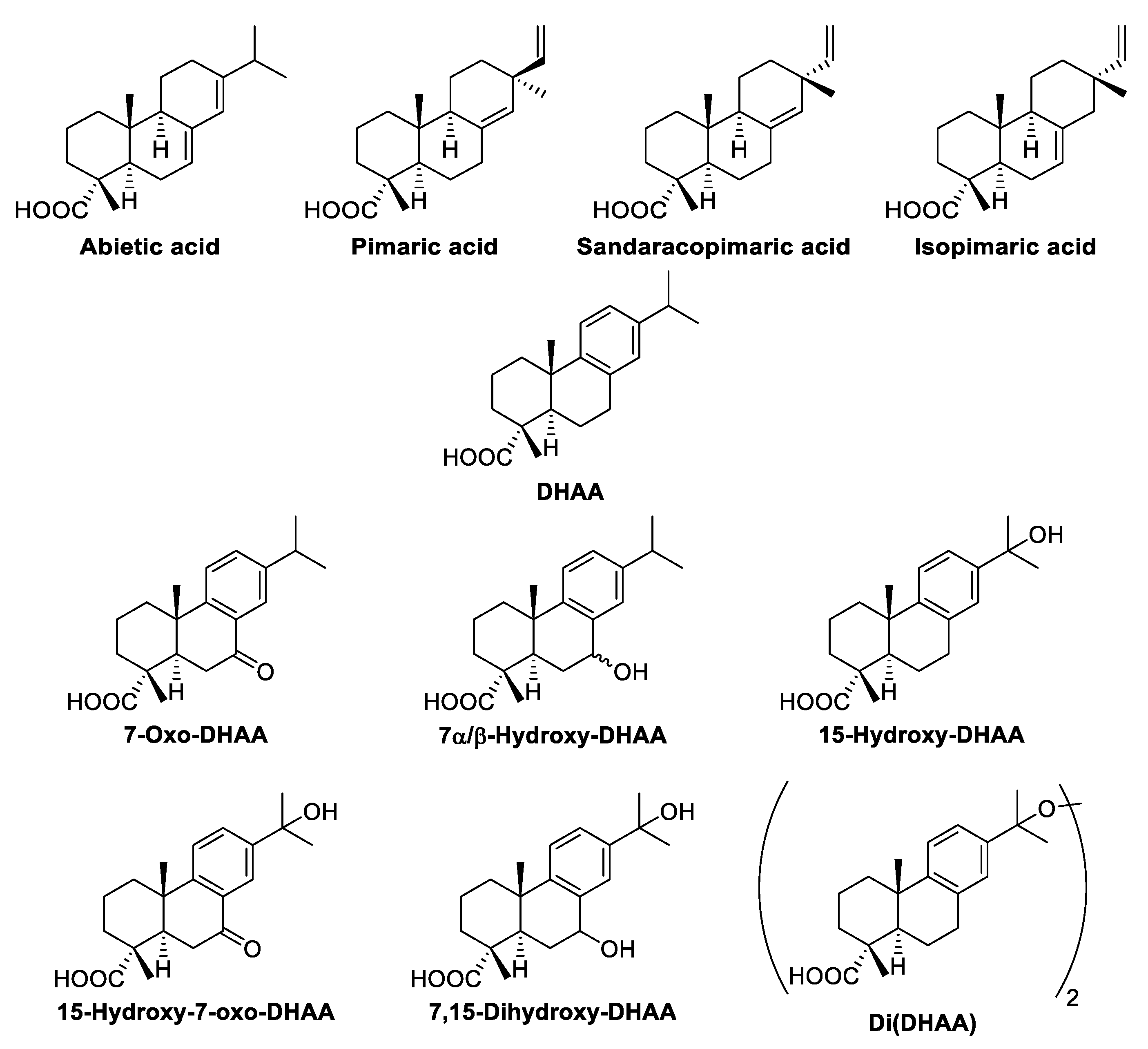
| 41.26 | 1 | Pimaric acid | 217 | 27 |
| 41.59 | 2 | Sandaracopimaric acid | 54 | 19 |
| 41.81 | 3 | Isopimaric acid | 128 | 120 |
| Abietane-type resin acids | 1954 | 794 | ||
| 42.07 | 4 | Di(dehydroabietic acid) | 18 | 4 |
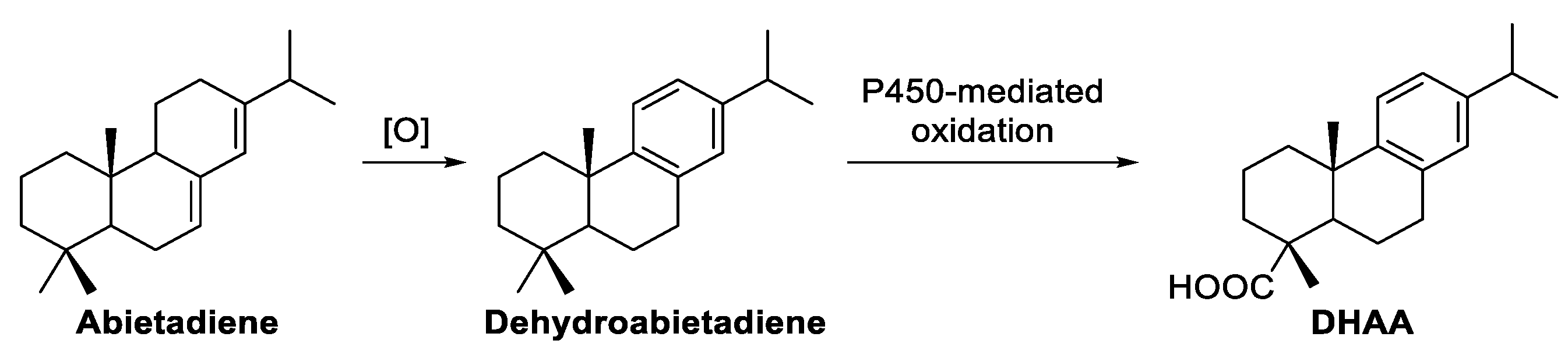
| 42.71 | 5 | Dehydroabietic acid | 954 | 452 |
| 43.41 | 6 | Abietic acid | 62 | 14 |
| 45.39 | 7 | 7β-Hydroxydehydroabietic acid | 99 | 39 |
| 45.81 | 8 | 7α-Hydroxydehydroabietic acid | 198 | 120 |
| 46.50 | 9 | 7-Oxodehydroabietic acid | 179 | 57 |
| 46.98 | 10 | 15-Hydroxydehydroabietic acid | 269 | 67 |
| 49.86 | 11 | 7,15-Dihydroxydehydroabietic acid | 100 | 24 |
| 50.28 | 12 | 15-Hydroxy-7-oxodehydroabietic acid | 75 | 17 |
| Monoterpenes | 55 | n.d. | ||
| 7.86 | 13 | Camphor | 2 | n.d. |
| 8.46 | 14 | Pinocarvone | 6 | n.d. |
| 11.30 | 15 | Geraniol | 4 | n.d. |
| 11.74 | 16 | Borneol | 10 | n.d. |
| 14.08 | 17 | Myrtenol | 4 | n.d. |
| 15.06 | 18 | α-Terpineol | 13 | n.d. |
| 22.07 | 19 | Terpin | 16 | n.d. |
| Sesquiterpenes | 32 | 5 | ||
| 17.47 | 20 | Longifolene | 21 | 2 |
| 22.67 | 21 | Caryophyllene oxide | 7 | 3 |
| 26.11 | 22 | Isolongifolol | 4 | n.d. |
| Pentacyclic triterpenes | 425 | n.d. | ||
| 73.75 | 23 | Betulin | 425 | n.d. |
| Aromatic compounds | 366 | 53 | ||
| Aldehydes | 37 | 9 | ||
| 15.83 | 24 | p-Hydroxybenzaldehyde | 4 | 3 |
| 20.97 | 25 | Vanillin | 33 | 6 |
| Acids | 83 | 29 | ||
| 28.39 | 26 | Vanillic acid | 6 | n.d. |
| 31.85 | 27 | Syringic acid | 1 | n.d. |
| 32.34 | 28 | (Z)-Ferulic acid | 3 | n.d. |
| 32.87 | 29 | p-Coumaric acid | 8 | n.d. |
| 36.51 | 30 | (E)-Ferulic acid | 58 | 29 |
| 38.07 | 31 | Caffeic acid | 7 | n.d. |
| Resinols | 246 | 15 | ||
| 61.66 | 32 | Pinoresinol | 246 | 15 |
| Fatty acids | 749 | 1146 | ||
| Saturated fatty acids | 670 | 1058 | ||
| 6.22 | 33 | Hexanoic acid | 3 | 4 |
| 12.88 | 34 | Octanoic acid | 1 | 4 |
| 16.27 | 35 | Nonanoic acid | 1 | 11 |
| 19.51 | 36 | Decanoic acid | tr | 3 |
| 30.87 | 37 | Tetradecanoic acid | 1 | 4 |
| 33.38 | 38 | Pentadecanoic acid | 1 | 2 |
| 35.79 | 39 | Hexadecanoic acid | 55 | 50 |
| 37.46 | 40 | Heptadecanoic acid | 4 | 4 |
| 40.30 | 41 | Octadecanoic acid | 23 | 42 |
| 44.46 | 42 | Eicosanoic acid | 67 | 156 |
| 48.32 | 43 | Docosanoic acid | 251 | 401 |
| 50.14 | 44 | Tricosanoic acid | 30 | 36 |
| 52.00 | 45 | Tetracosanoic acid | 213 | 326 |
| 56.05 | 46 | Hexacosanoic acid | 20 | 15 |
| Unsaturated fatty acids | 75 | 80 | ||
| 39.38 | 47 | (9Z,12Z)-Octadeca-9,12-dienoic acid | 22 | 11 |
| 39.58 | 48 | (9Z)-Octadec-9-enoic acid | 48 | 39 |
| 39.79 | 49 | (9E)-Octadec-9-enoic acid | 5 | 6 |
| 43.83 | 50 | (11Z)-Eicos-11-enoic acid | n.d. | 24 |
| Diacids | 4 | 8 | ||
| 29.42 | 51 | Nonanedioic acid | 4 | 8 |
| Long-chain aliphatic alcohols | 124 | 219 | ||
| 33.94 | 52 | Hexadecan-1-ol | 11 | 8 |
| 37.86 | 53 | Octadec-9-en-1-ol | 11 | 8 |
| 38.57 | 54 | Octadecan-1-ol | 6 | 8 |
| 46.78 | 55 | Docosan-1-ol | 34 | 71 |
| 50.47 | 56 | Tetracosan-1-ol | 62 | 124 |
| Sterols | 587 | 242 | ||
| Δ5-Sterols | 467 | 242 | ||
| 60.66 | 57 | Campesterol | 33 | 18 |
| 62.73 | 58 | β-Sitosterol | 434 | 224 |
| Δ4-3-keto-steroids | 120 | n.d. | ||
| 64.08 | 59 | Stigmast-4-en-3-one | 120 2 | n.d. |
| Others | 55 | 285 | ||
| 14.22 | 60 | Glycerol | 3 | 7 |
| 59.42 | 61 | 2,3-Dihydroxypropyl docosanoate | 52 | 91 |
| 64.08 | 62 | 2,3-Dihydroxypropyl tetracosanoate | 2 | 187 |
| Total | 4746 | 2910 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa, J.L.C.; Ramos, P.A.B.; Freire, C.S.R.; Silva, A.M.S.; Silvestre, A.J.D. Chemical Composition of Lipophilic Bark Extracts from Pinus pinaster and Pinus pinea Cultivated in Portugal. Appl. Sci. 2018, 8, 2575. https://doi.org/10.3390/app8122575
Sousa JLC, Ramos PAB, Freire CSR, Silva AMS, Silvestre AJD. Chemical Composition of Lipophilic Bark Extracts from Pinus pinaster and Pinus pinea Cultivated in Portugal. Applied Sciences. 2018; 8(12):2575. https://doi.org/10.3390/app8122575
Chicago/Turabian StyleSousa, Joana L. C., Patrícia A. B. Ramos, Carmen S. R. Freire, Artur M. S. Silva, and Armando J. D. Silvestre. 2018. "Chemical Composition of Lipophilic Bark Extracts from Pinus pinaster and Pinus pinea Cultivated in Portugal" Applied Sciences 8, no. 12: 2575. https://doi.org/10.3390/app8122575
APA StyleSousa, J. L. C., Ramos, P. A. B., Freire, C. S. R., Silva, A. M. S., & Silvestre, A. J. D. (2018). Chemical Composition of Lipophilic Bark Extracts from Pinus pinaster and Pinus pinea Cultivated in Portugal. Applied Sciences, 8(12), 2575. https://doi.org/10.3390/app8122575