Study on the Decomposition Mechanism of Natural Gas Hydrate Particles and Its Microscopic Agglomeration Characteristics
Abstract
:1. Introduction
2. Experimental Apparatus and Materials
2.1. The High-Pressure Hydrate Experimental Loop
2.2. The Hydrate Experimental Loop Instrumentation
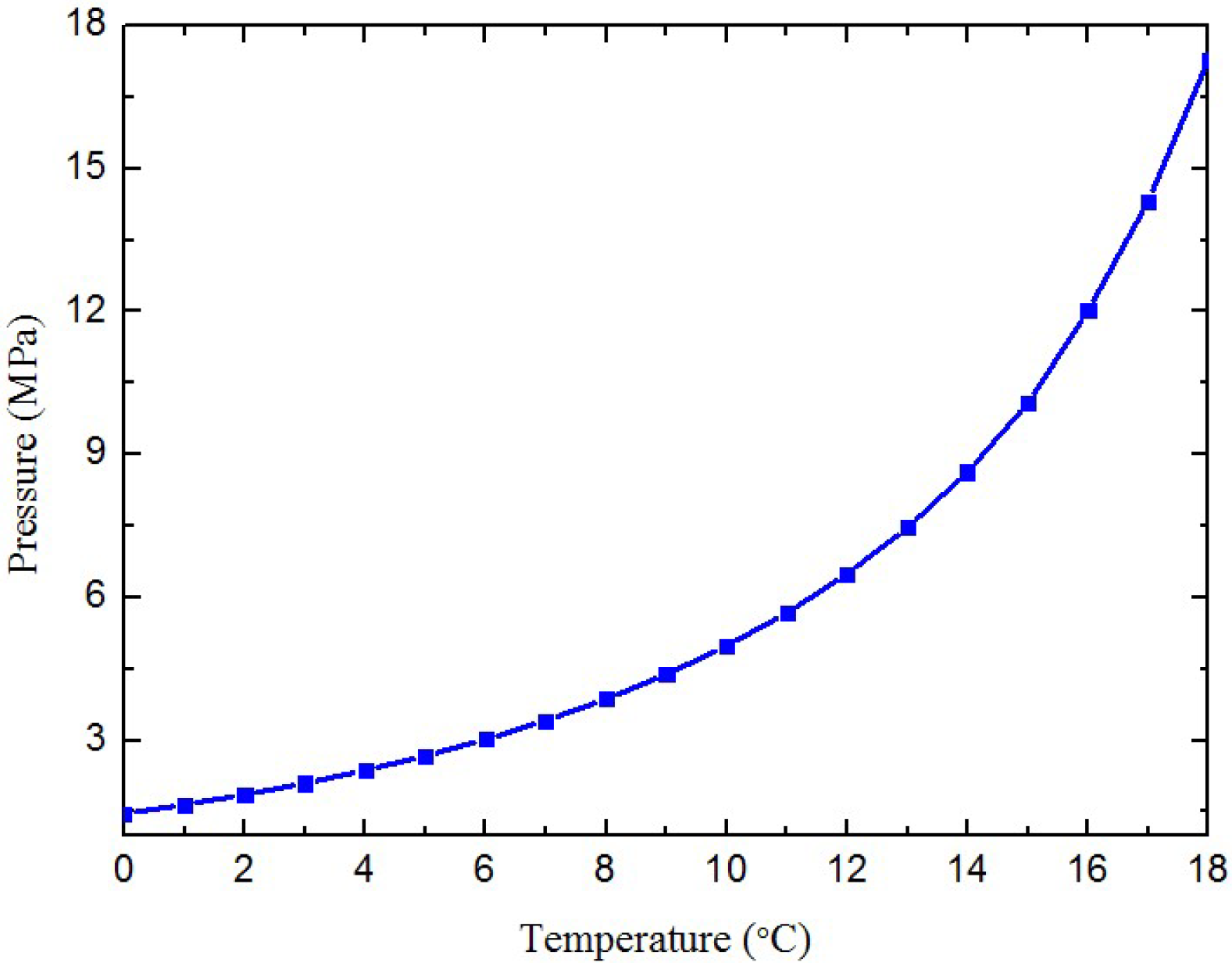
2.3. Fluids
2.4. Experimental Procedure
- (1)
- Evacuate the entire experimental loop until the vacuum degree reaches 0.09 MPa;
- (2)
- Load diesel and water (100 vol% liquid loading) with a specific water-cut for each test. Here water-cut is defined as the volume ratio of water to the total liquid. Diesel volume is fixed at 70 L during all these experiments. The gas-supply unit begins to inject gas into the separator at the room temperature (20 °C) to reach the aimed experimental pressure;
- (3)
- Circulate the water and oil mixture at a constant flow rate to form a homogeneous and stable emulsion with the specific AA dosage for each test. Stability of the water/oil emulsion referred to a relatively stable process (dynamic stability) according to the measured data from FBRM under shearing action. This is to say that the emulsion is regarded as stable when the average chord length of droplets fluctuated in ±0.2 μm within 2 h;
- (4)
- Decrease the temperature gradually to a specific value under the initial pressure and flow velocity. Open the data acquisition system to collect the temperature, pressure, pressure drop, flow rate, density, and the chord length continuously, during the hydrate formation process;
- (5)
- When the pressure, temperature, and flow rate of the loop are stable, maintain the stable situation for at least 5 more hours.
- (6)
- To dissociate the hydrate slurry, the system is heated at constant volume using the bath system. Set the bath temperature at 30 °C and collect all data during the hydrate decomposition process;
- (7)
- A round of hydrate formation and decomposition experiment is scheduled for completion when all measured data are stable at the end of the hydrate decomposition process.
3. Results and Discussion
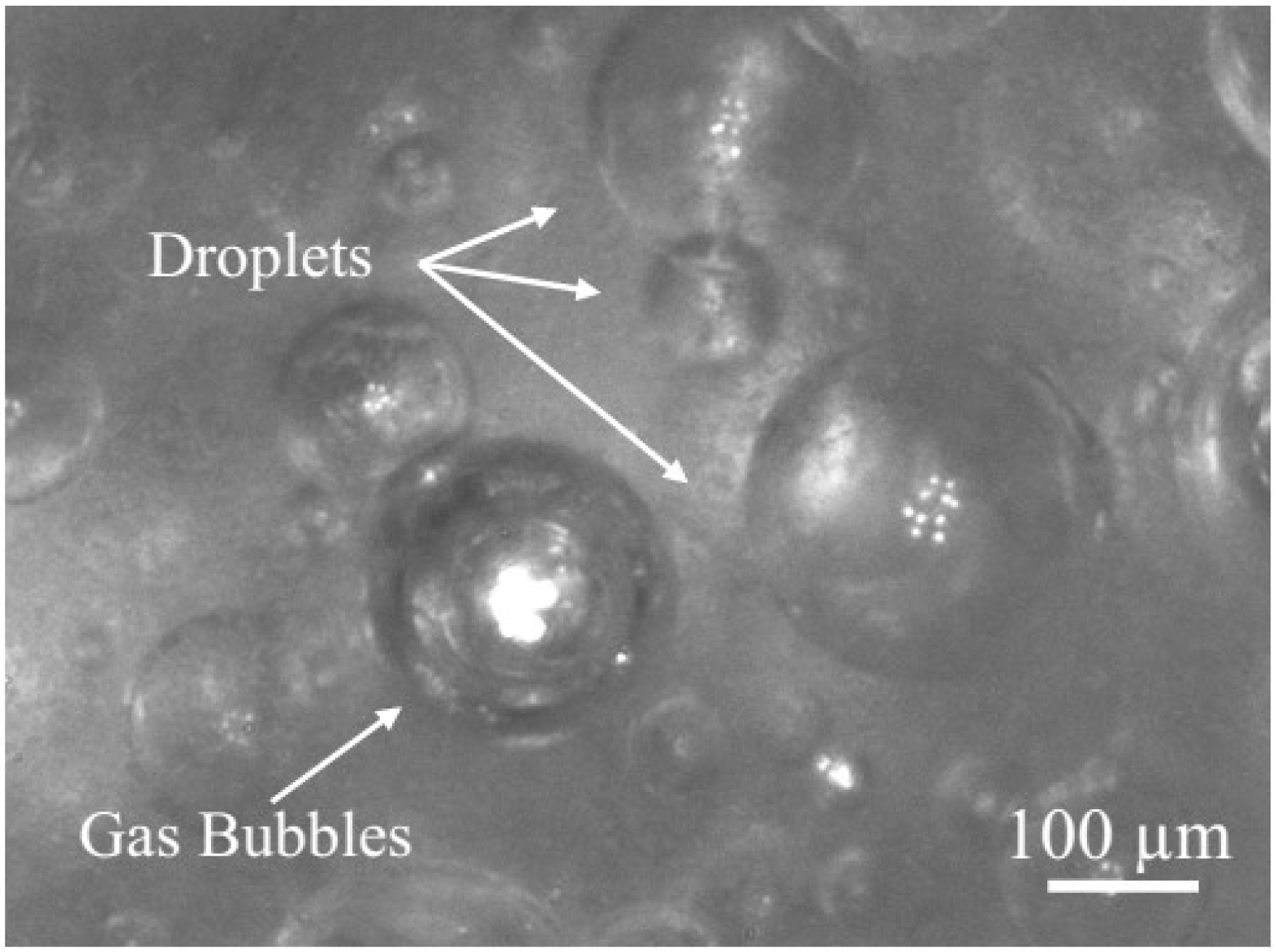
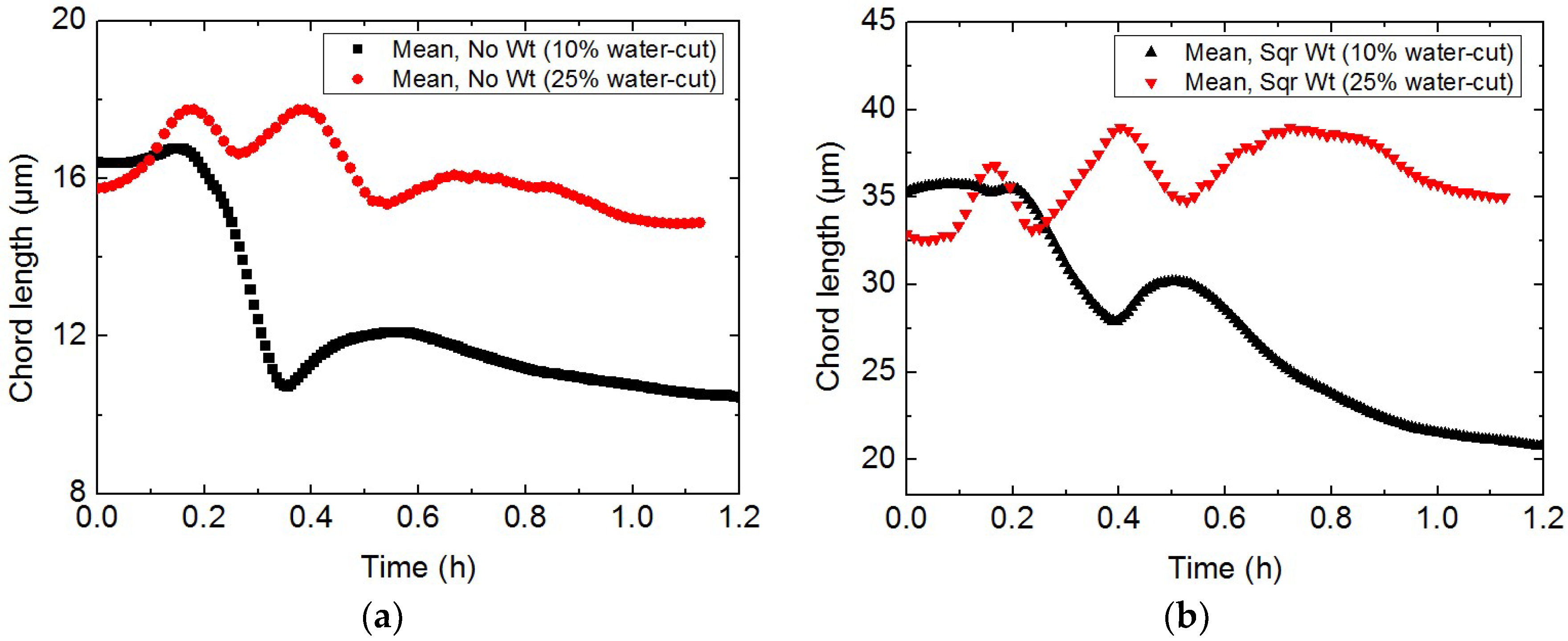
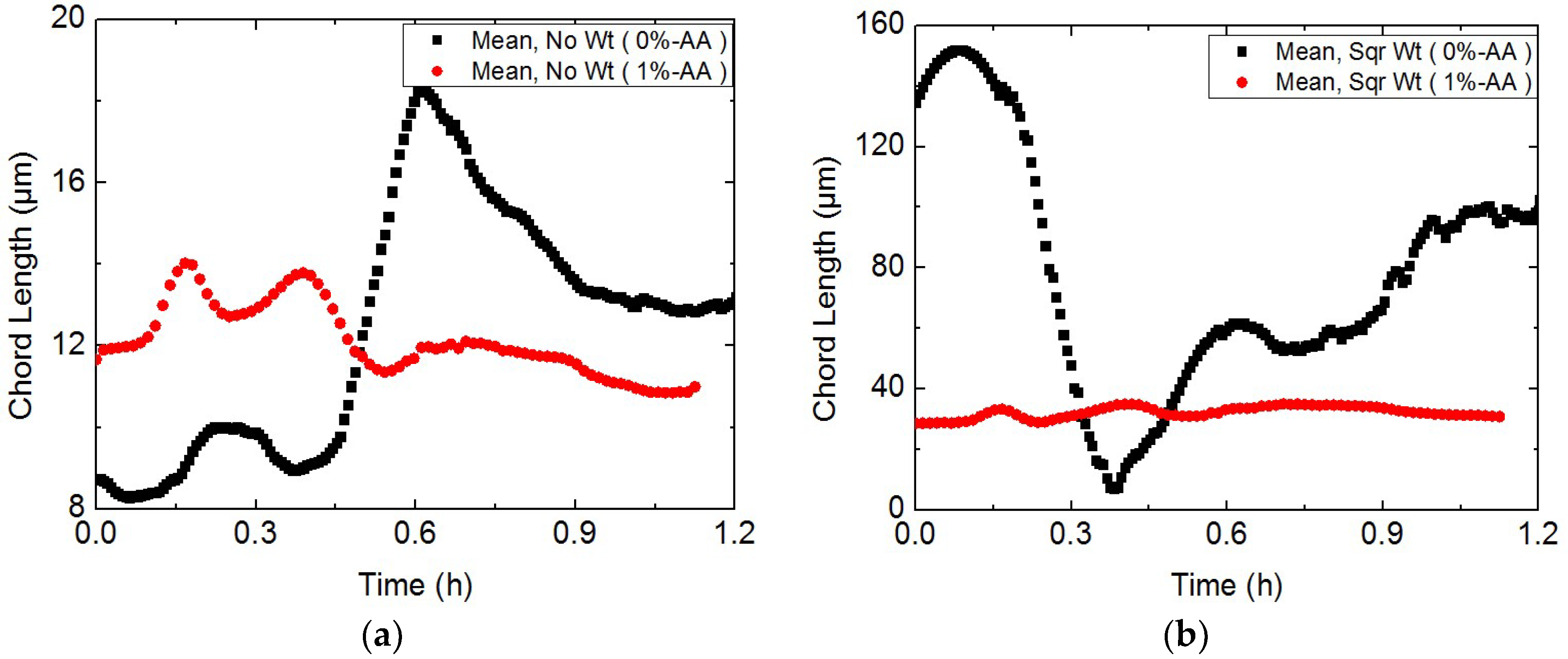
3.1. Study on Hydrate Slurry Dissociation Morphology
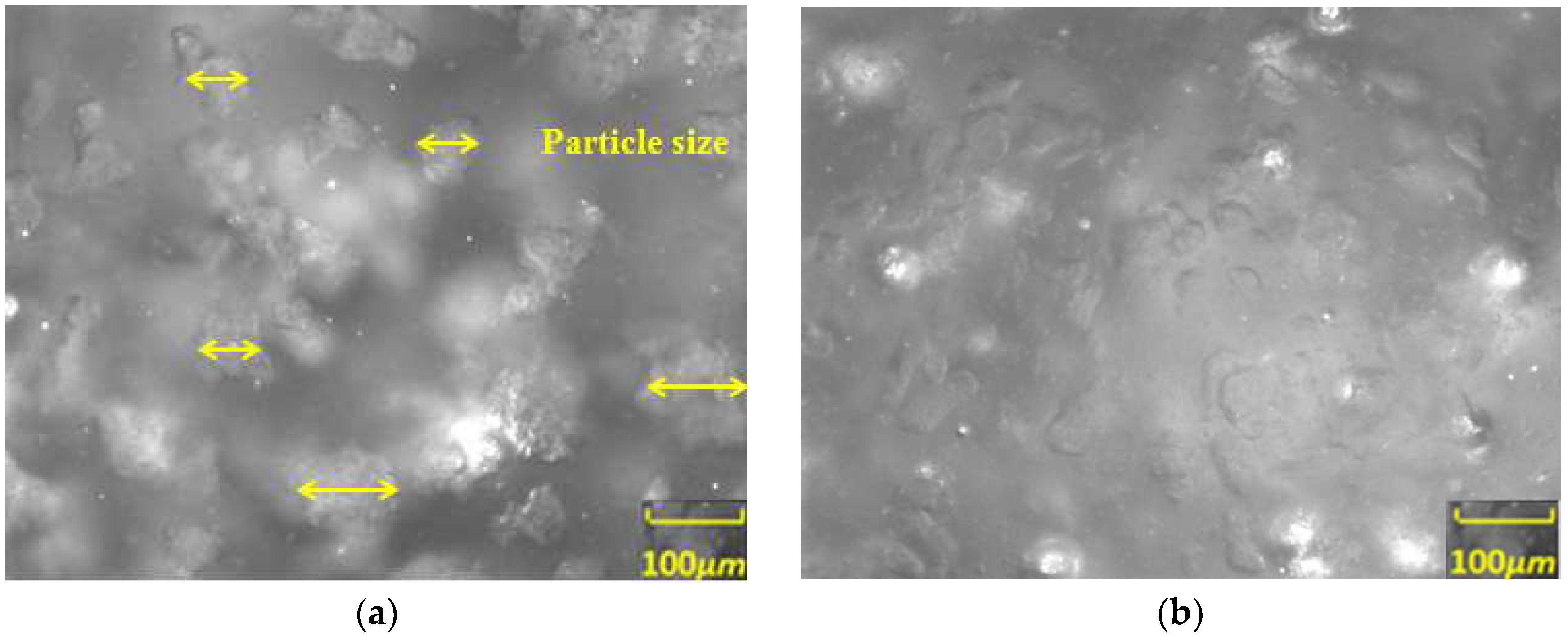
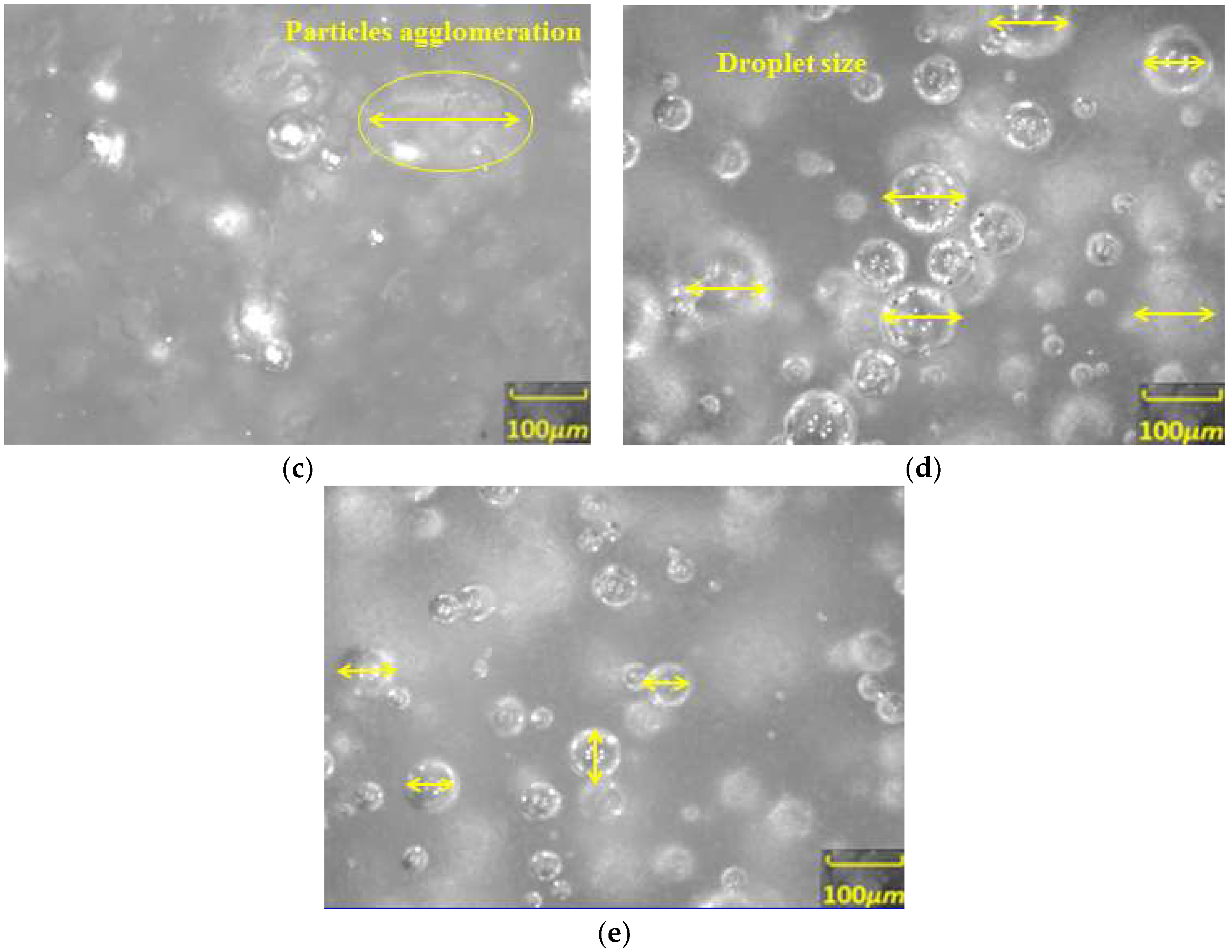
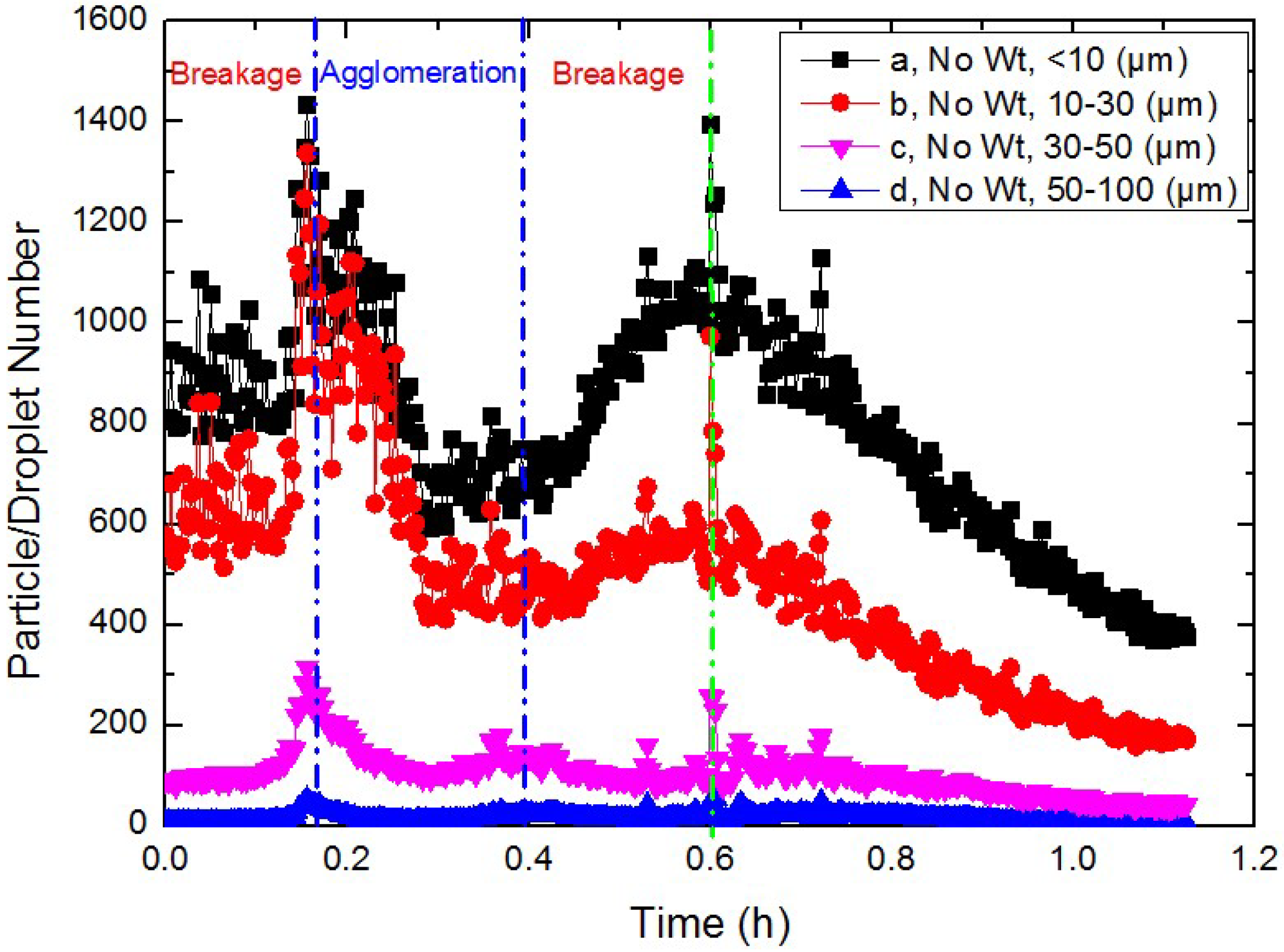
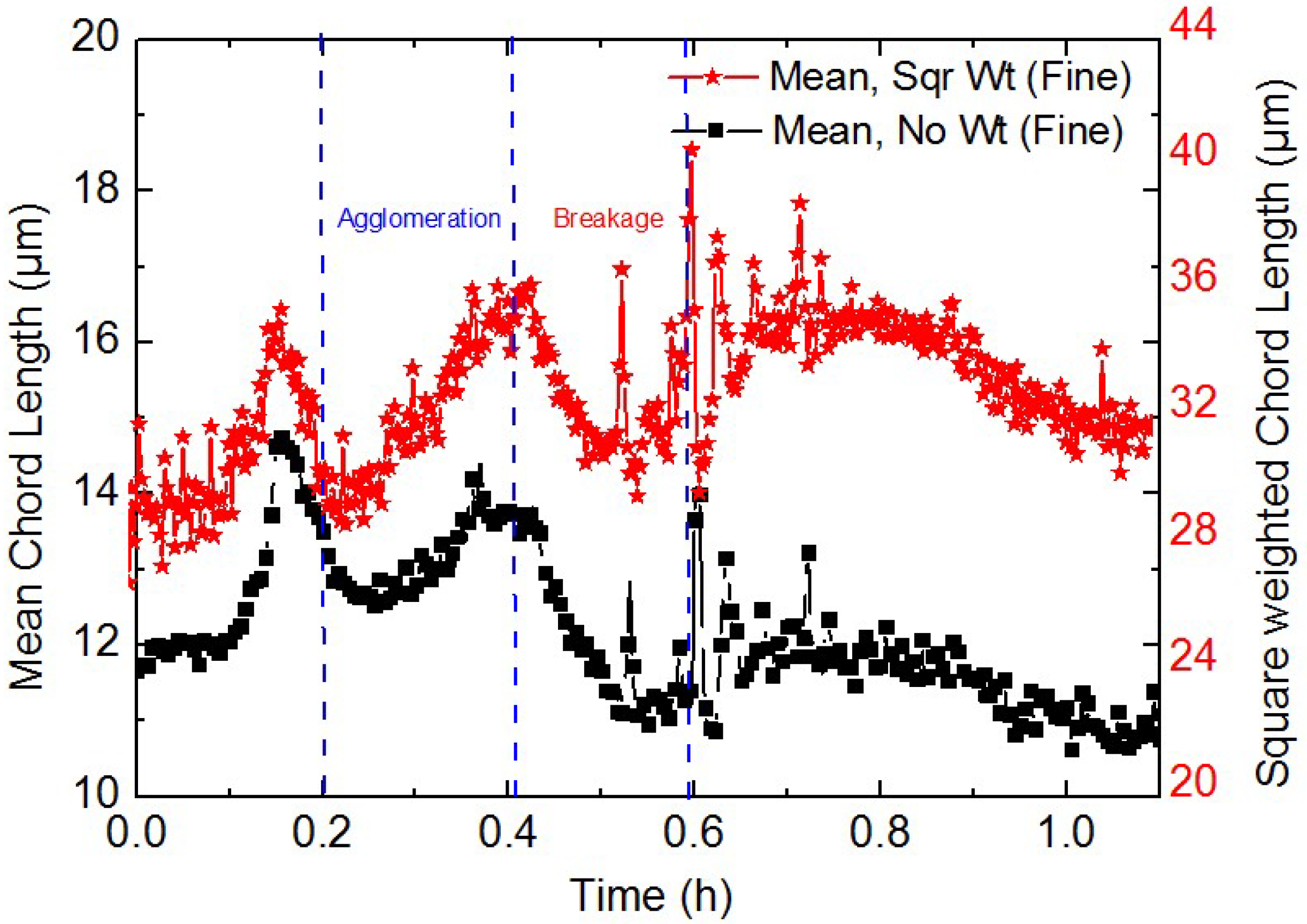
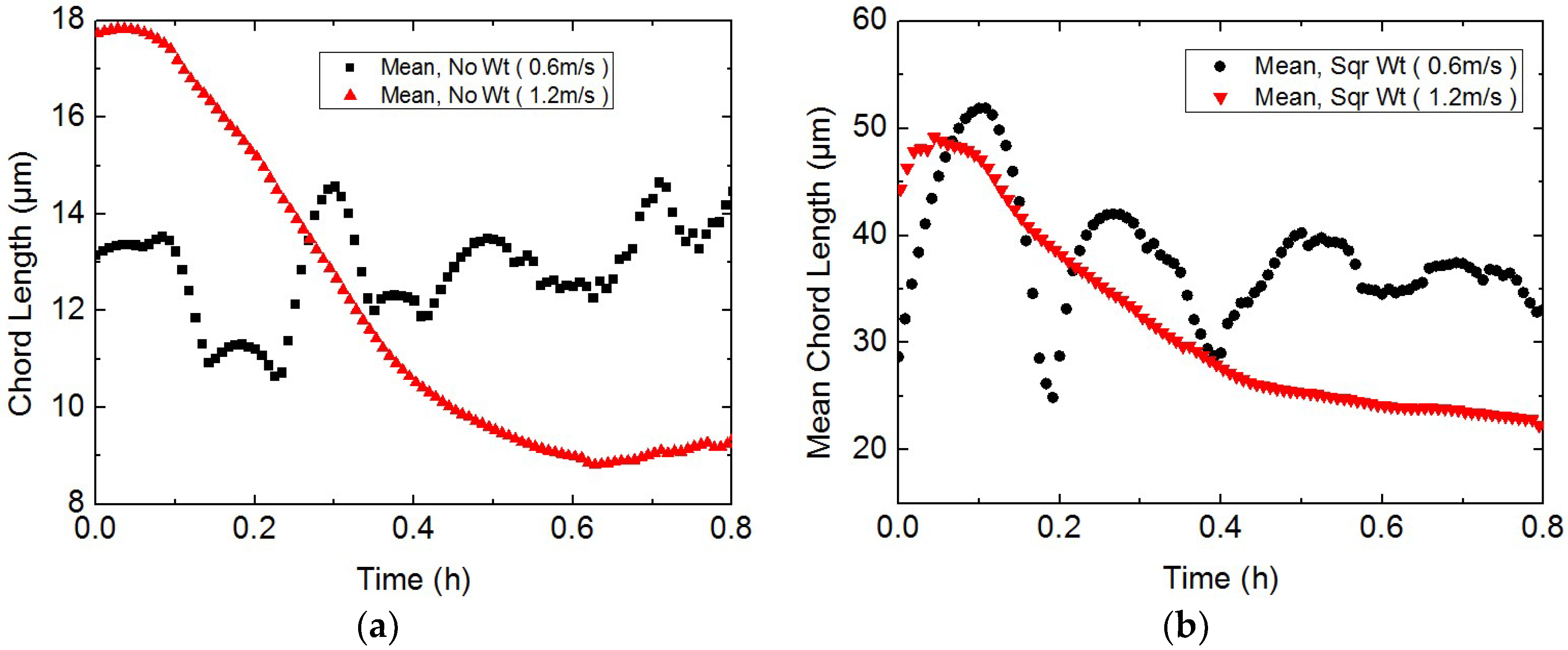
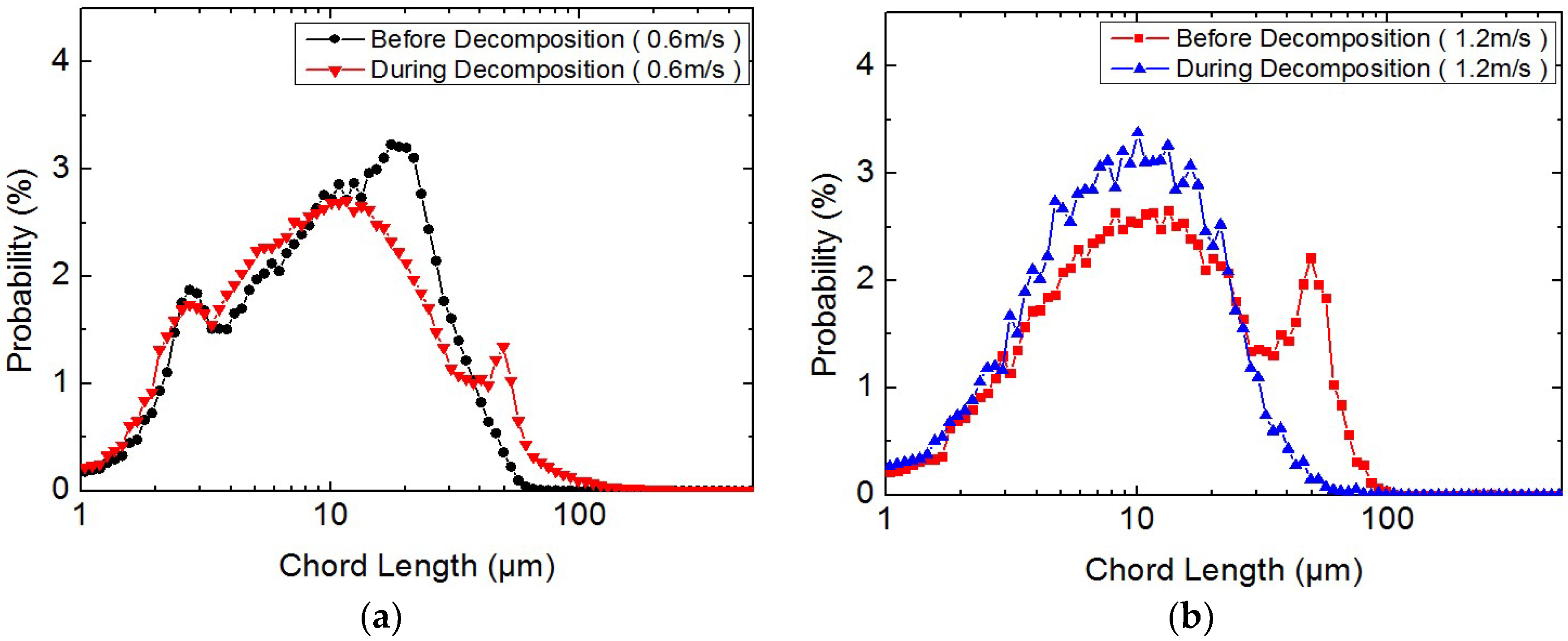
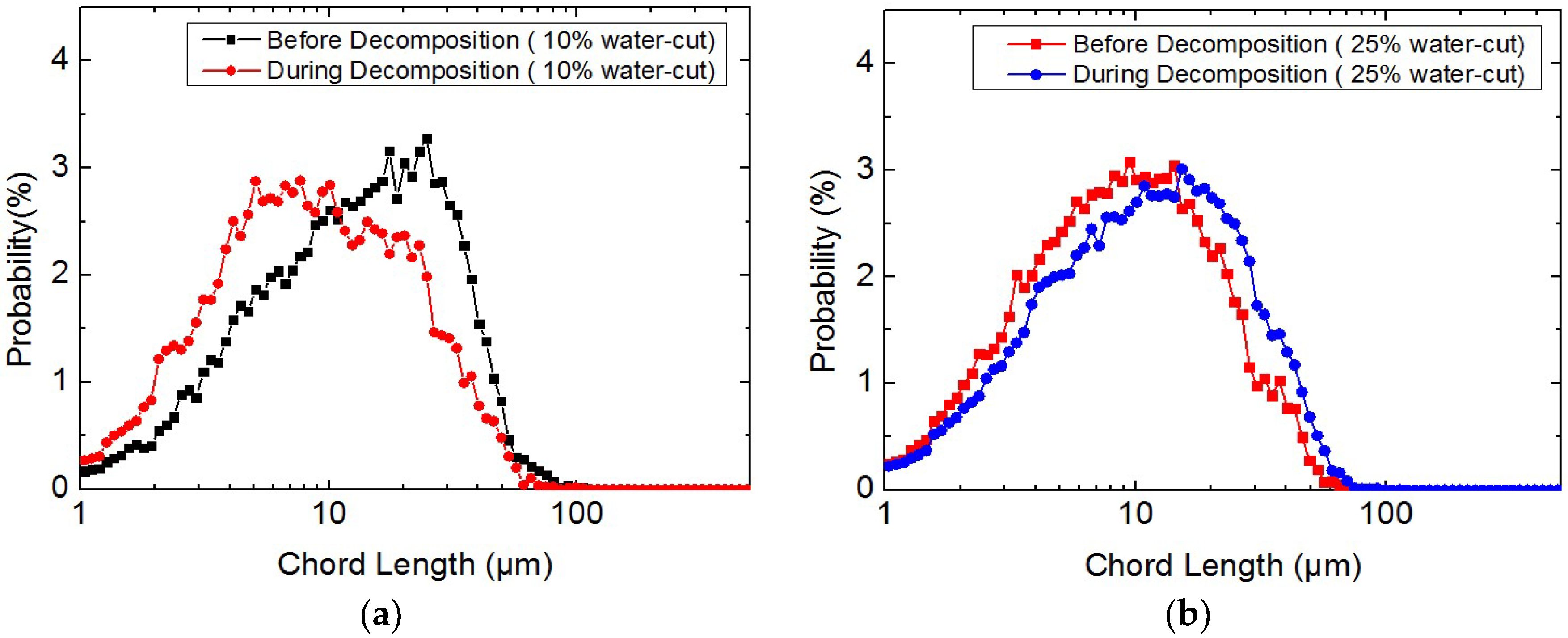
3.2. Study on the Micro-Particle Distribution Characteristics
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sloan, E.D.; Koh, C.A. Clathrate Hydrates of Natural Gases, 3rd ed.; CRC Press: New York, NY, USA, 2008. [Google Scholar]
- Sloan, E.D.; Koh, C.A.; Sum, A.K.; Ballard, A.L.; Creek, J.; Eaton, M.; Lachance, J.; Mcmullen, N.; Palermo, T.; Shoup, G.; et al. Natural Gas Hydrates in Flow Assurance; Elsevier: Amsterdam, The Netherlands; Boston, MA, USA; Heidelberg, Germany; London, UK; New York, NY, USA; Oxford, UK; Paris, France; San Diego, CA, USA; Singapore; Sydney, Australia; Tokyo, Japan, 2010. [Google Scholar]
- Hammerschmidt, T. Formation of Gas Hydrates in Natural Gas Transmission Lines. Ind. Eng. Chem. 1934, 26, 851–855. [Google Scholar] [CrossRef]
- Kim, H.; Park, J.; Seo, Y.; Ko, M. Hydrate risk management with aqueous ethylene glycol and electrolyte solutions in thermodynamically under-inhibition condition. Chem. Eng. Sci. 2017, 158, 172–180. [Google Scholar] [CrossRef]
- Zanota, M.L.; Dicharry, C.; Graciaa, A. Hydrate Plug Prevention by Quaternary Ammonium Salts. Energy Fuels 2005, 19, 584–590. [Google Scholar] [CrossRef]
- Davies, S.R.; Ivanic, J.; Sloan, E.D. Predictions of hydrate plug dissociation with electrical heating. In Proceeding of the Fifth International Conference on Gas Hydrates, Trondhelm, Norway, 13–16 June 2005. [Google Scholar]
- Kelkar, S.K.; Selim, M.S.; Sloan, E.D. Hydrate dissociation rates in pipelines. Fluid Phase Equilib. 1998, 150–151, 371–382. [Google Scholar] [CrossRef]
- Sohn, Y.; Kim, J.; Shin, K.; Chang, D.; Seo, Y.; Aman, Z.M.; May, E.F. Hydrate plug formation risk with varying water-cut and inhibitor concentrations. Chem. Eng. Sci. 2015, 126, 711–718. [Google Scholar] [CrossRef]
- Shi, B.; Song, F.; Lv, X.; Li, W.Q.; Wang, Y.; Ding, L.; Liu, Y.; Yang, J.H.; Wu, H.H.; Wang, W.; et al. Investigation on natural gas hydrate dissociation from a slurry to a water-in-oil emulsion in a high-pressure flow loop. Fuel 2018, 233, 743–758. [Google Scholar] [CrossRef]
- Wu, R.; Aman, Z.M.; May, E.F.; Kozielski, K.A.; Hartley, P.G.; Maeda, N.; Sum, A.K. The Effect of a Kinetic Hydrate Inhibitor PVCap on Cyclopentane Hydrate Cohesion Forces and Growth. Energy Fuels 2014, 28, 3632–3637. [Google Scholar] [CrossRef]
- Zhaoa, H.; Suna, M.; Firoozabadi, A. Anti-agglomeration of natural gas hydrates in liquid condensate and crude oil at constant pressure conditions. Fuel 2016, 180, 187–193. [Google Scholar] [CrossRef]
- Ding, L.; Shi, B.H.; Lv, X.F.; Liu, Y.; Wu, H.; Wang, W.; Gong, J. Investigation of natural gas hydrate slurry flow properties and flow patterns using a high pressure flow loop. Chem. Eng. Sci. 2016, 146, 199–206. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, B.; Ding, L.; Yong, Y.; Zhang, Y.; Ma, Q.; Lv, X.; Song, S.; Yang, J.; Wang, W. Investigation of Hydrate Agglomeration and Plugging Mechanism in Low-Wax-Content Water-in-Oil Emulsion Systems. Energy Fuels 2018, 32, 8986–9000. [Google Scholar] [CrossRef]
- Joshi, S.V.; Grasso, G.A.; Lafond, P.G.; Rao, I.; Webb, E.; Zerpa, L.E.; Sloan, E.D.; Koh, C.A.; Sum, A.K. Experimental flow loop investigations of gas hydrate formation in high water cut systems. Chem. Eng. Sci. 2013, 97, 198–209. [Google Scholar] [CrossRef]
- Yan, K.L.; Sun, C.Y.; Chen, J.; Chen, L.T.; Shen, D.J.; Liu, B.; Jia, M.L.; Niu, M.; Lv, Y.N.; Li, N. Flow characteristics and rheological properties of natural gas hydrate slurry in the presence of anti-agglomerant in a flow loop apparatus. Chem. Eng. Sci. 2014, 106, 99–108. [Google Scholar] [CrossRef]
- Majid, A.; Lee, W.; Srivastava, V.; Chen, L.; Warrier, P.; Grasso, G.; Vijayamohan, P.; Chaudhari, P.; Sloan, E.D.; Koh, C.A. Experimental investigation of gas-hydrate formation and particle transportability in fully and partially dispersed multiphase-flow systems using a high-pressure flow loop. SPE J. 2018, 23, 937–951. [Google Scholar] [CrossRef]
- Song, G.; Li, Y.; Wang, W.; Liu, S.; Wang, X.; Shi, Z.; Yao, S. Experimental investigation on the microprocess of hydrate particle agglomeration using a high-speed camera. Fuel 2018, 237, 475–485. [Google Scholar] [CrossRef]
- Shi, B.H.; Chai, S.; Wang, L.Y.; Lv, X.; Liu, H.S.; Wu, H.H.; Wang, W.; Yu, D.; Gong, J. Viscosity investigation of natural gas hydrate slurries with anti-agglomerants additives. Fuel 2016, 185, 323–338. [Google Scholar] [CrossRef]
- Lv, X.F.; Gong, J.; Li, W.Q.; Shi, B.; Yu, D.; Wu, H. Experimental Study on Natural Gas Hydrate Slurry Flow. SPE J. 2014, 19, 206–214. [Google Scholar] [CrossRef]
- Lv, X.F.; Shi, B.H.; Wang, Y.; Gong, J. Study on Gas Hydrate Formation and Hydrate Slurry Flow in a Multiphase Transportation System. Energy Fuels 2013, 27, 7294–7302. [Google Scholar] [CrossRef]
- Shi, B.H. Study on the Characteristics of Hydrate Growth and Flow in Gas—Condensate Multiphase Pipelines. Ph.D. Dissertation, China University of Petroleum, Beijing, China, 2012. [Google Scholar]
- Takeya, S.; Ripmeester, J.A. Dissociation behavior of clathrate hydrates to ice and dependence on guest molecules. Angew. Chem. Int. Ed. 2008, 47, 1276–1279. [Google Scholar] [CrossRef]
- Servio, P.; Englezos, P. A morphology study of methane and carbon dioxide gas hydrates formed from water droplets exposed to a gas atmosphere. In Proceedings of the 4th International Conference on Gas Hydrate, Yokohama, Japan, 19–23 May 2002. [Google Scholar]
- Gupta, A.; Moridis, G.J.; Kneafsey, T.J.; Sloan, E.D., Jr. Modeling pure methane hydrate dissociation using a numerical simulator from a novel combination of X-ray computed tomography and macroscopic data. Energy Fuels 2009, 23, 5958–5965. [Google Scholar] [CrossRef]
- Ilhan, S.; Kahruman, C.; Yusufoglu, I. Characterization of the thermal decomposition products of ammonium phosphomolybdate hydrate. J. Anal. Appl. Pyrolysis 2007, 78, 363–370. [Google Scholar] [CrossRef]
- Lee, J.D.; Susilo, R.; Englezos, P. Methane-ethane and methane-propane hydrate formation and decomposition on water droplets. Chem. Eng. Sci. 2005, 60, 4203–4212. [Google Scholar]
- Uchida, T.; Ebinuma, T.; Narita, H. Observations of CO2-hydrate decomposition and reformation processes. J. Cryst. Growth 2000, 217, 189–200. [Google Scholar] [CrossRef]
- Boxall, J.; Greaves, D.; Mulligan, J. Gas hydrate formation and dissociation from water-in-oil emulsions studied using PVM and FBRM Particle size analysis. In Proceedings of the 6th International Conference on Gas Hydrates, Vancouver, BC, Canada, 6–20 July 2008. [Google Scholar]
- Aman, Z.; Koh, C.A. Interfacial Phenomena in Gas Hydrate Systems. Chem. Soc. Rev. 2016, 45, 1678–1690. [Google Scholar] [CrossRef]
- Li, S.L.; Sun, C.Y.; Chen, G.J.; Li, Z.Y.; Ma, Q.L.; Yang, L.Y.; Sum, A.K. Measurements of Hydrate Film Fracture under Conditions Simulating the Rise of Hydrated Gas Bubbles in Deep Water. Chem. Eng. Sci. 2014, 116, 109–117. [Google Scholar] [CrossRef]
- Chen, G.J.; Guo, T.M. A New Approach to Gas Hydrate Modeling. Chem. Eng. J. 1998, 71, 145–151. [Google Scholar] [CrossRef]



| Composition | Mol % | Composition | Mol % |
|---|---|---|---|
| N2 | 1.53 | C3 | 3.06 |
| CO | 2.05 | iC4 | 0.33 |
| CO2 | 0.89 | iC5 | 0.04 |
| C1 | 89.02 | nC6+ | 0.01 |
| C2 | 3.07 | — | — |
| Composition | Mol % | Composition | Mol % |
|---|---|---|---|
| C11 | 0.89 | C16 | 6.83 |
| C12 | 3.36 | C17 | 7.99 |
| C13 | 5.38 | C18 | 7.46 |
| C14 | 6.2 | C19 | 6.38 |
| C15 | 6.78 | C20+ | 48.73 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, X.; Shi, B.; Zhou, S.; Wang, S.; Huang, W.; Sun, X. Study on the Decomposition Mechanism of Natural Gas Hydrate Particles and Its Microscopic Agglomeration Characteristics. Appl. Sci. 2018, 8, 2464. https://doi.org/10.3390/app8122464
Lv X, Shi B, Zhou S, Wang S, Huang W, Sun X. Study on the Decomposition Mechanism of Natural Gas Hydrate Particles and Its Microscopic Agglomeration Characteristics. Applied Sciences. 2018; 8(12):2464. https://doi.org/10.3390/app8122464
Chicago/Turabian StyleLv, Xiaofang, Bohui Shi, Shidong Zhou, Shuli Wang, Weiqiu Huang, and Xianhang Sun. 2018. "Study on the Decomposition Mechanism of Natural Gas Hydrate Particles and Its Microscopic Agglomeration Characteristics" Applied Sciences 8, no. 12: 2464. https://doi.org/10.3390/app8122464
APA StyleLv, X., Shi, B., Zhou, S., Wang, S., Huang, W., & Sun, X. (2018). Study on the Decomposition Mechanism of Natural Gas Hydrate Particles and Its Microscopic Agglomeration Characteristics. Applied Sciences, 8(12), 2464. https://doi.org/10.3390/app8122464





