Microbial Fuel Cells, Related Technologies, and Their Applications
Abstract
1. Introducing Microbial Electrochemical Systems for Bioremediation, Energy and Biofuel Production
2. The Living Building Blocks of MESs
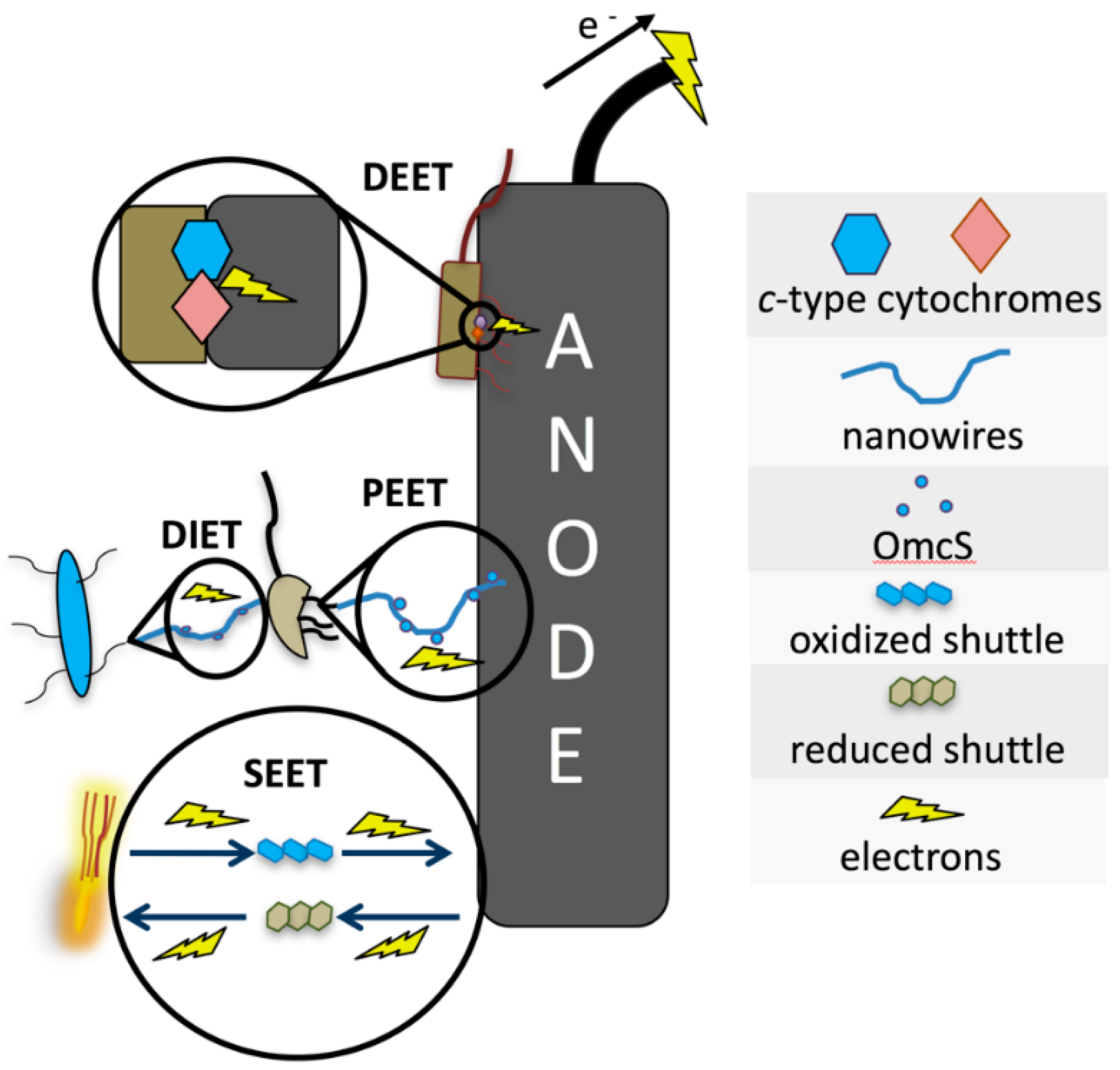
2.1. The Bacteria–Electrode Interface
2.2. Bacteria–Anode Interactions
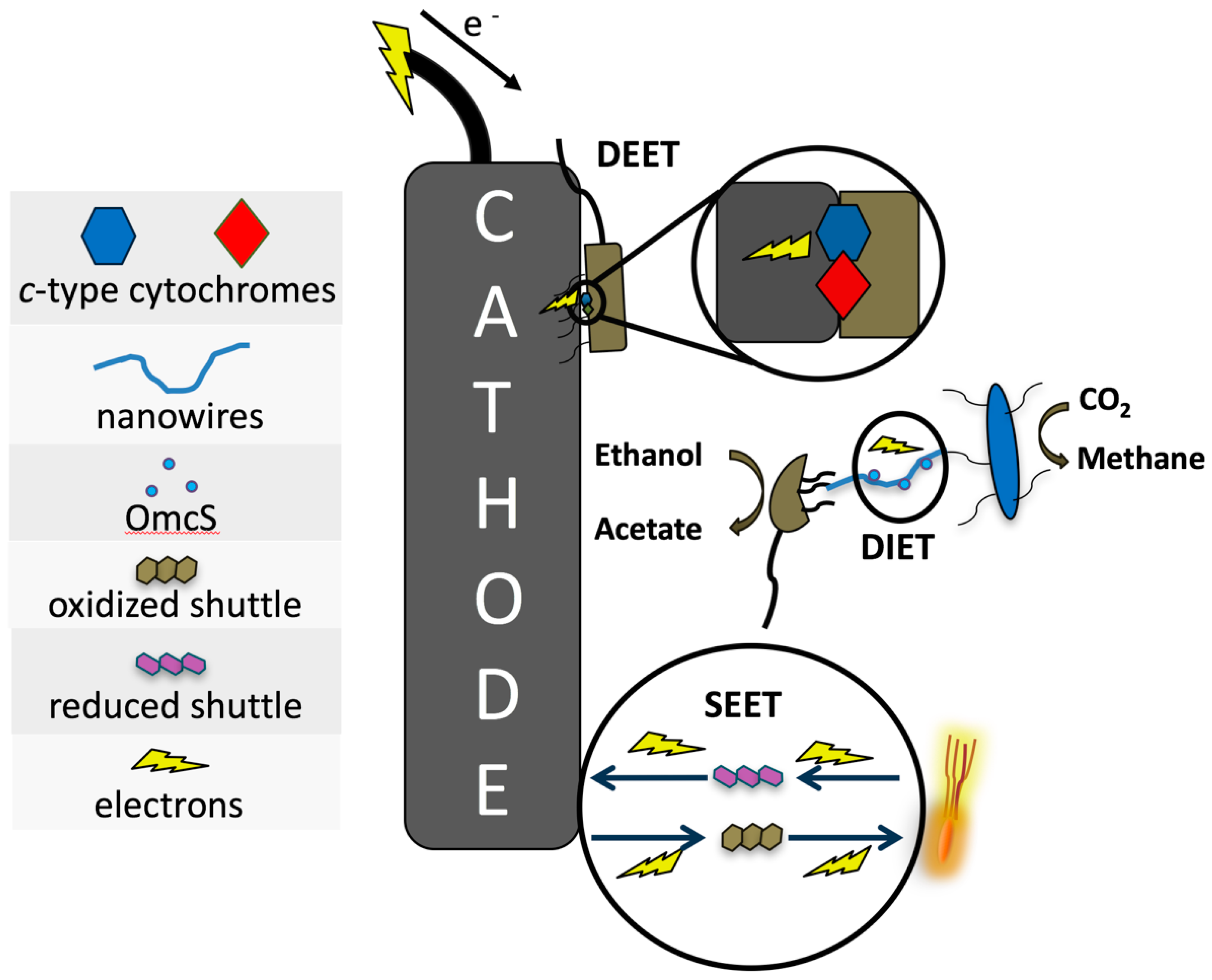
2.3. Direct Extracellular Electron Transfer (DEET)
2.4. Pilin-Mediated Extracellular Electron Transfer (PEET)
2.5. Shuttle-Mediated Extracellular Electron Transfer (SEET)
3. Bacteria–Cathode Interactions
4. The Electrogenic Biofilm Interface
5. Electrode Materials
5.1. Carbon-Based Electrodes
5.2. Other Materials
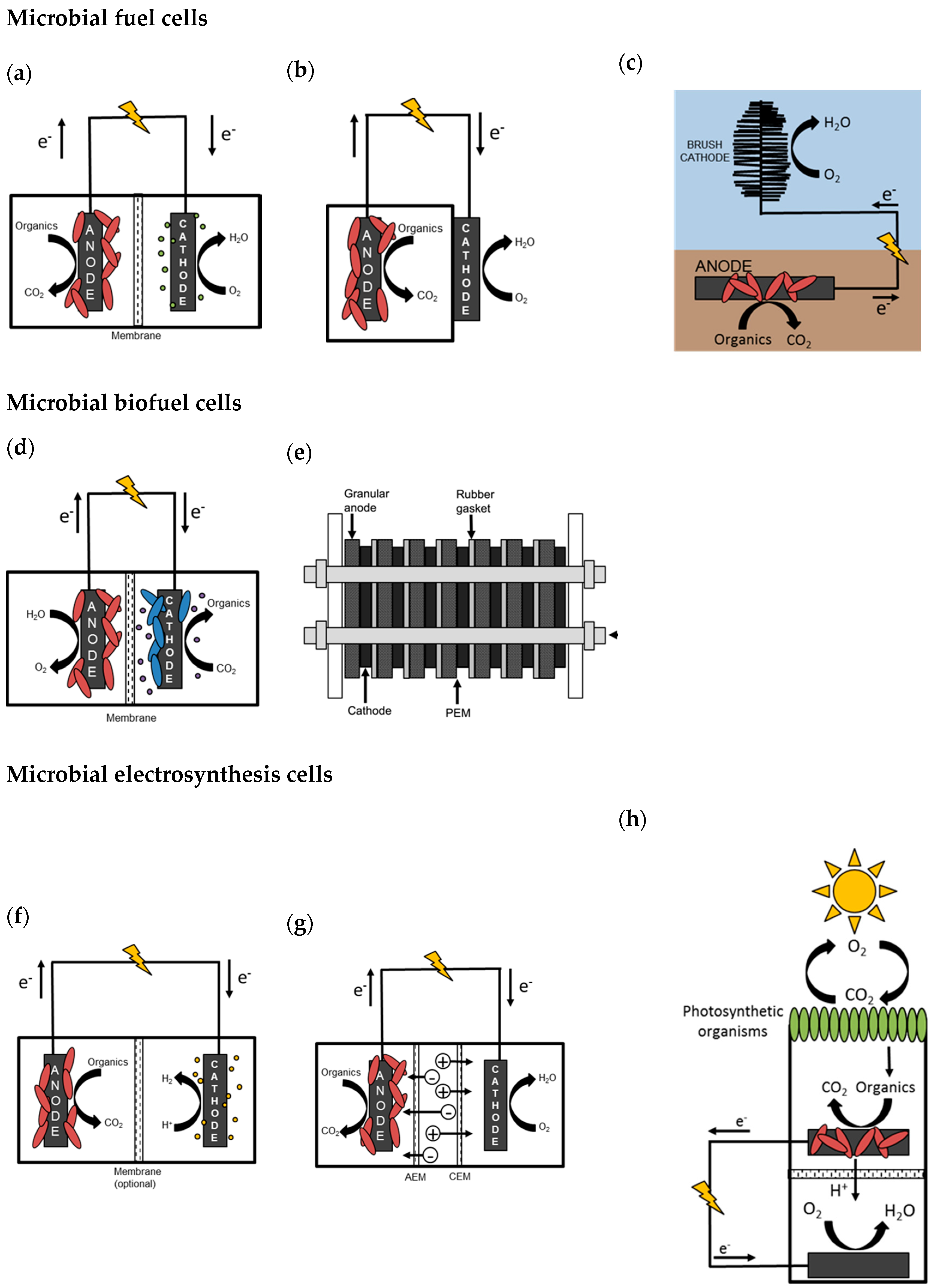
6. The Design and Application of MESs
6.1. MFCs for Power Production
6.2. Benthic and Sedimentary MFCs for Power and Biofuel Production
6.3. MECs for Biofuel Production and Chemical Synthesis
6.4. Microbial Solar Cells Enhance MES Functions
6.5. Microbial Desalination Cells May Supplement Strategies for Water Sustainability
6.6. Microbial Remediation Cells
7. Conclusions and Future Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Wang, H.; Ren, Z.J. A comprehensive review of microbial electrochemical systems as a platform technology. Biotechnol. Adv. 2013, 31, 1796–1807. [Google Scholar] [CrossRef] [PubMed]
- Franks, A.E.; Nevin, K.P. Microbial fuel cells, a current review. Energies 2010, 3, 899–919. [Google Scholar] [CrossRef]
- Khunjar, W.O.; Sahin, A.; West, A.C.; Chandran, K.; Banta, S. Biomass Production from Electricity Using Ammonia as an Electron Carrier in a Reverse Microbial Fuel Cell. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Nevin, K.P.; Woodard, T.L.; Franks, A.E.; Summers, Z.M.; Lovley, D.R. Microbial Electrosynthesis: Feeding Microbial Electrosynthesis: Feeding Microbes Electricity to Convert Carbon Dioxide and Water to Multicarbon Extracellular Organic. mBio 2010, 1, e00103-10. [Google Scholar] [CrossRef] [PubMed]
- Rabaey, K.; Rozendal, R.A. Microbial electrosynthesis—Revisiting the electrical route for microbial production. Nat. Rev. Microbiol. 2010, 8, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R. Electromicrobiology. Annu. Rev. Microbiol. 2012, 66, 391–409. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R.; Phillips, E.J. Novel mode of microbial energy metabolism: Organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl. Environ. Microbiol. 1988, 54, 1472–1480. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.M.; Bennetto, H.P. Microbial fuel cells: Electricity production from carbohydrates. Appl. Biochem. Biotechnol. 1993, 39, 27–40. [Google Scholar] [CrossRef]
- Kim, B.H.; Kim, H.J.; Hyun, M.S.; Park, D.H. Direct electrode reaction of Fe(III)-reducing bacterium, Shewanella putrefaciens. J. Microbiol. Biotechnol. 1999, 9, 127–131. [Google Scholar]
- Lovley, D.R. Bug juice: Harvesting electricity with microorganisms. Nat. Rev. Microbiol. 2006, 4, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R. Microbial fuel cells: Novel microbial physiologies and engineering approaches. Curr. Opin. Biotechnol. 2006, 17, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R. Powering microbes with electricity: Direct electron transfer from electrodes to microbes. Environ. Microbiol. Rep. 2011, 3, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Reimers, C.E.; Tender, L.M.; Fertig, S.; Wang, W. Harvesting energy from the marine sediment--water interface. Environ. Scie.Technol. 2001, 35, 192–195. [Google Scholar] [CrossRef]
- Tender, L.M.; Reimers, C.E.; Stecher, H.A.; Holmes, D.E.; Bond, D.R.; Lowy, D.A.; Pilobello, K.; Fertig, S.J.; Lovley, D.R. Harnessing microbially generated power on the seafloor. Nature Biotechnol. 2002, 20, 821–825. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Li, H.; Gu, T. A state of the art review on microbial fuel cells: A promising technology for wastewater treatment and bioenergy. Biotechnol. Adv. 2007, 25, 464–482. [Google Scholar] [CrossRef] [PubMed]
- Strik, D.P.B.T.B.; Timmers, R.A.; Helder, M.; Steinbusch, K.J.J.; Hamelers, H.V.M.; Buisman, C.J.N. Microbial solar cells: Applying photosynthetic and electrochemically active organisms. Trends Biotechnol. 2011, 29, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Rohrback, G.H.; Scott, W.R.; Canfield, J.H. Biochemical fuel cells. In Proceedings of the 16th Annual Power Sources Conference, Atlantic City, NJ, USA, 22–24 May 1962; Volume 18. [Google Scholar]
- Fortman, J.L.; Chhabra, S.; Mukhopadhyay, A.; Chou, H.; Lee, T.S.; Steen, E.; Keasling, J.D. Biofuel alternatives to ethanol: Pumping the microbial well. Trends Biotechnol. 2008, 26, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Liao, J.C. Engineering metabolic systems for production of advanced fuels. J. Ind. Microbiol. Biotechnol. 2009, 36, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Wang, H.; Hassett, D.J.; Gu, T. Recent advances in microbial fuel cells (MFCs) and microbial electrolysis cells (MECs) for wastewater treatment, bioenergy and bioproducts. J. Chem. Technol. Biotechnol. 2013, 88, 508–518. [Google Scholar] [CrossRef]
- Bajracharya, S.; Sharma, M.; Mohanakrishna, G.; Dominguez Benneton, X.; Strik, D.P.B.T.B.; Sarma, P.M.; Pant, D. An overview on emerging bioelectrochemical systems (BESs): Technology for sustainable electricity, waste remediation, resource recovery, chemical production and beyond. Renew. Energy 2016, 98, 153–170. [Google Scholar] [CrossRef]
- Anderson, R.T.; Vrionis, H.A.; Ortiz-Bernad, I.; Resch, C.T.; Long, P.E.; Dayvault, R.; Karp, K.; Marutzky, S.; Metzler, D.R.; Peacock, A.; et al. Stimulating the In Situ Activity of Geobacter species to remove uranium from the groundwater of a uranium-contaminated aquifer. Appl. Environ. Microbiol. 2003, 69, 5884–5891. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.H.; Nevin, K.P.; Franks, A.; Englert, A.; Long, P.E.; Lovley, D.R. Electrode-based approach for monitoring in situ microbial activity during subsurface bioremediation. Environ. Sci. Technol. 2010, 44, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.J.; Lee, C.; Chang, J.; Liao, Q.; Su, A. Treatment of Sulfate/Sulfide-Containing Wastewaters Using a Microbial Fuel Cell: Single and Two-Anode Systems. Int. J. Green Energy 2015, 12, 998–1004. [Google Scholar] [CrossRef]
- Pikaar, I.; Rozendal, R.A.R.A.; Yuan, Z.; Keller, J.J.; Rabaey, K. Electrochemical sulfide removal from synthetic and real domestic wastewater at high current densities. Water Res. 2011, 45, 2281–2289. [Google Scholar] [CrossRef] [PubMed]
- Rabaey, K.; Van de Sompel, K.; Maignien, L.; Boon, N.; Aelterman, P.; Clauwaert, P.; De Schamphelaire, L.; Pham, H.T.; Vermeulen, J.; Verhaege, M.; et al. Microbial Fuel Cells for Sulfide removal. Environ. Sci. Technol. 2006, 40, 5218–5224. [Google Scholar] [CrossRef] [PubMed]
- Rohrback, G.H. Biological electrical power generation. U.S. Patent 3,228,799, 1 November 1966. [Google Scholar]
- Davis, J.B.; Yarbrough, H.F. Biochemical fuel cell. U.S. Patent 3,331,705, 18 July 1967. [Google Scholar]
- Angenent, L.; Zhen, H. Upflow microbial fuel cell (UMFC). U.C. Patent 20,060,147,763, 6 July 2006. [Google Scholar]
- Swift, J.A.; Butler, M.A.; Wallace, S.J. Microbial fuel cell and method. U.C. Patent 7,807,303, 5 October 2010. [Google Scholar]
- Bond, D.R.; Holmes, D.E.; Tender, L.M.; Lovley, D.R. Electrode-reducing microorganisms that harvest energy from marine sediments. Science 2002, 295, 483–485. [Google Scholar] [CrossRef] [PubMed]
- Bond, D.R.; Lovley, D.R. Electricity Production by Geobacter sulfurreducens Attached to Electrodes. Appl. Environ. Microbiol. 2003, 69, 1548–1555. [Google Scholar] [CrossRef] [PubMed]
- Villano, M.; Aulenta, F.; Ciucci, C.; Ferri, T.; Giuliano, A.; Majone, M. Bioelectrochemical reduction of CO2 to CH4 via direct and indirect extracellular electron transfer by a hydrogenophilic methanogenic culture. Bioresour. Technol. 2010, 101, 3085–3090. [Google Scholar] [CrossRef] [PubMed]
- RA, R.; AW, J.; HV, H.; CJ, B. Hydrogen production with a microbial biocathode. Environ. Sci. Technol. 2008, 42, 629–634. [Google Scholar]
- Lovley, D.R.; Ueki, T.; Zhang, T.; Malvankar, N.S.; Shrestha, P.M.; Flanagan, K.A.; Aklujkar, M.; Butler, J.E.; Giloteaux, L.; Rotaru, A.E.; et al. Geobacter: The microbe electric’s physiology, ecology, and practical applications. Adv. Microb. Physiol. 2011, 59, 1–100. [Google Scholar] [CrossRef] [PubMed]
- Semenec, L.; Franks, A.E. The microbiology of microbial electrolysis cells. Microbiol. Aust. 2014, 35, 201–206. [Google Scholar] [CrossRef]
- Qiao, Y.; Qiao, Y.J.; Zou, L.; Ma, C.X.; Liu, J.H. Real-time monitoring of phenazines excretion in Pseudomonas aeruginosa microbial fuel cell anode using cavity microelectrodes. Bioresour. Technol. 2015, 198, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Bond, D.R.; Lovley, D.R. Evidence for Involvement of an Electron Shuttle in Electricity Generation by Geothrix fermentans Evidence for Involvement of an Electron Shuttle in Electricity Generation by Geothrix fermentans. Appl. Environ. Microbiol. 2005, 71, 2186–2189. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Kim, B.H.; Kim, H.S.; Kim, H.J.; Kim, G.; Kim, M.; Chang, I.S.; Park, Y.K.; Chang, H.I. A novel electrochemically active and Fe(III)-reducing bacterium phylogenetically related to Clostridium butyricum isolated from a microbial fuel cell. Scanning 2001, 297–306. [Google Scholar] [CrossRef]
- Angenent, L.T.; Karim, K.; Al-Dahhan, M.H.; Wrenn, B.A.; Domíguez-Espinosa, R. Production of bioenergy and biochemicals from industrial and agricultural wastewater. Trends Biotechnol. 2004, 22, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.E.; Bond, D.R.; Lovley, D.R. Electron Transfer by Desulfobulbus propionicus to Fe(III) and Graphite Electrodes. Appl. Environ. Microbiol. 2004, 70, 1234. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Li, J.; Qin, D.; Chen, L.; Zhao, F.; Chen, S.; Hu, H.; Yu, C.P. Characterization of exoelectrogenic bacteria enterobacter strains isolated from a microbial fuel cell exposed to copper shock load. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Gregory, K.B.; Bond, D.R.; Lovley, D.R. Graphite electrodes as electron donors for anaerobic respiration. Environ. Microbiol. 2004, 6, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Gregory, K.B.; Lovley, D.R. Remediation and recovery of uranium from contaminated subsurface environments with electrodes. Environ. Sci. Technol. 2005, 39, 8943–8947. [Google Scholar] [CrossRef] [PubMed]
- Dumas, C.; Basseguy, R.; Bergel, A. Microbial electrocatalysis with Geobacter sulfurreducens biofilm on stainless steel cathodes. Electrochim. Acta 2008, 53, 2494–2500. [Google Scholar] [CrossRef]
- Lovley, D.R.; Nevin, K.P. Electrobiocommodities: Powering microbial production of fuels and commodity chemicals from carbon dioxide with electricity. Curr. Opin. Biotechnol. 2013, 24, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Schievano, A.; Pant, D. Electro-stimulated microbial factory for value added product synthesis. Bioresour. Technol. 2015, 213, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Singh, L.; Zularisam, A.W. Exoelectrogens: Recent advances in molecular drivers involved in extracellular electron transfer and strategies used to improve it for microbial fuel cell applications. Renew. Sustain. Energy Rev. 2016, 56, 1322–1336. [Google Scholar] [CrossRef]
- Zhang, Y.-C.; Jiang, Z.-H.; Liu, Y. Application of electrochemically active bacteria as anodic biocatalyst in microbial fuel cells. Chin. J. Anal. Chem. 2015, 43, 155–163. [Google Scholar] [CrossRef]
- Inoue, K.; Leang, C.; Franks, A.E.; Woodard, T.L.; Nevin, K.P.; Lovley, D.R. Specific localization of the c-type cytochrome OmcZ at the anode surface in current-producing biofilms of Geobacter sulfurreducens. Environ. Microbiol. Rep. 2011, 3, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Nevin, K.P.; Kim, B.C.; Glaven, R.H.; Johnson, J.P.; Woodward, T.L.; Methé, B.A.; Didonato, R.J.; Covalla, S.F.; Franks, A.E.; Liu, A.; et al. Anode biofilm transcriptomics reveals outer surface components essential for high density current production in Geobacter sulfurreducens fuel cells. PLoS ONE 2009, 4. [Google Scholar] [CrossRef] [PubMed]
- Leang, C.; Qian, X.; Mester, T.; Lovley, D.R. Alignment of the c-type cytochrome OmcS along pili of Geobacter sulfurreducens. Appl. Environ. Microbiol. 2010, 76, 4080–4084. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Reguera, G.; Mester, T.; Lovley, D.R. Evidence that OmcB and OmpB of Geobacter sulfurreducens are outer membrane surface proteins. FEMS Microbiol. Lett. 2007, 277, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Mehta, T.; Coppi, M.V.; Childers, S.E.; Lovley, D.R. Outer membrane c -Type cytochromes required for Fe(III) and Mn (IV) oxide reduction in Geobacter sulfurreducens. Appl. Environ. Microbiol. 2005, 71, 8634–8641. [Google Scholar] [CrossRef] [PubMed]
- Steidl, R.J.; Lampa-Pastirk, S.; Reguera, G. Mechanistic stratification in electroactive biofilms of Geobacter sulfurreducens mediated by pilus nanowires. Nat. Commun. 2017, 8, 15474. [Google Scholar] [CrossRef] [PubMed]
- Reguera, G.; Nevin, K.P.; Nicoll, J.S.; Covalla, S.F.; Woodard, T.L.; Lovley, D.R. Biofilm and nanowire production leads to increased current in Geobacter sulfurreducens fuel cells. Appl. Environ. Microbiol. 2006, 72, 7345–7348. [Google Scholar] [CrossRef] [PubMed]
- Malvankar, N.S.; Tuominen, M.T.; Lovley, D.R. Biofilm conductivity is a decisive variable for high-current-density Geobacter sulfurreducens microbial fuel cells. Energy Environ. Sci. 2012, 5, 5790. [Google Scholar] [CrossRef]
- Liu, X.; Tremblay, P.L.; Malvankar, N.S.; Nevin, K.P.; Lovley, D.R.; Vargas, M. A Geobacter sulfurreducens strain expressing Pseudomonas aeruginosa type IV pili localizes OmcS on pili but is deficient in Fe(III) oxide reduction and current production. Appl. Environ. Microbiol. 2014, 80, 1219–1224. [Google Scholar] [CrossRef] [PubMed]
- Malvankar, N.S.; Vargas, M.; Nevin, K.P.; Franks, A.E.; Leang, C.; Kim, B.-C.; Inoue, K.; Mester, T.; Covalla, S.F.; Johnson, J.P.; et al. Tunable metallic-like conductivity in microbial nanowire networks. Nat. Nanotechnol. 2011, 6, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Malvankar, N.S.; Vargas, M.; Nevin, K.; Tremblay, P.; Evans-lutterodt, K.; Nykypanchuk, D. Structural basis for metallic-like conductivity in microbial nanowires. mBio 2015, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Malvankar, N.S.; Tuominen, M.T.; Lovley, D.R. Comment on “On electrical conductivity of microbial nanowires and biofilms” by S. M. Strycharz-Glaven, R. M.; Snider, A. Guiseppi-Elie and L. M. Tender, Energy Environ. Sci., 2011, 4, 4366. Energy Environ. Sci. 2012, 5, 6247–6249. [Google Scholar] [CrossRef]
- Strycharz-Glaven, S.M.; Snider, R.M.; Guiseppi-Elie, A.; Tender, L.M. On the electrical conductivity of microbial nanowires and biofilms. Energy Environ. Sci. 2011, 4, 4366. [Google Scholar] [CrossRef]
- Strycharz, S.M.; Tender, L.M. Reply to the ‘comment on “On electrical conductivity of microbial nanowires and biofilms”’ by N. S. Malvankar, M. T. Tuominen and D. R. Lovley, Energy Environ. Sci., 2012, 5, doi:10.1039/c2ee02613a. Energy Environ. Sci. 2012, 5, 6250–6255. [Google Scholar] [CrossRef]
- Bond, D.R.; Strycharz-Glaven, S.M.; Tender, L.M.; Torres, C.I. On electron transport through geobacter biofilms. ChemSusChem 2012, 5, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Boesen, T.; Nielsen, P. Molecular dissection of bacterial nanowires. mBio 2013, 4, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Adhikari, R.Y.; Malvankar, N.S.; Ward, J.E.; Woodard, T.L.; Nevin, K.P.; Lovley, R. Expressing the Geobacter metallireducens PilA in Geobacter sulfurreducens Yields Pili with Exceptional Conductivity. mBio 2017, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pirbadian, S.; Barchinger, S.E.; Leung, K.M.; Byun, H.S.; Jangir, Y.; Bouhenni, R.A.; Reed, S.B.; Romine, M.F.; Saffarini, D.A.; Shi, L.; et al. Shewanella oneidensis MR-1 nanowires are outer membrane and periplasmic extensions of the extracellular electron transport components. Proc. Natl. Acad. Sci. USA 2014, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Pirbadian, S.; El-Naggar, M.Y. Multistep hopping and extracellular charge transfer in microbial redox chains. Phys. Chem. Chem. Phys. 2012, 14, 13802. [Google Scholar] [CrossRef] [PubMed]
- Marsili, E.; Baron, D.B.; Shikhare, I.D.; Coursolle, D.; Gralnick, J.A.; Bond, D.R. Shewanella secretes flavins that mediate extracellular electron transfer. Proc. Natl. Acad. Sci. USA 2008, 105, 3968–3973. [Google Scholar] [CrossRef] [PubMed]
- Daghio, M.; Gandolfi, I.; Bestetti, G.; Franzetti, A.; Guerrini, E.; Cristiani, P. Anodic and cathodic microbial communities in single chamber microbial fuel cells. New Biotechnol. 2015, 32, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, M.; Aulenta, F.; Villano, M.; Angenent, L.T. Cathodes as electron donors for microbial metabolism: Which extracellular electron transfer mechanisms are involved? Bioresour. Technol. 2011, 102, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Choi, O.; Sang, B.-I. Extracellular electron transfer from cathode to microbes: Application for biofuel production. Biotechnol. Biofuels 2016, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Kumar, P. Future microbial applications for bioenergy production: A perspective. Front. Microbiol. 2017, 8, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Rotaru, A.E.; Shrestha, P.M.; Liu, F.; Ueki, T.; Nevin, K.; Summers, Z.M.; Lovley, D.R. Interspecies electron transfer via hydrogen and formate rather than direct electrical connections in cocultures of Pelobacter carbinolicus and Geobacter sulfurreducens. Appl. Environ. Microbiol. 2012, 78, 7645–7651. [Google Scholar] [CrossRef] [PubMed]
- Sieber, J.R.; McInerney, M.J.; Gunsalus, R.P. Genomic Insights into Syntrophy: The Paradigm for Anaerobic Metabolic Cooperation. Annu. Rev. Microbiol. 2012, 66, 429–452. [Google Scholar] [CrossRef] [PubMed]
- Summers, Z.M.; Fogarty, H.E.; Leang, C.; Franks, A.E.; Malvankar, N.S.; Lovley, D.R. Direct exchange of electrons within aggregates of an evolved syntrophic coculture of anaerobic bacteria. Science 2010, 330, 1413–1415. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, P.M.; Rotaru, A.E.; Aklujkar, M.; Liu, F.; Shrestha, M.; Summers, Z.M.; Malvankar, N.; Flores, D.C.; Lovley, D.R. Syntrophic growth with direct interspecies electron transfer as the primary mechanism for energy exchange. Environ. Microbiol. Rep. 2013, 5, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Rotaru, A.E.; Shrestha, P.M.; Liu, F.; Markovaite, B.; Chen, S.; Nevin, K.P.; Lovley, D.R. Direct interspecies electron transfer between Geobacter metallireducens and Methanosarcina barkeri. Appl. Environ. Microbiol. 2014, 80, 4599–4605. [Google Scholar] [CrossRef] [PubMed]
- Semenec, L.; Laloo, A.E.; Schulz, B.L.; Vergara, I.A.; Bond, P.L.; Franks, A.E. Deciphering the electric code of Geobacter sulfurreducens in cocultures with Pseudomonas aeruginosa via SWATH-MS proteomics. Bioelectrochemistry 2017, 119, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Kato, S. Influence of Anode Potentials on Current Generation and Extracellular Electron Transfer Paths of Geobacter Species. Int. J. Mol. Sci. 2017, 18, 108. [Google Scholar] [CrossRef] [PubMed]
- Commault, A.S.; Lear, G.; Weld, R.J. Maintenance of Geobacter-dominated biofilms in microbial fuel cells treating synthetic wastewater. Bioelectrochemistry 2015, 106, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R. Happy together: Microbial communities that hook up to swap electrons. ISME J. 2017, 11, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Liesack, W. Differential assemblage of functional units in paddy soil microbiomes. PLoS ONE 2015, 10, e0122221. [Google Scholar] [CrossRef] [PubMed]
- Hori, T.; Noll, M.; Igarashi, Y.; Friedrich, M.W.; Conrad, R. Identification of acetate-assimilating microorganisms under methanogenic conditions in anoxic rice field soil by comparative stable isotope probing of RNA. Appl. Environ. Microbiol. 2007, 73, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Rotaru, A.-E.; Shrestha, P.M.; Malvankar, N.S.; Liu, F.; Fan, W.; Nevin, K.P.; Lovley, D.R. Promoting interspecies electron transfer with biochar. Sci. Rep. 2014, 4, 5019. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Rotaru, A.E.; Liu, F.; Philips, J.; Woodard, T.L.; Nevin, K.P.; Lovley, D.R. Carbon cloth stimulates direct interspecies electron transfer in syntrophic co-cultures. Bioresour. Technol. 2014, 173, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Li, L.L.; Tong, Z.H.; Fang, C.Y.; Chu, J.; Yu, H.Q. Response of anaerobic granular sludge to single-wall carbon nanotube exposure. Water Res. 2015, 70, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Chi, M.; Luo, J.; He, H.; Jin, T. An overview of electrode materials in microbial fuel cells. J. Power Sources 2011, 196, 4427–4435. [Google Scholar] [CrossRef]
- Wei, J.; Liang, P.; Huang, X. Recent progress in electrodes for microbial fuel cells. Bioresour. Technol. 2011, 102, 9335–9344. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cheng, S.; Feng, Y.; Merrill, M.D.; Saito, T.; Logan, B.E. Use of carbon mesh anodes and the effect of different pretreatment methods on power production in microbial fuel cells. Environ. Sci. Technol. 2009, 43, 6870–6874. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Rahunen, N.; Varcoe, J.R.; Chandra, A.; Avignone-Rossa, C.; Thumser, A.E.; Slade, R.C.T. Activated carbon cloth as anode for sulfate removal in a microbial fuel cell. Environ. Sci. Technol. 2008, 42, 4971–4976. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Harnisch, F.; Fricke, K.; Schröder, U.; Climent, V.; Feliu, J.M. The study of electrochemically active microbial biofilms on different carbon-based anode materials in microbial fuel cells. Biosens. Bioelectron. 2010, 25, 2167–2171. [Google Scholar] [CrossRef] [PubMed]
- Ieropoulos, I.; Greenman, J.; Melhuish, C. Microbial fuel cells based on carbon veil electrodes: Stack configuration and scalability. Int. J. Energy Res. 2008, 32, 1228–1240. [Google Scholar] [CrossRef]
- Sonawane, J.M.; Yadav, A.; Ghosh, P.C.; Adeloju, S.B. Recent advances in the development and utilization of modern anode materials for high performance microbial fuel cells. Biosens. Bioelectron. 2017, 90, 558–576. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.; Cheng, S.; Watson, V.; Estadt, G. Graphite Fiber Brush Anodes for Increased Power Production in Air-Cathode Microbial Fuel Cells Graphite Fiber Brush Anodes for Increased Power Production in Air-Cathode Microbial Fuel Cells. Environ. Sci. Technol. 2007, 41, 3341–3346. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E. Chapter 9: Materials for BES. In Bioelectrochemical Systems; Rabaey, K., Angenent, L.T., Schroder, U., Keller, J., Eds.; IWA Publishing: London, UK, 2010; p. 520. ISBN 9781843392330. [Google Scholar]
- Jiang, D.; Li, B. Granular activated carbon single-chamber microbial fuel cells (GAC-SCMFCs): A design suitable for large-scale wastewater treatment processes. Biochem. Eng. J. 2009, 47, 31–37. [Google Scholar] [CrossRef]
- Dumas, C.; Mollica, A.; Féron, D.; Basséguy, R.; Etcheverry, L.; Bergel, A. Marine microbial fuel cell: Use of stainless steel electrodes as anode and cathode materials. Electrochim. Acta 2007, 53, 468–473. [Google Scholar] [CrossRef]
- Bergel, A.; Féron, D.; Mollica, A. Catalysis of oxygen reduction in PEM fuel cell by seawater biofilm. Electrochem. Commun. 2005, 7, 900–904. [Google Scholar] [CrossRef]
- Richter, H.; McCarthy, K.; Nevin, K.P.; Johnson, J.P.; Rotello, V.M.; Lovley, D.R. Electricity Generation by Geobacter sulfurreducensAttached to Gold Electrodes. Langmuir 2008, 24, 4376–4379. [Google Scholar] [CrossRef] [PubMed]
- Ter Heijne, A.; Hamelers, H.V.M.; Saakes, M.; Buisman, C.J.N. Performance of non-porous graphite and titanium-based anodes in microbial fuel cells. Electrochim. Acta 2008, 53, 5697–5703. [Google Scholar] [CrossRef]
- Fan, Y.; Xu, S.; Schaller, R.; Jiao, J.; Chaplen, F.; Liu, H. Biosens. Bioelectron. Nanoparticle decorated anodes for enhanced current generation in microbial electrochemical cells. Biosens. Bioelectron. 2011, 26, 1908–1912. [Google Scholar] [CrossRef] [PubMed]
- He, Z. Microbial Fuel Cells: Now Let us Talk about Energy. Environ. Sci. Technol. 2013, 47, 332–333. [Google Scholar] [CrossRef] [PubMed]
- Khera, J.; Chandra, A. Microbial fuel cells: Recent trends. Proc. Natl. Acad. Sci. India Sect. A Phys. Sci. 2012, 82, 31–41. [Google Scholar] [CrossRef]
- Janicek, A.; Fan, Y.; Liu, H. Design of microbial fuel cells for practical application: A review and analysis of scale-up studies. Biofuels 2014, 5, 79–92. [Google Scholar] [CrossRef]
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial fuel cells: Methodology and technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Logan, B.E. Electricity Generation Using an Air-Cathode Single Chamber Microbial Fuel Cell in the Presence and Absence of a Proton Exchange Membrane. Environ. Sci. Technol. 2004, 38, 4040–4046. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Hu, H.; Liu, H. Enhanced Coulombic efficiency and power density of air-cathode microbial fuel cells with an improved cell configuration. J. Power Sources 2007, 171, 348–354. [Google Scholar] [CrossRef]
- Rahimnejad, M.; Ghoreyshi, A.A.; Najafpour, G.D.; Younesi, H.; Shakeri, M. A novel microbial fuel cell stack for continuous production of clean energy. Int. J. Hydrogen Energy 2012, 37, 5992–6000. [Google Scholar] [CrossRef]
- Aelterman, P.; Rabaey, K.; Pham, H.T.; Boon, N.; Verstraete, W. Continuous electricity generation at high voltages and currents using stacked microbial fuel cells. Environ. Sci. Technol. 2006, 40, 3388–3394. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.E.; Logan, B.E. Voltage reversal during microbial fuel cell stack operation. J. Power Sources 2007, 167, 11–17. [Google Scholar] [CrossRef]
- Choi, S.; Chae, J. An array of microliter-sized microbial fuel cells generating 100 uw of power. Sens. Actuators A Phys. 2012, 177, 10–15. [Google Scholar] [CrossRef]
- Boghani, H.C.; Papaharalabos, G.; Michie, I.; Fradler, K.R.; Dinsdale, R.M.; Guwy, A.J.; Ieropoulos, I.; Greenman, J.; Premier, G.C. Controlling for peak power extraction from microbial fuel cells can increase stack voltage and avoid cell reversal. J. Power Sources 2014, 269, 363–369. [Google Scholar] [CrossRef]
- Papaharalabos, G.; Greenman, J.; Stinchcombe, A.; Horsfield, I.; Melhuish, C.; Ieropoulos, I. Dynamic electrical reconfiguration for improved capacitor charging in microbial fuel cell stacks. J. Power Sources 2014, 272, 34–38. [Google Scholar] [CrossRef]
- Wu, S.; Li, H.; Zhou, X.; Liang, P.; Zhang, X.; Jiang, Y.; Huang, X. A novel pilot-scale stacked microbial fuel cell for efficient electricity generation and wastewater treatment. Water Res. 2016, 98, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Sugnaux, M.; Happe, M.; Cachelin, C.P.; Gloriod, O.; Huguenin, G.; Blatter, M.; Fischer, F. Two stage bioethanol refining with multi litre stacked microbial fuel cell and microbial electrolysis cell. Bioresour. Technol. 2016, 221, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Donovan, C.; Dewan, A.; Heo, D.; Beyenal, H. Batteryless, wireless sensor powered by a sediment microbial fuel cell. Environ. Sci. Technol. 2008, 42, 8591–8596. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Drott, E.; Grisdela, P.; Lee, J.; Lee, C.; Lowy, D.A.; Gray, S.; Tender, L.M. A self-assembling self-repairing microbial photoelectrochemical solar cell. Energy Environ. Sci. 2009, 2, 292. [Google Scholar] [CrossRef]
- Li, H.; Tian, Y.; Qu, Y.; Qiu, Y.; Liu, J.; Feng, Y. A Pilot-scale Benthic Microbial Electrochemical System (BMES) for Enhanced Organic Removal in Sediment Restoration. Sci. Rep. 2017, 7, 39802. [Google Scholar] [CrossRef] [PubMed]
- Schrader, P.S.; Reimers, C.E.; Girguis, P.; Delaney, J.; Doolan, C.; Wolf, M.; Green, D. Independent benthic microbial fuel cells powering sensors and acoustic communications with the MARS underwater observatory. J. Atmos. Ocean. Technol. 2016, 33, 607–617. [Google Scholar] [CrossRef]
- Arias-Thode, Y.M.; Hsu, L.; Anderson, G.; Babauta, J.; Fransham, R.; Obraztsova, A.; Tukeman, G.; Chadwick, D.B. Demonstration of the SeptiStrand benthic microbial fuel cell powering a magnetometer for ship detection. J. Power Sources 2017, 356, 419–429. [Google Scholar] [CrossRef]
- Nevin, K.P.; Hensley, S.A.; Franks, A.E.; Summers, Z.M.; Ou, J.; Woodard, T.L.; Snoeyenbos-West, O.L.; Lovley, D.R. Electrosynthesis of organic compounds from carbon dioxide is catalyzed by a diversity of acetogenic microorganisms. Appl. Environ. Microbiol. 2011, 77, 2882–2886. [Google Scholar] [CrossRef] [PubMed]
- Soussan, L.; Riess, J.; Erable, B.; Delia, M.L.; Bergel, A. Electrochemical reduction of CO2 catalysed by Geobacter sulfurreducens grown on polarized stainless steel cathodes. Electrochem. Commun. 2013, 28, 27–30. [Google Scholar] [CrossRef]
- Villano, M.; Monaco, G.; Aulenta, F.; Majone, M. Electrochemically assisted methane production in a biofilm reactor. J. Power Sources 2011, 196, 9467–9472. [Google Scholar] [CrossRef]
- Zhen, G.; Lu, X.; Kobayashi, T.; Kumar, G.; Xu, K. Promoted electromethanosynthesis in a two-chamber microbial electrolysis cells (MECs) containing a hybrid biocathode covered with graphite felt (GF). Chem. Eng. J. 2016, 284, 1146–1155. [Google Scholar] [CrossRef]
- Zhen, G.; Kobayashi, T.; Lu, X.; Kumar, G.; Xu, K. Chemosphere Biomethane recovery from Egeria densa in a microbial electrolysis cell- assisted anaerobic system: Performance and stability assessment. Chemosphere 2016, 149, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Xing, D.; Ren, Z.J. Microbial community structure accompanied with electricity production in a constructed wetland plant microbial fuel cell. Bioresour. Technol. 2015, 195, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Wetser, K.; Sudirjo, E.; Buisman, C.J.N.; Strik, D.P.B.T.B. Electricity generation by a plant microbial fuel cell with an integrated oxygen reducing biocathode. Appl. Energy 2015, 137, 151–157. [Google Scholar] [CrossRef]
- Moqsud, M.A.; Yoshitake, J.; Bushra, Q.S.; Hyodo, M.; Omine, K.; Strik, D. Compost in plant microbial fuel cell for bioelectricity generation. Waste Manag. 2015, 36, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Huang, X.; Liang, P.; Xiao, K.; Zhou, Y.; Zhang, X.; Logan, B.E. A New Method for Water Desalination Using Microbial Desalination Cells. Environ. Sci. Technol. 2009, 43, 7148–7152. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Logan, B.E. Microbial desalination cells for energy production and desalination. Desalination 2013, 308, 122–130. [Google Scholar] [CrossRef]
- Morris, J.M.; Jin, S.; Crimi, B.; Pruden, A. Microbial fuel cell in enhancing anaerobic biodegradation of diesel. Chem. Eng. J. 2009, 146, 161–167. [Google Scholar] [CrossRef]
- Srikanth, S.; Kumar, M.; Singh, D.; Singh, M.P.; Das, B.P. Electro-biocatalytic treatment of petroleum refinery wastewater using microbial fuel cell (MFC) in continuous mode operation. Bioresour. Technol. 2016, 221, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Colantonio, N.; Kim, Y. Cadmium (II) removal mechanisms in microbial electrolysis cells. J. Hazard. Mater. 2016, 311, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Shelobolina, E.S.; Vrionis, H.A.; Findlay, R.H.; Lovley, D.R. Geobacter uraniireducens sp. nov., isolated from subsurface sediment undergoing uranium bioremediation. Int. J. Syst. Evol. Microbiol. 2008, 58, 1075–1078. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.; Tian, X.; Li, Z.; Guo, J.; Guo, Y.; Yue, L.; Ping, J.; Duan, L. The effects of different electron donors and electron acceptors on perchlorate reduction and bioelectricity generation in a microbial fuel cell. Int. J. Hydrog. Energy 2017, 1–9. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drendel, G.; Mathews, E.R.; Semenec, L.; Franks, A.E. Microbial Fuel Cells, Related Technologies, and Their Applications. Appl. Sci. 2018, 8, 2384. https://doi.org/10.3390/app8122384
Drendel G, Mathews ER, Semenec L, Franks AE. Microbial Fuel Cells, Related Technologies, and Their Applications. Applied Sciences. 2018; 8(12):2384. https://doi.org/10.3390/app8122384
Chicago/Turabian StyleDrendel, Gene, Elizabeth R. Mathews, Lucie Semenec, and Ashley E. Franks. 2018. "Microbial Fuel Cells, Related Technologies, and Their Applications" Applied Sciences 8, no. 12: 2384. https://doi.org/10.3390/app8122384
APA StyleDrendel, G., Mathews, E. R., Semenec, L., & Franks, A. E. (2018). Microbial Fuel Cells, Related Technologies, and Their Applications. Applied Sciences, 8(12), 2384. https://doi.org/10.3390/app8122384




