Designing Magnetic Layered Double Hydroxides and Two-Dimensional Magnetic Nano-Nets of Cobalt Ferrite through a Novel Approach
Abstract
1. Introduction
2. Materials and Methods
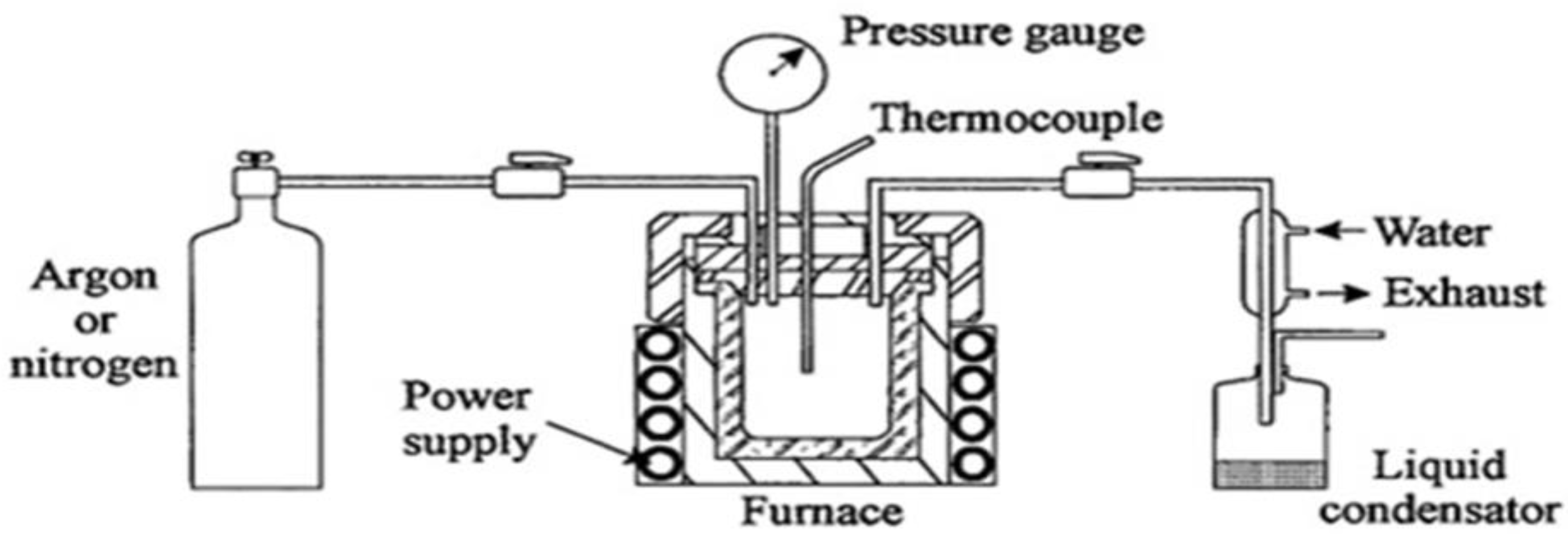
2.1. Preparation of the Cobalt Ferrite-Supported LDH
2.2. Transformation of the Supported LDH to Nano-Nets
2.3. Preparation of Nanoparticles
2.4. Physical Characterization
3. Results
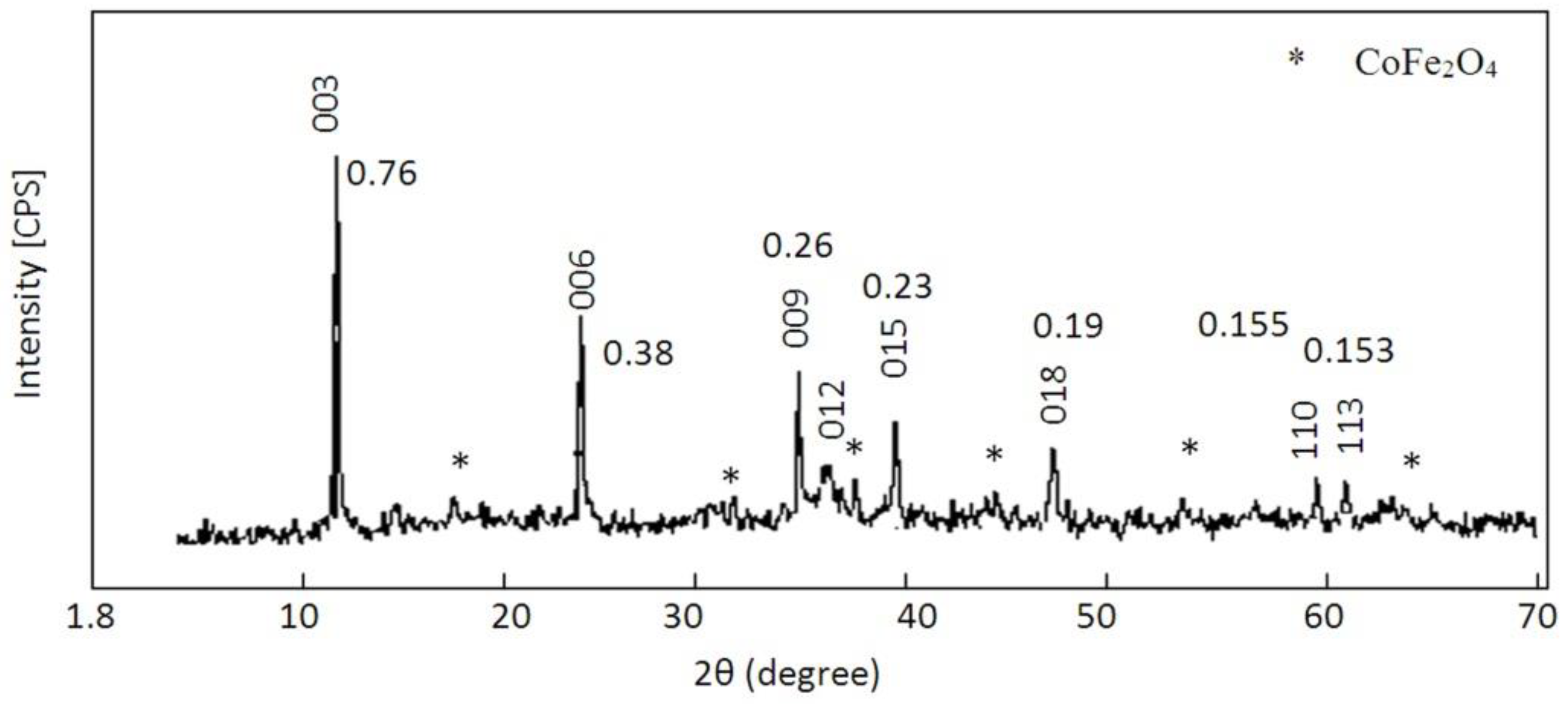
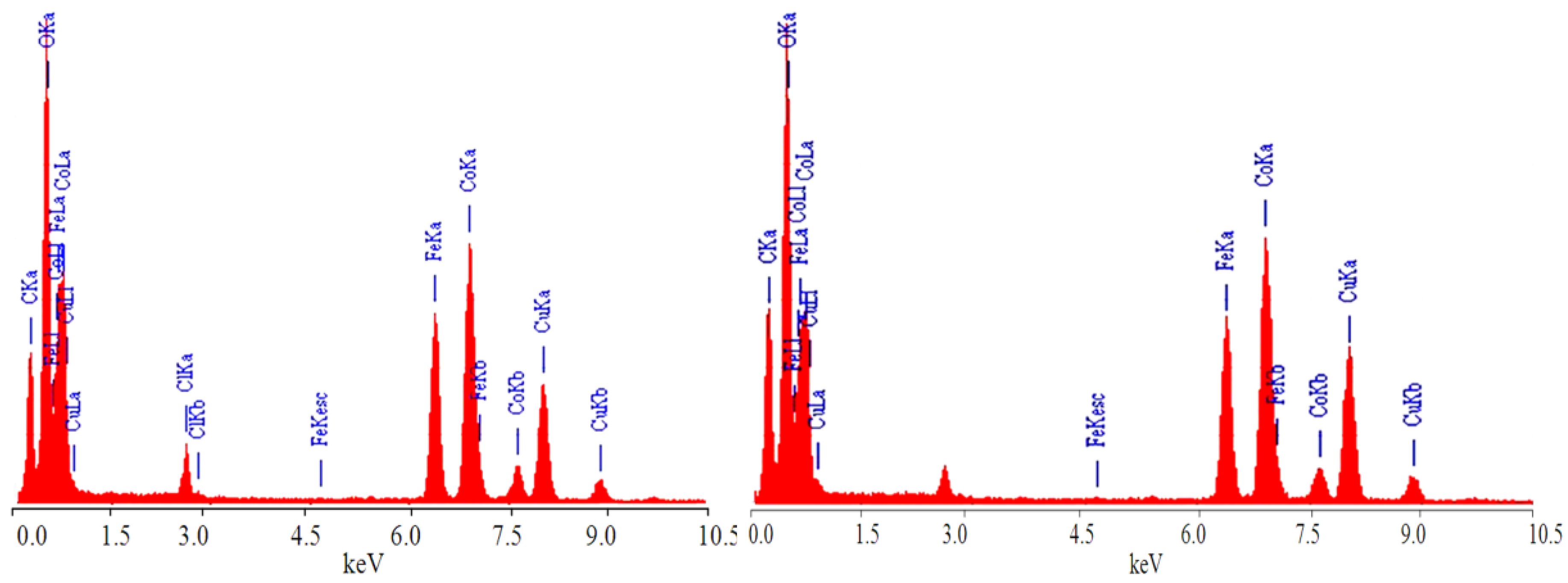
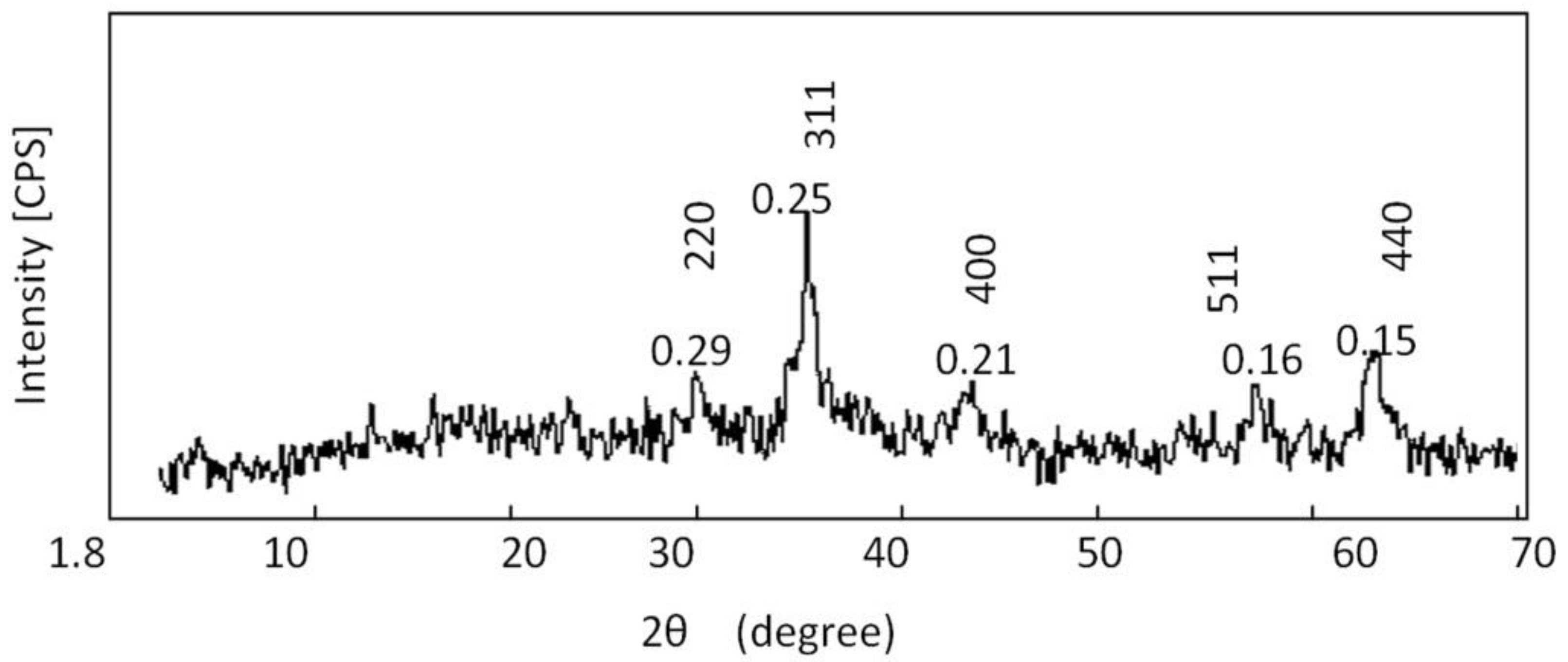
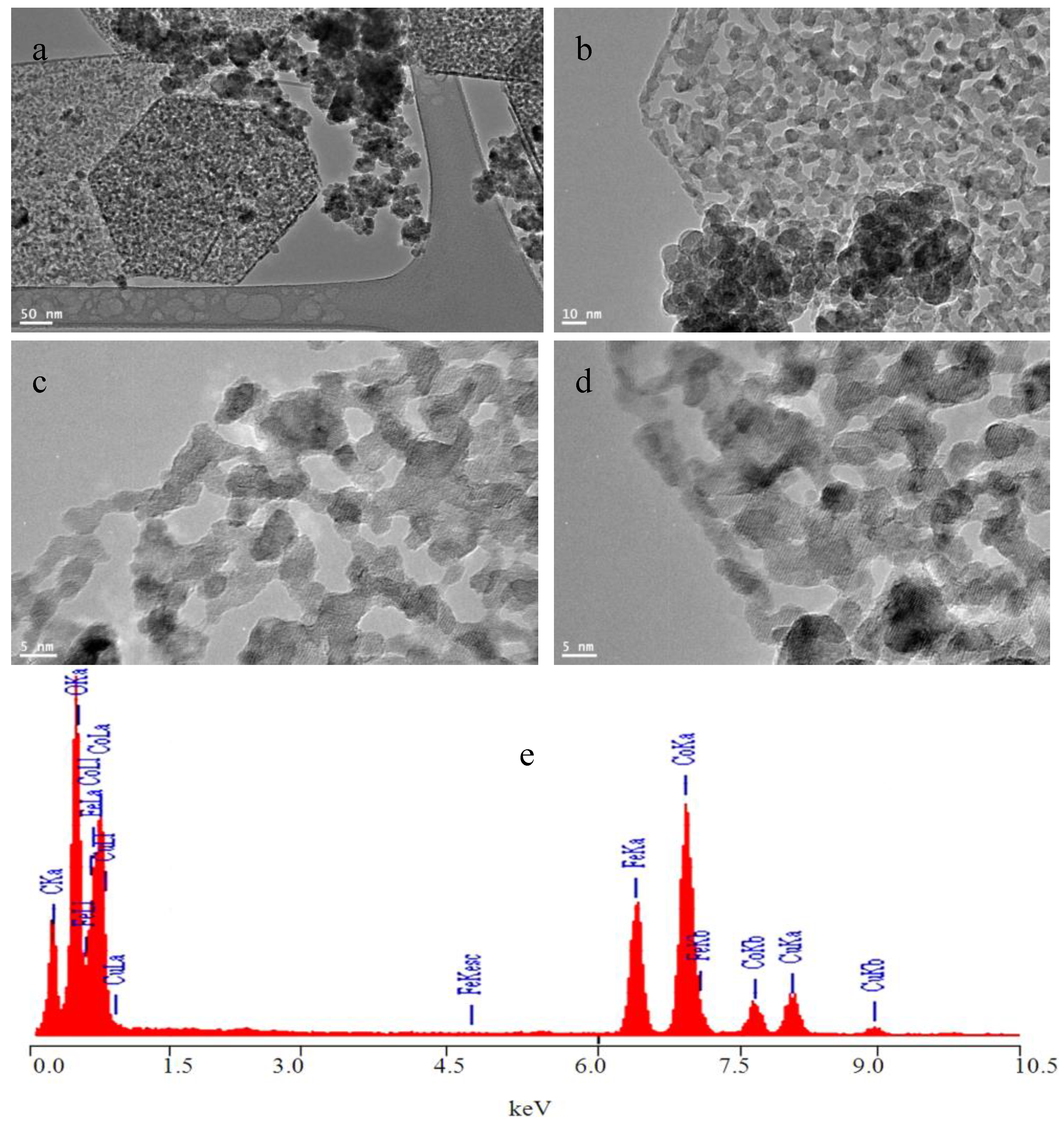
3.1. Supporting Cobalt Ferrite Nanoparticles over Co-Fe LDH
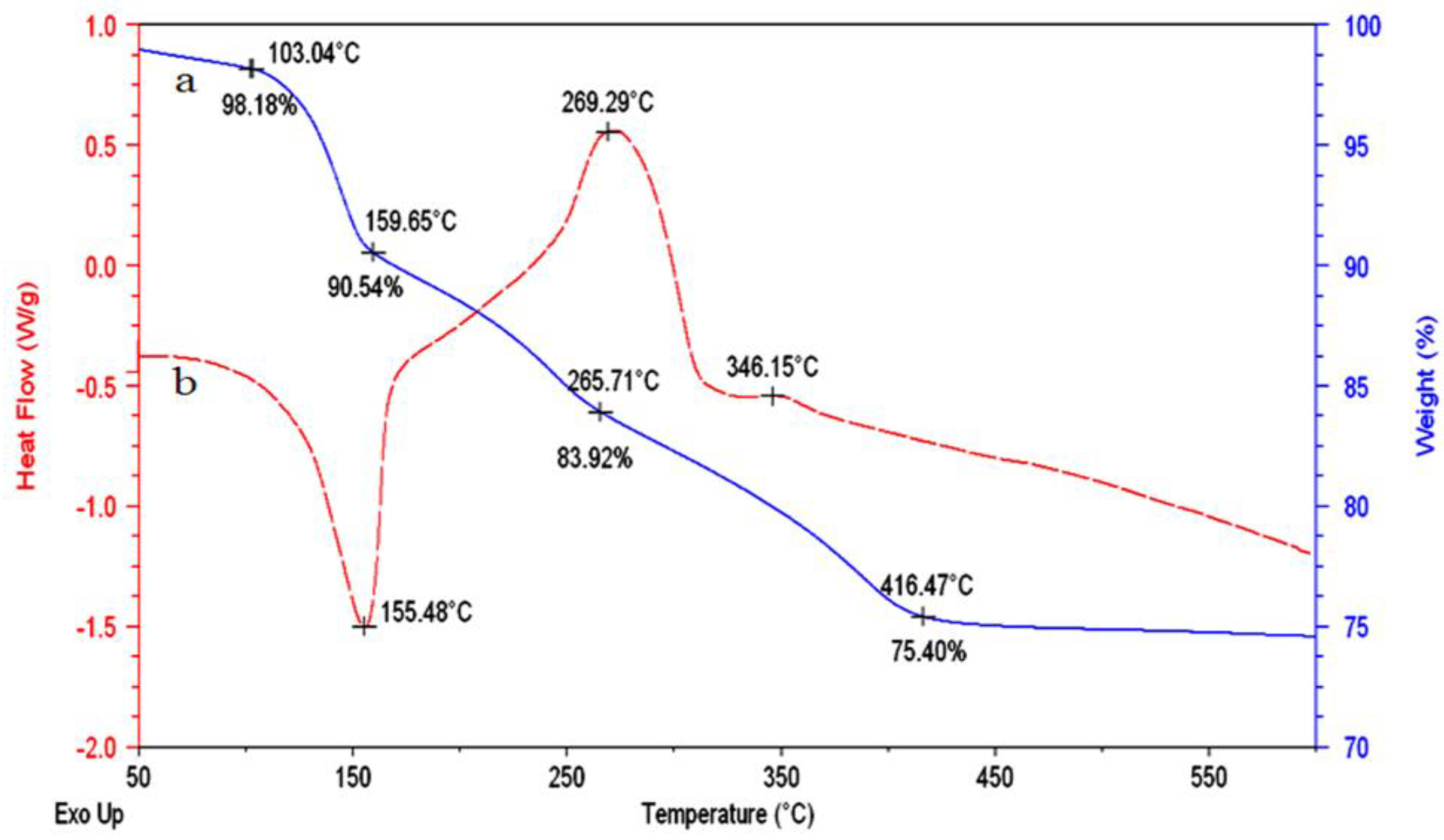
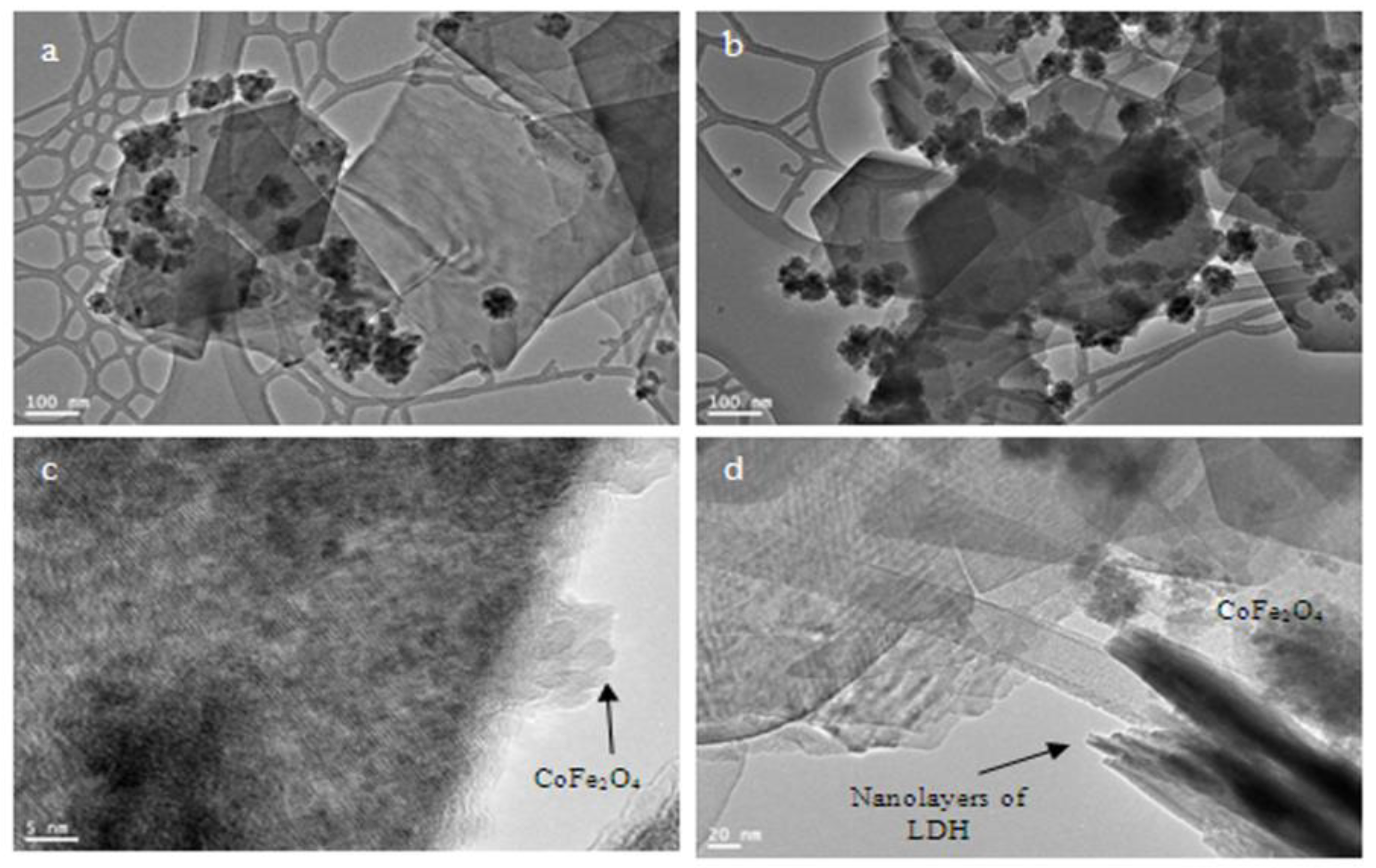
3.2. Transformation of the Nanolayers to the Nano-Nets
3.3. Mechanism of the Nano-Nets Formation
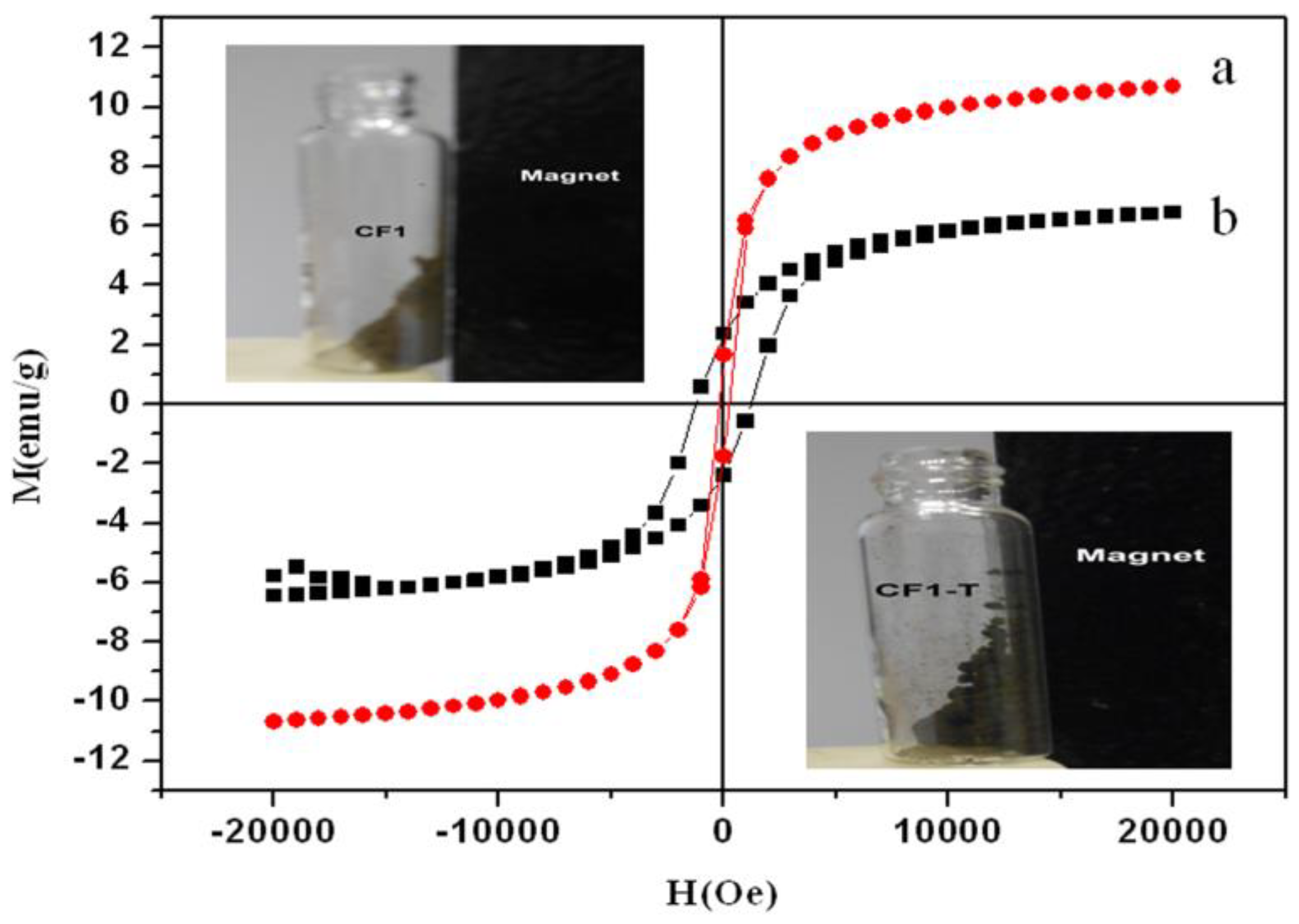
3.4. Magnetic Properties
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tran, H.D.; Li, D.; Kaner, R.B. One-Dimensional Conducting Polymer Nanostructures: Bulk Synthesis and Applications. Adv. Mater. 2009, 21, 1487–1499. [Google Scholar] [CrossRef]
- Wang, X.F.; Ding, B.; Yu, J.Y.; Si, Y.; Yang, S.B.; Sun, G. Electro-netting: Fabrication of two-dimensional nano-nets for highly sensitive trimethylamine sensing. Nanoscale 2011, 3, 911–915. [Google Scholar] [CrossRef] [PubMed]
- Saber, O.; Aljaafari, A.; Osama, M.; Alabdulgader, H. Accelerating the photocatalytic degradation of green pollutants through new coating technique for carbon nanotubes with nanolayered structures and nanocomposites. Chem. Open 2018, 7, 833–841. [Google Scholar]
- Saber, O.; Alomair, H.A.; Abu-Abdeen, M.; Aljaafari, A. Fast degradation of green pollutants through nanonets and nanofibers of the Al-doped zinc oxide. Acta Metall. Sin. Engl. 2018, 31, 533–546. [Google Scholar] [CrossRef]
- Cao, H.Q.; Zheng, H.; Liu, K.Y.; Fu, R.P. Single-Crystalline Semiconductor In(OH)3 Nanocubes with Bifunctions: Superhydrophobicity and Photocatalytic Activity. Cryst. Growth Des. 2010, 10, 597–601. [Google Scholar] [CrossRef]
- Wang, M.R.; He, J.H.; Yu, J.Y.; Pan, N. Lattice Boltzmann modeling of the effective thermal conductivity for fibrous materials. Int. J. Therm. Sci. 2007, 46, 848–855. [Google Scholar] [CrossRef]
- Kodama, T.; Kitayama, Y.; Tsuji, M.; Tamaura, Y. Characterization of Ultrafine NixFe3−xO4 Particles Synthesized by Co-Precipitation: Size Regulation and Magnetic Properties. J. Magn. Soc. Jpn. 1996, 20, 305–308. [Google Scholar] [CrossRef]
- Giri, A.K.; Kirkpatric, E.M.; Moongkhmklang, P.; Majctich, S.A. Photomagnetism and structure in cobalt ferrite nanoparticles. Appl. Phys. Lett. 2002, 80, 2341–2343. [Google Scholar] [CrossRef]
- Giri, A.K.; Pellerin, K.; Pongsaksawad, W.; Sorescu, M.; Majetich, S.A. Effect of light on the magnetic properties of cobalt ferrite nanoparticles. IEEE Trans. Magn. 2000, 36, 3029–3031. [Google Scholar] [CrossRef]
- Chen, Y.; Ruan, M.; Jiang, Y.F.; Cheng, S.G.; Li, W. The Synthesis and Thermal Effect of CoFe2O4 Nanoparticles. J. Alloys Compd. 2010, 493, L36–L38. [Google Scholar] [CrossRef]
- Morais, P.C.; Garg, V.K.; Oliveira, A.C.; Silva, L.P.; Azevedo, R.B.; Silva, A.M.L.; Lima, E.C.D. Synthesis and characterization of size-controlled cobalt-ferrite-based ionic ferrofluids. J. Magn. Magn. Mater. 2001, 225, 37–40. [Google Scholar] [CrossRef]
- Pillai, V.; Shah, D.O. Synthesis of High-Coercivity Cobalt Perrite Particles Using Water-in-Oil Microemulsions. J. Magn. Magn. Mater. 1996, 163, 243–248. [Google Scholar] [CrossRef]
- Saber, O.; Mohamed, N.H.; Aljaafari, A. Synthesis of Magnetic Nanoparticles and Nanosheets for Oil Spill Removal. Nanosci. Nanotechnol. Asia 2015, 5, 32–43. [Google Scholar] [CrossRef]
- Wu, M.; Xiong, Y.; Peng, Z.; Jang, N.; Qi, H.; Chen, Q. The enhanced coercivity for the magnetite/silica nanocomposite at room temperature. Mater. Res. Bull. 2004, 39, 1875–1880. [Google Scholar] [CrossRef]
- Wanger, J.; Authenrieth, T.; Hempelmann, R. Core shell particles consisting of cobalt ferrite and silica as model ferrofluids [CoFe2O4–SiO2 core shell particles. J. Magn. Magn. Mater. 2002, 252, 4–6. [Google Scholar]
- Huang, X.H.; Chen, Z.H. Sol-gel preparation and characterization of CoFe2O4–SiO2 nanocomposites. Solid State Commun. 2004, 132, 845–850. [Google Scholar] [CrossRef]
- Gharagozlou, M. Study on the influence of annealing temperature and ferrite content on the structural and magnetic properties of x(NiFe2O4)/(100−x)SiO2. J. Alloys Compd. 2010, 495, 217–223. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Lu, M.; Dong, H.; Hua, J.; Xu, S.C.; Li, H.B. Magnetic and Mössbauer spectroscopy studies of NiFe2O4/SiO2 nanocomposites synthesized by sol-gel method. J. Sol.-Gel Sci. Technol. 2015, 28, 191–196. [Google Scholar] [CrossRef]
- Nadeem, K.; Traussnig, T.; Letofsky-Papst, I.; Krenn, H.; Brossmann, U. Sol-gel synthesis and characterization of single-phase Ni ferrite nanoparticles dispersed in SiO2 matrix. J. Alloys Compd. 2010, 493, 385–390. [Google Scholar] [CrossRef]
- Rohilla, S.; Kumar, S.; Aghamkar, P.; Sunder, S.; Agarwal, A. Investigations on structural and magnetic properties of cobalt ferrite/silica Nanocomposites prepared by the coprecipitation method. J. Magn. Magn. Mater. 2011, 323, 897–902. [Google Scholar] [CrossRef]
- Wanga, X.; Dinga, B.; Yuc, J.; Yange, J. Large-scale fabrication of two-dimensional spider-web-like gelatin nano-nets via electro-netting. Colloids Surf. B 2011, 86, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Pant, H.R.; Pant, B.; Pokharel, P.; Kim, H.J.; Tijing, L.D.; Park, C.H.; Lee, D.S.; Kim, H.Y.; Kim, C.S. PhotocatalyticTiO2–RGO/nylon-6 spider-wave-like nano-nets via electrospinning and hydrothermal treatment. J. Membr. Sci. 2013, 429, 225–234. [Google Scholar] [CrossRef]
- Hyde, S.T.; Friedrichs, O.D. From untangled graphs and nets to tangled materials. Solid State Sci. 2011, 13, 676–683. [Google Scholar] [CrossRef]
- Saber, O. Improvement of optical properties and thermal stability of poly vinyl alcohol using salicylic acid confined in nanohybrid material. Polym. Bull. 2012, 68, 209–222. [Google Scholar] [CrossRef]
- Meng, W.Q.; Li, F.; Evans, D.G.; Duan, X. Preparation of magnetic material containing MgFe2O4 spinel ferrite from a Mg–Fe(III) layered double hydroxide intercalated by hexacyanoferrate (III) ions. Mater. Chem. Phys. 2004, 86, 1–4. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, D.; Zhang, S.; Maa, Y.; Yang, W.; Wang, Y.; Awaji, S.; Watanabe, K. Effect of high magnetic field annealing on the microstructure and magnetic properties of Co–Fe layered double hydroxide. J. Magn. Magn. Mater. 2010, 322, 3023–3027. [Google Scholar] [CrossRef]
- Chen, W.Q.; Wang, J.; Ma, K.Y.; Li, M.; Guo, S.H.; Liu, F.; Cheng, J.P. Hierarchical NiCo2O4@Co-Fe LDH core-shell nanowire arrays for high-performance supercapacitor. Appl. Surf. Sci. 2018, 451, 280–288. [Google Scholar] [CrossRef]
- Saber, O.; El-Brolossy, T.; AlJaafari, A. Improvement of photocatalytic degradation of naphthol green B under solar light using aluminum doping of zinc oxide nanoparticles. Water Air Soil Pollut. 2012, 223, 4615–4626. [Google Scholar] [CrossRef]
- Saber, O. Preparation and characterization of a new layered double hydroxide, Co–Zr–Si. J. Colloid Interface Sci. 2006, 297, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarin, M.; Amighian, J.; Darsheshdar, E. Magnetic and structural studies of nickel-substituted cobalt ferrite nanoparticles, synthesized by the sol–gel method. J. Magn. Magn. Mater. 2014, 350, 19–22. [Google Scholar] [CrossRef]
- Velinov, N.; Koleva, K.; Tsonchev, T.; Paneva, D.; Manova, E.; Tenchev, K.; Kunev, B.; Genova, I.; Mitov, I. Copper-cobalt ferrites as catalysts for methanol decomposition. Cent. Eur. J. Chem. 2014, 12, 250–259. [Google Scholar] [CrossRef]
- Porter, J.R.; De Jonghe, L.C.; De Jonghe. Hydrogen reduction of cobalt ferrite. Metall. Trans. B 1981, 12, 299–309. [Google Scholar] [CrossRef]
- Nejati, K.; Davaran, S.; Baggalzadeh, R. Synthesis and investigation of magnetic nanocomposite of Fe3O4 with cetirizine-intercalated layered double hydroxide. Superlattices Microstruct. 2014, 75, 257–267. [Google Scholar] [CrossRef]
- Nadeem, K.; Zeb, F.; Azeem Abid, M.; Mumataz, M.; Rehman, A.M. Effect of amorphous silica matrix on structural, magnetic, and dielectric properties of cobalt ferrite/silica nanocomposites. J. Non-Cryst. Solids 2014, 400, 45–50. [Google Scholar] [CrossRef]
- Gozuak, F.; Koseoglu, Y.; Baykal, A.; Kavas, H. Synthesis and characterization of CoxZn1−xFe2O4 magnetic nanoparticles via a PEG-assisted route. J. Magn. Magn. Mater. 2009, 321, 2170–2217. [Google Scholar] [CrossRef]
- Saber, O. New trend for synthesizing of magnetic nanorods with titanomaghemite structure. J. Magn. Magn. Mater. 2016, 410, 10–17. [Google Scholar] [CrossRef]
- Herrera, A.P.; Polo-Corrales, L.; Chavez, E.; Cabarcas-Bolivar, J.; Uwakweh, O.N.C.; Rinaldi, C. Influence of aging time of oleate precursor on the magnetic relaxation of cobalt ferrite nanoparticles synthesized by the thermal decomposition method. J. Magn. Magn. Mater. 2013, 328, 41–52. [Google Scholar] [CrossRef]
- Chinnasamy, C.N.; Jeyadevan, B.; Shinoda, K.; Tohji, K.; Djayaprawira, D.J.; Takahashi, M.; Joseyphus, R.J.; Narayanasamy, A. Unusually high coercivity and critical single-domain size of nearly monodispersed CoFe2O4 nanoparticles. Appl. Phys. Lett. 2003, 83, 2862–2864. [Google Scholar] [CrossRef]
- Cannas, C.; Musinu, A.; Ardu, A.; Orru, F.; Peddis, G.; Casu, M.; Sanna, R.; Angius, F.; Diaz, G.; Piccaluga, G. CoFe2O4 and CoFe2O4/SiO2 Core/Shell Nanoparticles: Magnetic and Spectroscopic Study. Chem. Mater. 2010, 22, 3353–3361. [Google Scholar] [CrossRef]
- Girgis, E.; Adel, D.; Tharwat, C.; Attallah, O.; Rao, K.V. Cobalt ferrite nanotubes and porous nanorods for dye removal. Adv. Nano Res. 2015, 3, 111–121. [Google Scholar] [CrossRef]

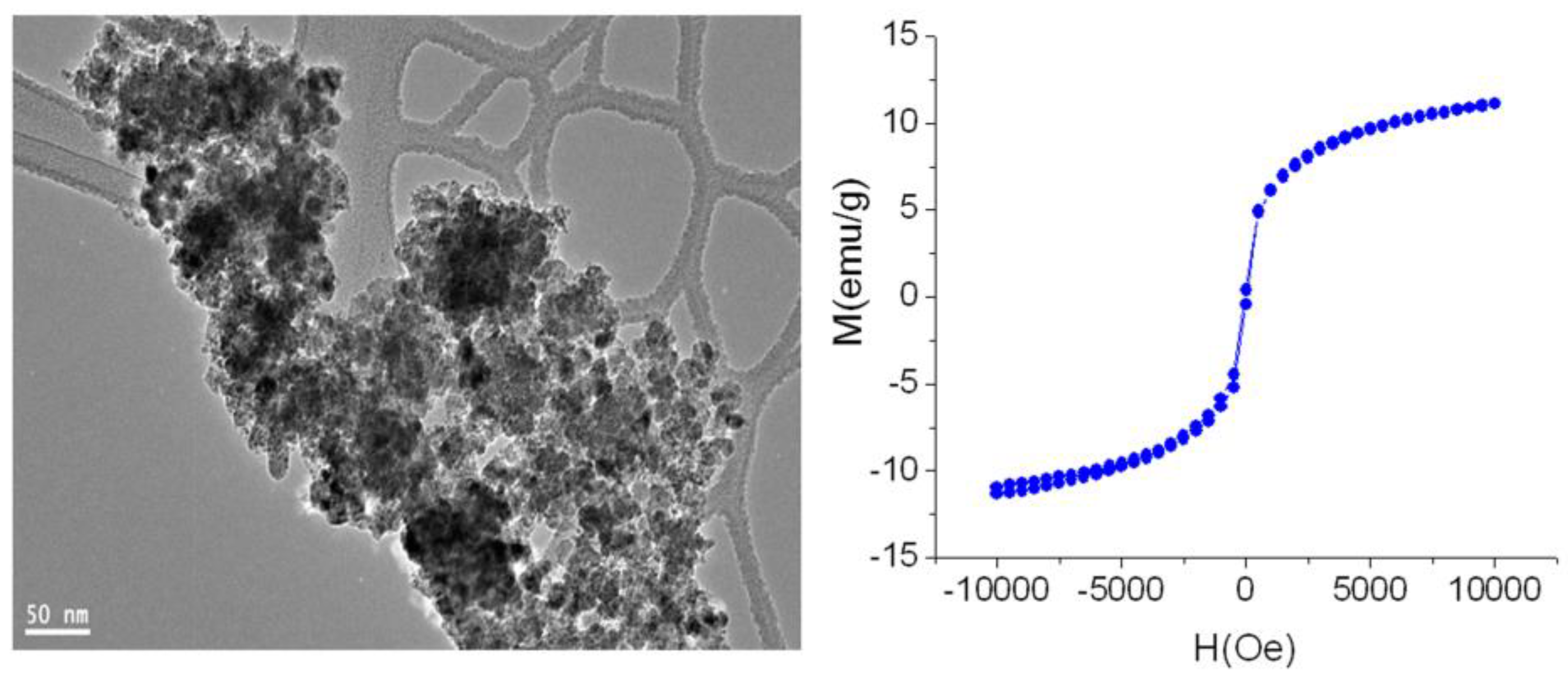
| Samples | Ms (emu g−1) | Mr (emu g−1) | Hc (Oe) | QS(Mr/Ms) |
|---|---|---|---|---|
| CoFe2O4/Co-Fe LDH | 10.69 ± 0.05 | 1.36 ± 0.007 | 2.60 ± 0.01 | 0.13 |
| CoFe2O4 nano-nets | 6.44 ± 0.03 | 2.30 ± 0.01 | 1277 ± 6 | 0.36 |
| CoFe2O4 nanoparticles | 11 ± 0.05 | 0.25 ± 0.001 | 35 ± 0.2 | 0.023 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saber, O.; Aljaafari, A.; Asiri, S.; Batoo, K.M. Designing Magnetic Layered Double Hydroxides and Two-Dimensional Magnetic Nano-Nets of Cobalt Ferrite through a Novel Approach. Appl. Sci. 2018, 8, 2099. https://doi.org/10.3390/app8112099
Saber O, Aljaafari A, Asiri S, Batoo KM. Designing Magnetic Layered Double Hydroxides and Two-Dimensional Magnetic Nano-Nets of Cobalt Ferrite through a Novel Approach. Applied Sciences. 2018; 8(11):2099. https://doi.org/10.3390/app8112099
Chicago/Turabian StyleSaber, Osama, Abdullah Aljaafari, Sarah Asiri, and Khalid M. Batoo. 2018. "Designing Magnetic Layered Double Hydroxides and Two-Dimensional Magnetic Nano-Nets of Cobalt Ferrite through a Novel Approach" Applied Sciences 8, no. 11: 2099. https://doi.org/10.3390/app8112099
APA StyleSaber, O., Aljaafari, A., Asiri, S., & Batoo, K. M. (2018). Designing Magnetic Layered Double Hydroxides and Two-Dimensional Magnetic Nano-Nets of Cobalt Ferrite through a Novel Approach. Applied Sciences, 8(11), 2099. https://doi.org/10.3390/app8112099







