Effects of Silk-Worm Excrement Biochar Combined with Different Iron-Based Materials on the Speciation of Cadmium and Lead in Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Method
2.3. Items for Determination
2.4. Data Processing
3. Results and Discussion
3.1. The Effect of Different Treatments on Cd and Pb in Soil
3.1.1. The Effect of Different Treatments on Cd in Soil
3.1.2. The Effect of Different Treatments on Pb in Soil
3.2. The Effect of Different Treatments on Soil Property and Soil Crystal Structure
3.2.1. The Effect of Different Treatments on Soil pH Value
3.2.2. The Effect of Different Treatments on Soil CEC
3.2.3. The Effect of Different Treatments on the Soil Crystal Structure
3.3. Correlation between the Speciation of PTMs and Soil Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Race, M.; Ferraro, A.; Fabbricino, M.; La, A.M.; Panico, A.; Spasiano, D.; Tognacchini, A.; Pirozzi, F. Ethylenediamine-N,N’-Disuccinic Acid (EDDS)-Enhanced Flushing Optimization for Contaminated Agricultural Soil Remediation and Assessment of Prospective Cu and Zn Transport. Int. J. Environ. Res. Public Health 2018, 15, 543. [Google Scholar] [CrossRef] [PubMed]
- Gurung, B.; Race, M.; Fabbricino, M.; Komínková, D.; Libralato, G.; Siciliano, A.; Guida, M. Assessment of metal pollution in the Lambro Creek (Italy). Ecotoxicol. Environ. Saf. 2018, 148, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Raicevic, S.; Kaludjerovicradoicic, T.; Zouboulis, A.I. In situ stabilization of toxic metals in polluted soils using phosphates: Theoretical prediction and experimental verification. J. Hazard. Mater. 2005, 117, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Shyleshchandran, M.N.; Mohan, M.; Ramasamy, E.V. Risk assessment of heavy metals in Vembanad Lake sediments (south-west coast of India), based on acid-volatile sulfide (AVS)-simultaneously extracted metal (SEM) approach. Environ. Sci. Pollut. Res. 2018, 25, 7333–7345. [Google Scholar] [CrossRef] [PubMed]
- Gustavsson, P. Strategies for Primary Prevention of Occupational Cancer. In Occupational Cancers; Anttila, S., Boffetta, P., Eds.; Springer: London, UK, 2014; pp. 565–572. [Google Scholar]
- Arito, H. Risk assessment of hazardous substances revisited. Ind. Health 2015, 53, 193–195. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhu, G.; Jin, T.; Lei, L.; Liang, Y. Bone mineral density is related with previous renal dysfunction caused by cadmium exposure. Environ. Toxicol. Pharmacol. 2011, 32, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, H.; Aoshima, K.; Oguma, E.; Sasaki, S.; Miyamoto, K.; Hosoi, Y.; Katoh, T.; Kayama, F. Latest status of cadmium accumulation and its effects on kidneys, bone, and erythropoiesis in inhabitants of the formerly cadmium-polluted Jinzu River Basin in Toyama, Japan, after restoration of rice paddies. Int. Arch. Occup. Environ. Health 2010, 83, 953–970. [Google Scholar] [CrossRef] [PubMed]
- Ke, S.; Cheng, X.Y.; Zhang, N.; Hu, H.G.; Yan, Q.; Hou, L.L.; Sun, X.; Chen, Z.N. Cadmium contamination of rice from various polluted areas of China and its potential risks to human health. Environ. Monit. Assess. 2015, 187, 408. [Google Scholar] [CrossRef] [PubMed]
- Wallin, M.; Sallsten, G.; Fabricius-Lagging, E.; Öhrn, C.; Lundh, T.; Barregard, L. Kidney cadmium levels and associations with urinary calcium and bone mineral density: A cross-sectional study in Sweden. Environ. Health 2013, 12, 22. [Google Scholar] [CrossRef] [PubMed]
- Harvey, P.J.; Taylor, M.P.; Handley, H.K. Widespread Environmental Contamination Hazards in Agricultural Soils from the Use of Lead Joints in Above Ground Large-Scale Water Supply Pipelines. Water Air Soil Pollut. 2015, 226, 178. [Google Scholar] [CrossRef]
- Han, C.; Wang, L.; Gong, Z.; Huaxia, X.U. Chemical forms of soil heavy metals and their environmental significance. Chin. J. Ecol. 2005, 24, 1499–1502. [Google Scholar]
- Santona, L.; Castaldi, P.; Melis, P. Evaluation of the interaction mechanisms between red muds and heavy metals. J. Hazard. Mater. 2006, 136, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Shiowatana, J.; McLaren, R.G.; Chanmekha, N.; Samphao, A. Fractionation of Arsenic in Soil by a Continuous-Flow Sequential Extraction Method. J. Environ. Qual. 2001, 30, 1940–1949. [Google Scholar] [CrossRef] [PubMed]
- Buekers, J. Fixation of Cadmium, Copper, Nickel and Zinc in Soil: Kinetics, Mechanisms and Its Effect on Metal Bioavailability. Ph.D. Thesis, Katholieke Universiteit Leuven, Leuven, Belgium, 2007. [Google Scholar]
- Levy, D.B.; Barbarick, K.A.; Siemer, E.G.; Sommers, L.E. Distribution and Partitioning of Trace Metals in Contaminated Soils near Leadville, Colorado. J. Environ. Qual. 1992, 21, 185–195. [Google Scholar] [CrossRef]
- Li, H.; Ye, X.; Geng, Z.; Zhou, H.; Guo, X.; Zhang, Y.; Zhao, H.; Wang, G. The influence of biochar type on long-term stabilization for Cd and Cu in contaminated paddy soils. J. Hazard. Mater. 2016, 304, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Sun, G.; Xu, Y.; Liu, W.; Liang, X.; Wang, L. Evaluation of the effectiveness of sepiolite, bentonite, and phosphate amendments on the stabilization remediation of cadmium-contaminated soils. J. Environ. Manag. 2016, 166, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Pan, G.; Li, L.; Yan, J.; Zhang, A.; Bian, R.; Chang, A. The reduction of wheat cd uptake in contaminated soil via biochar amendment: A two-year field experiment. Bioresources 2012, 7, 5666–5676. [Google Scholar] [CrossRef]
- Sohi, S.P.; Krull, E.; Lopez-Capel, E.; Bol, R. Chapter 2—A Review of Biochar and Its Use and Function in Soil. Adv. Agron. 2010, 105, 47–82. [Google Scholar]
- Houben, D.; Evrard, L.; Sonnet, P. Beneficial effects of biochar application to contaminated soils on the bioavailability of Cd, Pb and Zn and the biomass production of rapeseed (Brassica napus L.). Biomass Bioenergy 2013, 57, 196–204. [Google Scholar] [CrossRef]
- Biederman, L.A.; Harpole, W.S. Biochar and its effects on plant productivity and nutrient cycling: A meta-analysis. Glob. Change Biol. Bioenergy 2014, 6, 172–175. [Google Scholar] [CrossRef]
- Jeffery, S.; Verheijen, F.G.A.; Velde, M.V.D.; Bastos, A.C. A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agric. Ecosyst. Environ. 2011, 144, 175–187. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Yang, H.; Yan, G.; Xu, Z.; Chen, C.; Zhang, D. Biochar nutrient availability rather than its water holding capacity governs the growth of both C3 and C4 plants. J. Soils Sediments 2016, 16, 801–810. [Google Scholar] [CrossRef]
- Haefele, S.M.; Konboon, Y.; Wongboon, W.; Amarante, S.; Maarifat, A.A.; Pfeiffer, E.M.; Knoblauch, C. Effects and fate of biochar from rice residues in rice-based systems. Field Crops Res. 2011, 121, 430–440. [Google Scholar] [CrossRef]
- Seidel, H.; Görsch, K.; Amstätter, K.; Mattusch, J. Immobilization of arsenic in a tailings material by ferrous iron treatment. Water Res. 2005, 39, 4073–4082. [Google Scholar] [CrossRef] [PubMed]
- Subacz, J.L.; Barnett, M.O.; Jardine, P.M.; Stewart, M.A. Decreasing arsenic bioaccessibility/bioavailability in soils with iron amendments. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2007, 42, 1317–1329. [Google Scholar] [CrossRef] [PubMed]
- Feng, B. Research on groundwater arsenic removal technology. Public Technol. 2010, 24, 21–23. (In Chinese) [Google Scholar]
- Xenidis, A.; Stouraiti, C.; Papassiopi, N. Stabilization of Pb and As in soils by applying combined treatment with phosphates and ferrous iron. J. Hazard. Mater. 2010, 177, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Muehe, E.M.; Obst, M.; Hitchcock, A.; Tyliszczak, T.; Behrens, S.; Schröder, C.; Byrne, J.M.; Michel, F.M.; Krämer, U.; Kappler, A. Fate of Cd during Microbial Fe(III) Mineral Reduction by a Novel and Cd-Tolerant Geobacter Species. Environ. Sci. Technol. 2013, 47, 14099. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Du, X.; Weng, L.; Van Riemsdijk, W.H. Assessment of In Situ Immobilization of Lead (Pb) and Arsenic (As) in Contaminated Soils with Phosphate and Iron: Solubility and Bioaccessibility. Water Air Soil Pollut. 2010, 213, 95–104. [Google Scholar] [CrossRef]
- Shao, G.; Chen, M.; Wang, D.; Xu, C.; Mou, R.; Cao, Z.; Zhang, X. Using iron fertilizer to control Cd accumulation in rice plants: A new promising technology. Sci. China Life Sci. 2008, 51, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Warren, G.P.; Alloway, B.J. Reduction of arsenic uptake by lettuce with ferrous sulfate applied to contaminated soil. J. Environ. Qual. 2003, 32, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Qiu-Jun, L.I.; Da-Rong, L.I.; Wang, Y.H.; Feng, Z.Y.; Ning, X.J.; Li-Xiang, W.U. Effects of Three Kinds of Organic Materials on Physicochemical Properties and Available Heavy Metals in Soil. J. Soil Water Conserv. 2013, 6, 036. [Google Scholar]
- Lu, H.; Li, Z.; Fu, S.; Méndez, A.; Gascó, G.; Paz-Ferreiro, J. Effect of Biochar in Cadmium Availability and Soil Biological Activity in an Anthrosol Following Acid Rain Deposition and Aging. Water Air Soil Pollut. 2015, 226, 164. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, C.; Pan, L.; Yang, W.; Li, C.; Xu, G.; Li, F.; Lu, M. Effects of Amendments and Water Conditions on the Chemical Speciation of Cd and Pb in Contaminated Paddy Soil in a Mining Area. J. Soil Contam. 2016, 25, 717–726. [Google Scholar] [CrossRef]
- SBTS. GB-T 12496.7-1999 Test methods of wooden activated carbon—determination of pH [S]. China Stand. Press Beijing 1999. (In Chinese) [Google Scholar]
- SBTS. GB-T 12496.3-1999 Test methods of wooden activated carbon—determination of ash content [S]. China Stand. Press Beijing 1999. (In Chinese) [Google Scholar]
- Gillman, G.P.; Sumpter, E.A. Modification to the compulsive exchange method for measuring exchange characteristics of soils. Soil Res. 1986, 24, 61–66. [Google Scholar] [CrossRef]
- ISO. Soil Quality—Determination of Ph; ISO: Geneva, Switzerland, 1994. [Google Scholar]
- Boehm, H.P. Some aspects of the surface chemistry of carbon blacks and other carbons. Carbon 1994, 32, 759–769. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Zhu, Q.; Wu, J.; Wang, L.; Yang, G.; Zhang, X. Effect of Biochar on Heavy Metal Speciation of Paddy Soil. Water Air Soil Pollut. 2015, 226, 429. [Google Scholar] [CrossRef]
- Adriano, D.C.; Wenzel, W.W.; Vangronsveld, J.; Bolan, N.S. Role of assisted natural remediation in environmental cleanup. Geoderma 2004, 122, 121–142. [Google Scholar] [CrossRef]
- Wu, B. The immobilization remediation of Cadmium, Lead and Arsenic in contaminated soils. Level of Master Thesis, Central South University, Changsha, China, 2014. (In Chinese). [Google Scholar]
- Tsuji, M.; Tamaura, Y. Thermodynamic study of M+/H+ exchanges on cryptomelane-type manganic acid. Solvent Extr. Ion Exch. 2000, 18, 187–202. [Google Scholar] [CrossRef]
- Chao, X.U.; Lin, X.B.; Qi-Tang, W.U.; Tang, H.T.; Liao, Y.L.; Qin, X.B. Impacts of Biochar on Availability of Heavy Metals and Nutrient Content of Contaminated Soil Under Waterlogged Conditions. J. Soil Water Conserv. 2012, 26, 194–198. [Google Scholar]
- Cao, X.; Harris, W. Properties of dairy-manure-derived biochar pertinent to its potential use in remediation. Bioresour. Technol. 2010, 101, 5222–5228. [Google Scholar] [CrossRef] [PubMed]
- Fellet, G.; Marchiol, L.; Delle, V.G.; Peressotti, A. Application of biochar on mine tailings: Effects and perspectives for land reclamation. Chemosphere 2011, 83, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Soo, L.S.; Yang, J.E.; Ro, H.M.; Han, L.Y.; Sik, O.Y. Effects of soil dilution and amendments (mussel shell, cow bone, and biochar) on Pb availability and phytotoxicity in military shooting range soil. Ecotoxicol. Environ. Saf. 2012, 79, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Uchimiya, M.; Lima, I.M.; Klasson, K.T.; Wartelle, L.H. Contaminant immobilization and nutrient release by biochar soil amendment: Roles of natural organic matter. Chemosphere 2010, 80, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Chapman, H.D. Cation-Exchange Capacity 1; U.S. Salinity Laboratory: Riverside, CA, USA, 2011; Volume 44, p. 202. [Google Scholar]
- Schulz, H.; Glaser, B. Effects of biochar compared to organic and inorganic fertilizers on soil quality and plant growth in a greenhouse experiment. J. Plant Nutr. Soil Sci. 2012, 175, 410–422. [Google Scholar] [CrossRef]
- Singh, B.; Singh, B.P.; Cowie, A.L.; Krull, E.; Singh, B.; Joseph, S. Characterisation and evaluation of biochars for their application as a soil amendment. Soil Res. 2010, 48, 516–525. [Google Scholar] [CrossRef]
- Ma, L.Q.; Choate, A.L.; Rao, G.N. Effects of Incubation and Phosphate Rock on Lead Extractability and Speciation in Contaminated Soils. J. Environ. Qual. 1997, 26, 801–807. [Google Scholar] [CrossRef]
- Mustafa, G.; Singh, B.; Kookana, R.S. Cadmium desorption from goethite in the presence of desferrioxamine B and oxalic acid. Proceedings of 3rd Australian New Zealand Soils Conference, Sydney, Australia, 5–9 December 2004. [Google Scholar]
- And, S.S.; Mcbride, M.; Hendershot, W. Lead Phosphate Solubility in Water and Soil Suspensions. Environ. Sci. Technol. 1998, 32, 388–393. [Google Scholar]
- Sauvé, S.; Mcbride, M.; Hendershot, W. Soil Solution Speciation of Lead(II): Effects of Organic Matter and pH. Soil Sci. Soc. Am. J. 1998, 62, 618–621. [Google Scholar] [CrossRef]
- Wang, S.; Xu, Y.; Norbu, N.; Wang, Z. Remediation of Biochar on Heavy Metal Polluted Soils; IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2018; p. 042113. [Google Scholar]
- Lehmann, J.; Gaunt, J.; Rondon, M. Bio-char Sequestration in Terrestrial Ecosystems—A Review. Mitig. Adapt. Strateg. Glob. Change 2006, 11, 403–427. [Google Scholar] [CrossRef]
- Rees, F.; Simonnot, M.O.; Morel, J.L. Short-term effects of biochar on soil heavy metal mobility are controlled by intra-particle diffusion and soil pH increase. Eur. J. Soil Sci. 2014, 65, 149–161. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef] [PubMed]


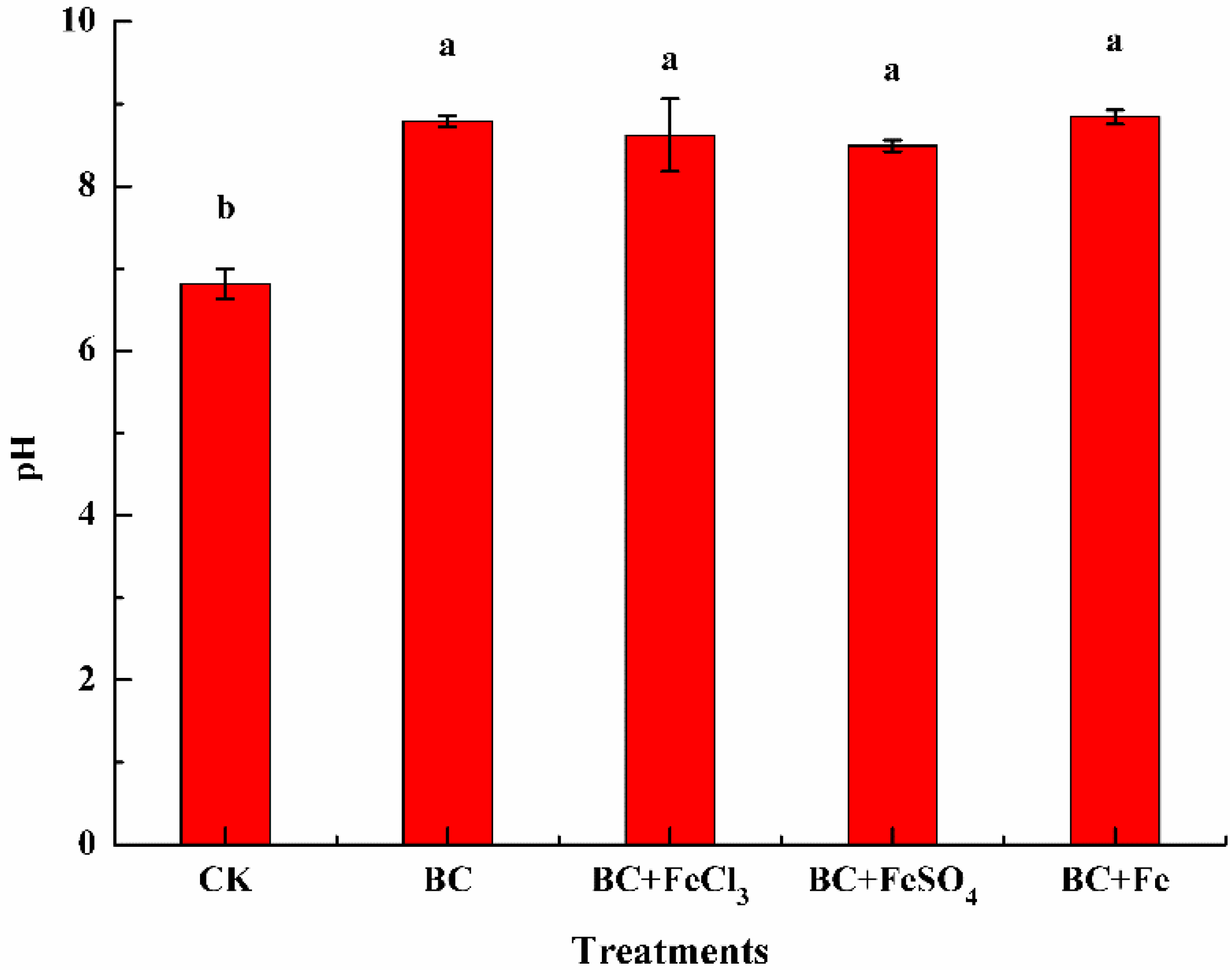
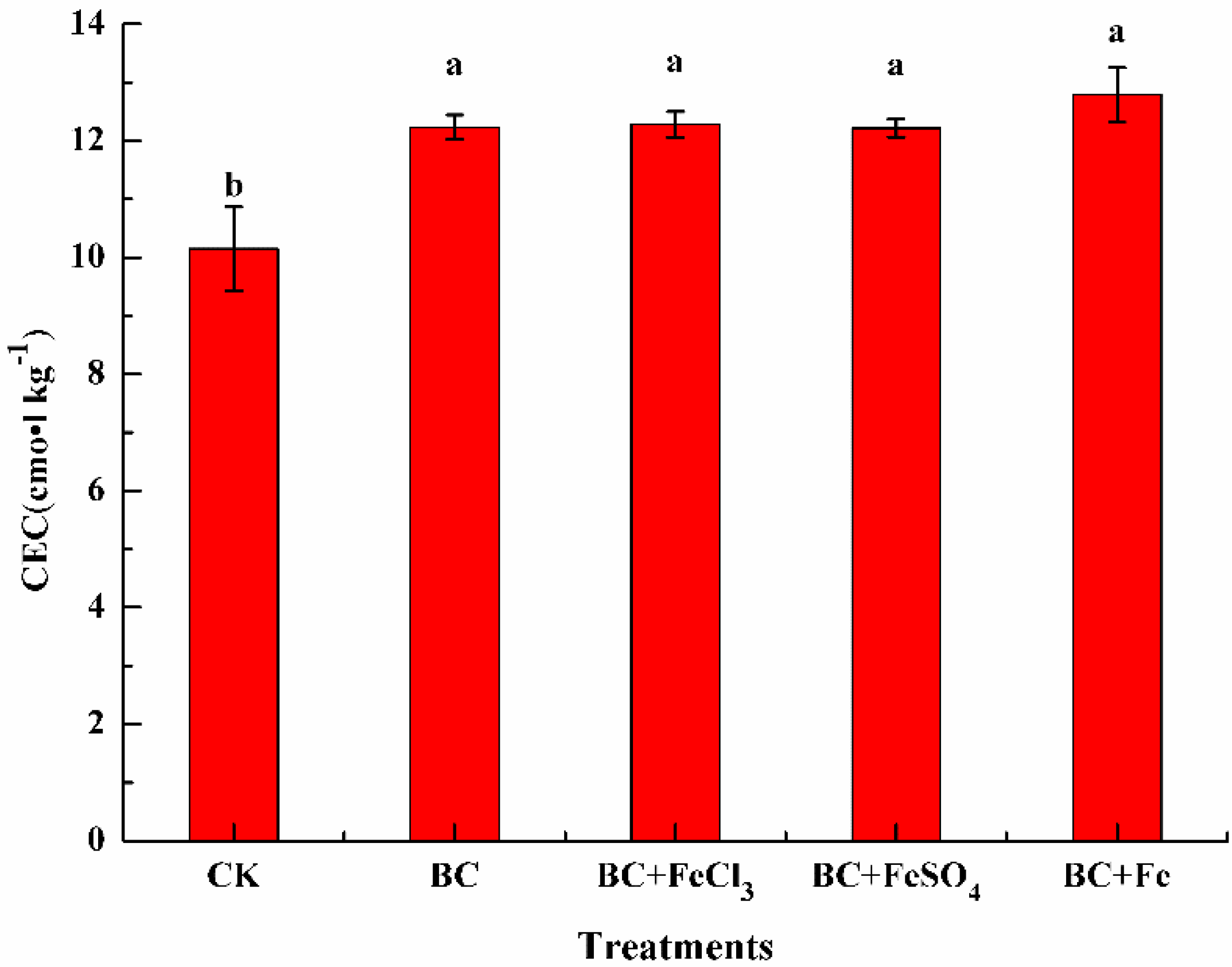
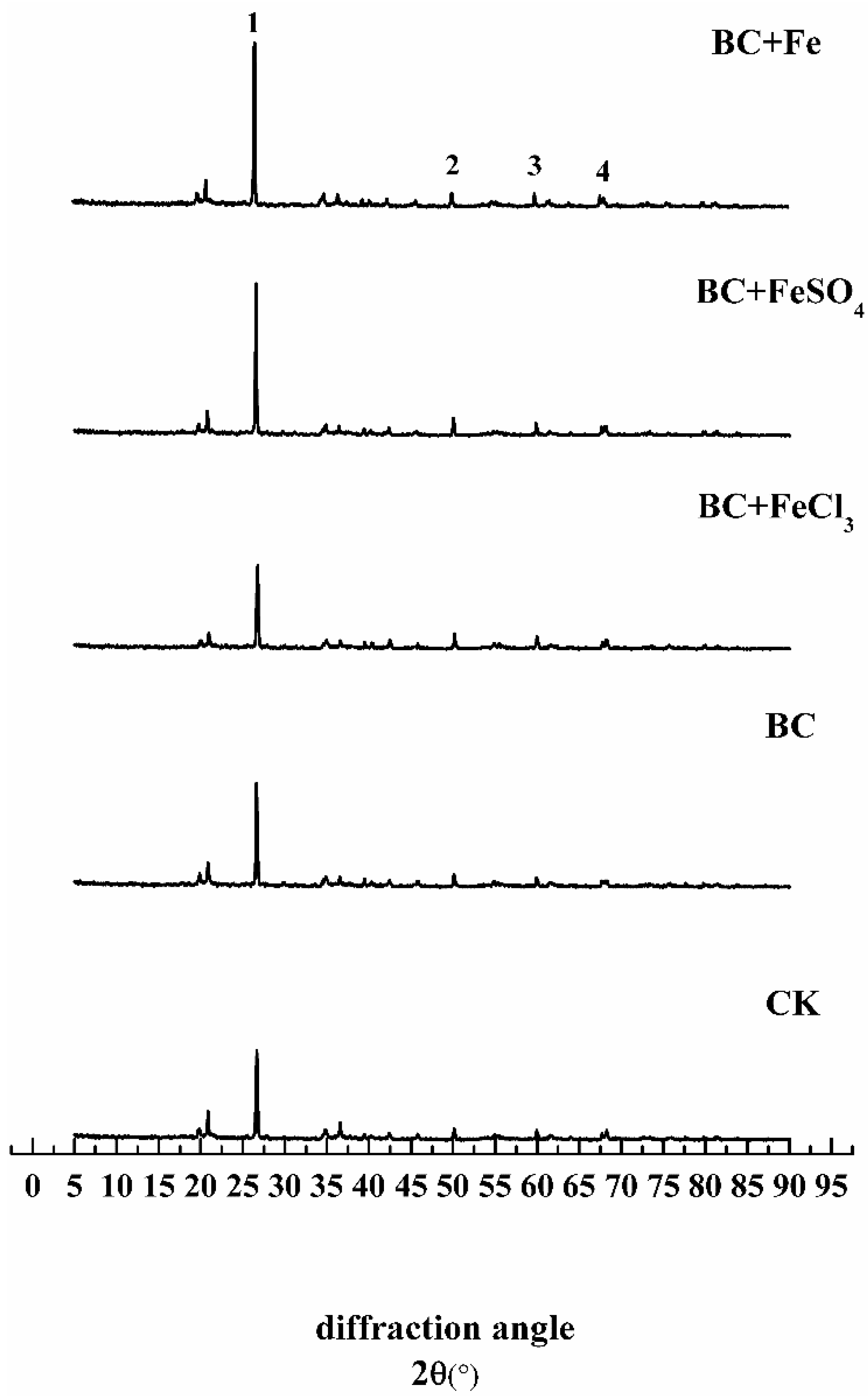
| Item | pH | Cation Exchange Capacity (cmol·kg−1) | Total Lead (mg·kg−1) | Total Cadmium (mg·kg−1) |
|---|---|---|---|---|
| Paddy soil | 6.76 | 10.86 | 1320.29 | 52.24 |
| Control sample | 8.60 | 13.42 | 0.61 | 41.57 |
| Material | pH | Cation Exchange Capacity (cmol·kg−1) | Alkaline Functional Groups (mmol·g−1) | Acid Functional Groups (mmol·g−1) |
|---|---|---|---|---|
| BC | 10.20 | 71.58 | 4.02 | 0.59 |
| Index | The Speciation of Cd | ||||
|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | |
| pH | −0.816 ** | −0.729 ** | 0.059 | 0.705 ** | 0.142 |
| CEC | −0.792 ** | −0.839 ** | 0.126 | 0.630 * | 0.225 |
| Index | The Speciation of Pb | ||||
| F1 | F2 | F3 | F4 | F5 | |
| pH | −0.929 ** | −0.323 | 0.156 | 0.692 ** | 0.919 ** |
| CEC | −0.860 ** | −0.207 | 0.325 | 0.651 ** | 0.861 ** |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bian, P.; Zhang, J.; Zhang, C.; Huang, H.; Rong, Q.; Wu, H.; Li, X.; Xu, M.; Liu, Y.; Ren, S. Effects of Silk-Worm Excrement Biochar Combined with Different Iron-Based Materials on the Speciation of Cadmium and Lead in Soil. Appl. Sci. 2018, 8, 1999. https://doi.org/10.3390/app8101999
Bian P, Zhang J, Zhang C, Huang H, Rong Q, Wu H, Li X, Xu M, Liu Y, Ren S. Effects of Silk-Worm Excrement Biochar Combined with Different Iron-Based Materials on the Speciation of Cadmium and Lead in Soil. Applied Sciences. 2018; 8(10):1999. https://doi.org/10.3390/app8101999
Chicago/Turabian StyleBian, Pengyang, Jingjing Zhang, Chaolan Zhang, He Huang, Qun Rong, Haixia Wu, Xue Li, Mengmeng Xu, Yu Liu, and Siwei Ren. 2018. "Effects of Silk-Worm Excrement Biochar Combined with Different Iron-Based Materials on the Speciation of Cadmium and Lead in Soil" Applied Sciences 8, no. 10: 1999. https://doi.org/10.3390/app8101999
APA StyleBian, P., Zhang, J., Zhang, C., Huang, H., Rong, Q., Wu, H., Li, X., Xu, M., Liu, Y., & Ren, S. (2018). Effects of Silk-Worm Excrement Biochar Combined with Different Iron-Based Materials on the Speciation of Cadmium and Lead in Soil. Applied Sciences, 8(10), 1999. https://doi.org/10.3390/app8101999




