Development of Sustainable Technology to Mitigate VOCs Pollution from Air Using a Continuous System Composed of Fe-ZSM-5 Coated on Polypropylene Tubes Coupled with UV Irradiation at the Pilot Scale
Abstract
Featured Application
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Coating of Fe-ZSM-5 on Polyethylene Tubes
2.3. VOCs Adsorption Test on Coated Tubes
2.4. Semi Pilot Reactor Design and Setup
2.5. Measurement of VOCs
2.6. Design Parameters
3. Results and Discussion
3.1. Coating of Fe-ZSM-5 on Polymeric Woven Tubes
3.2. VOC Removal on Tubes
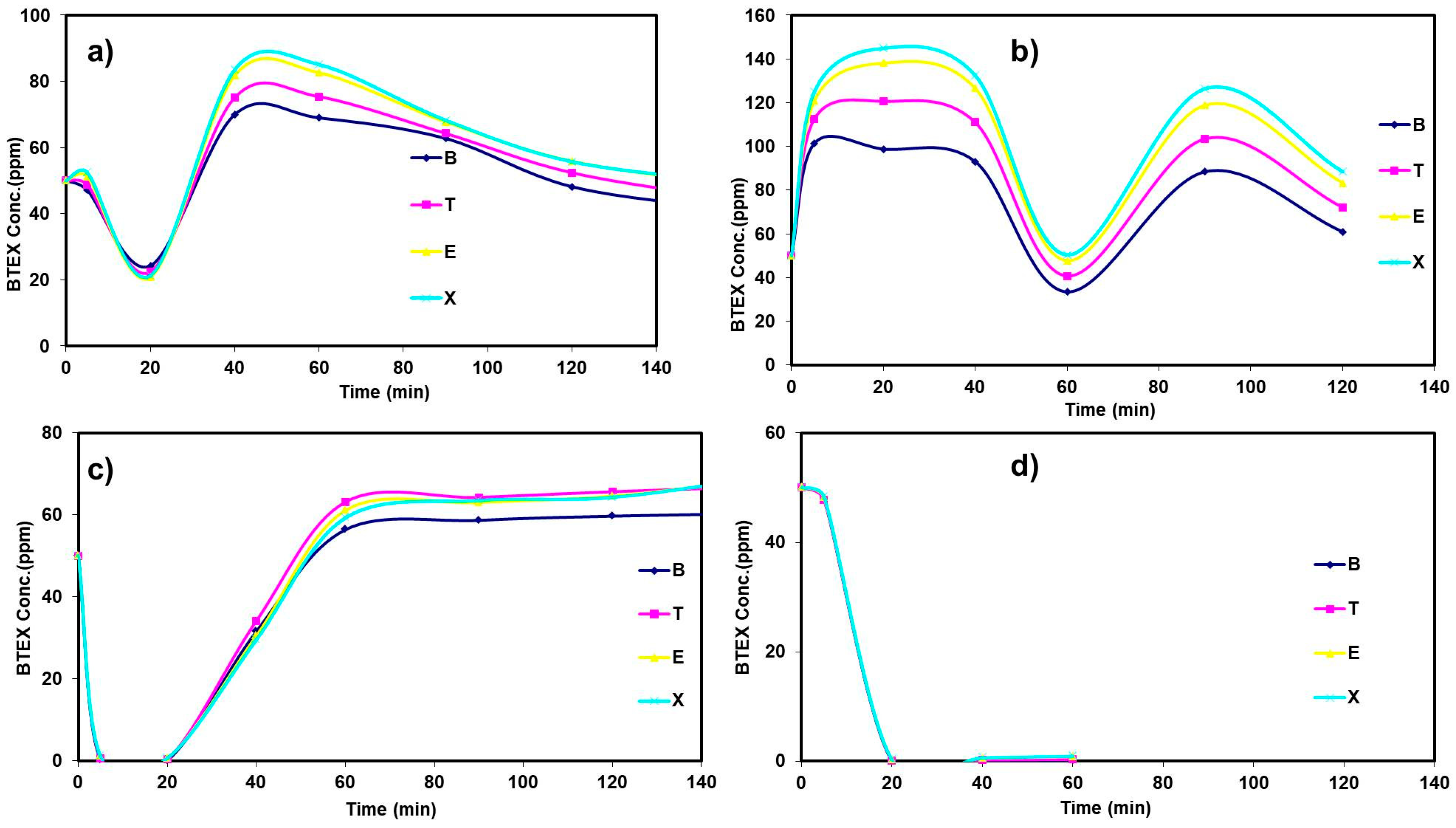
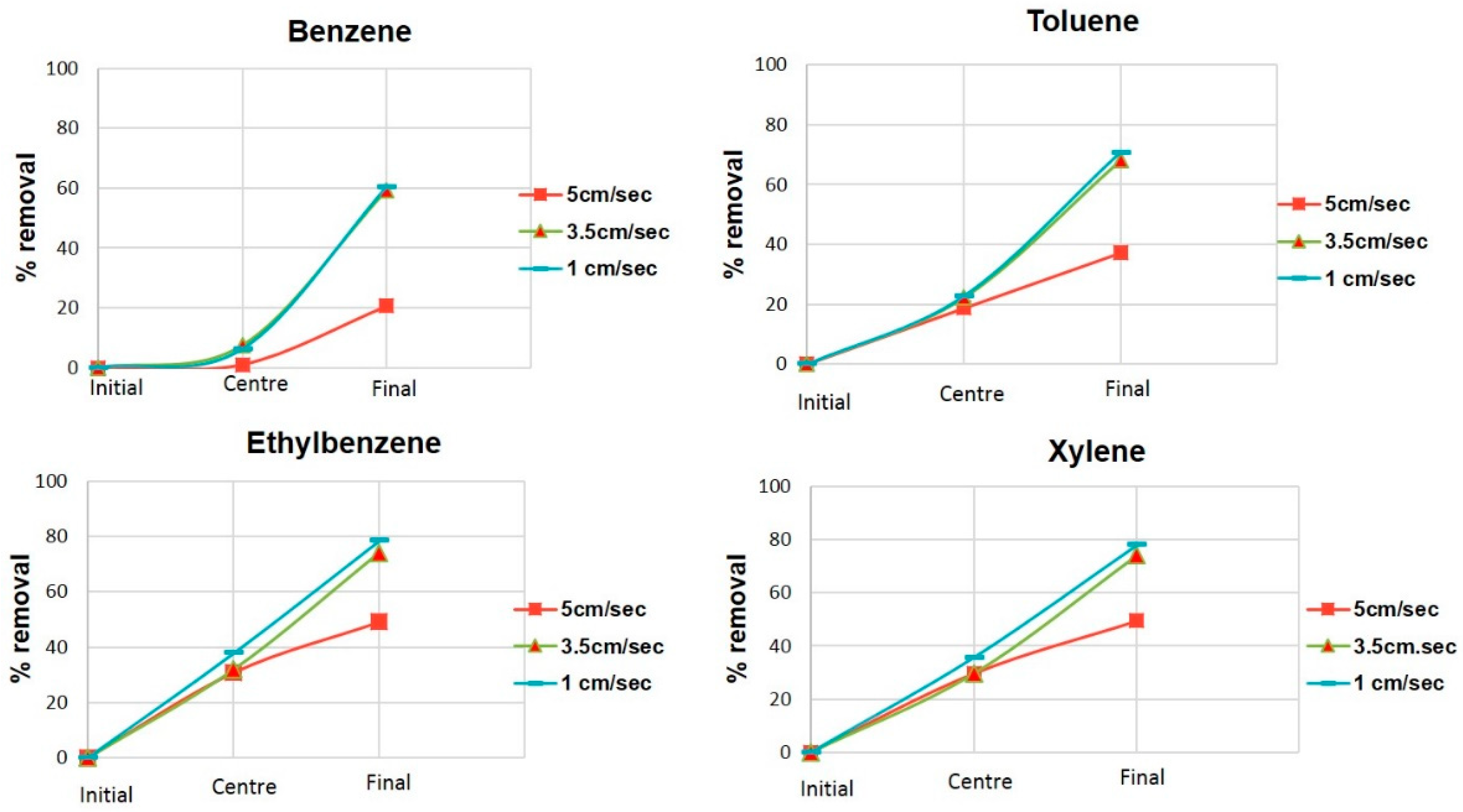
3.3. Semi Pilot Plant Operation: Linear Velocity Optimization
3.4. Blank Test of Semi Pilot Reactor
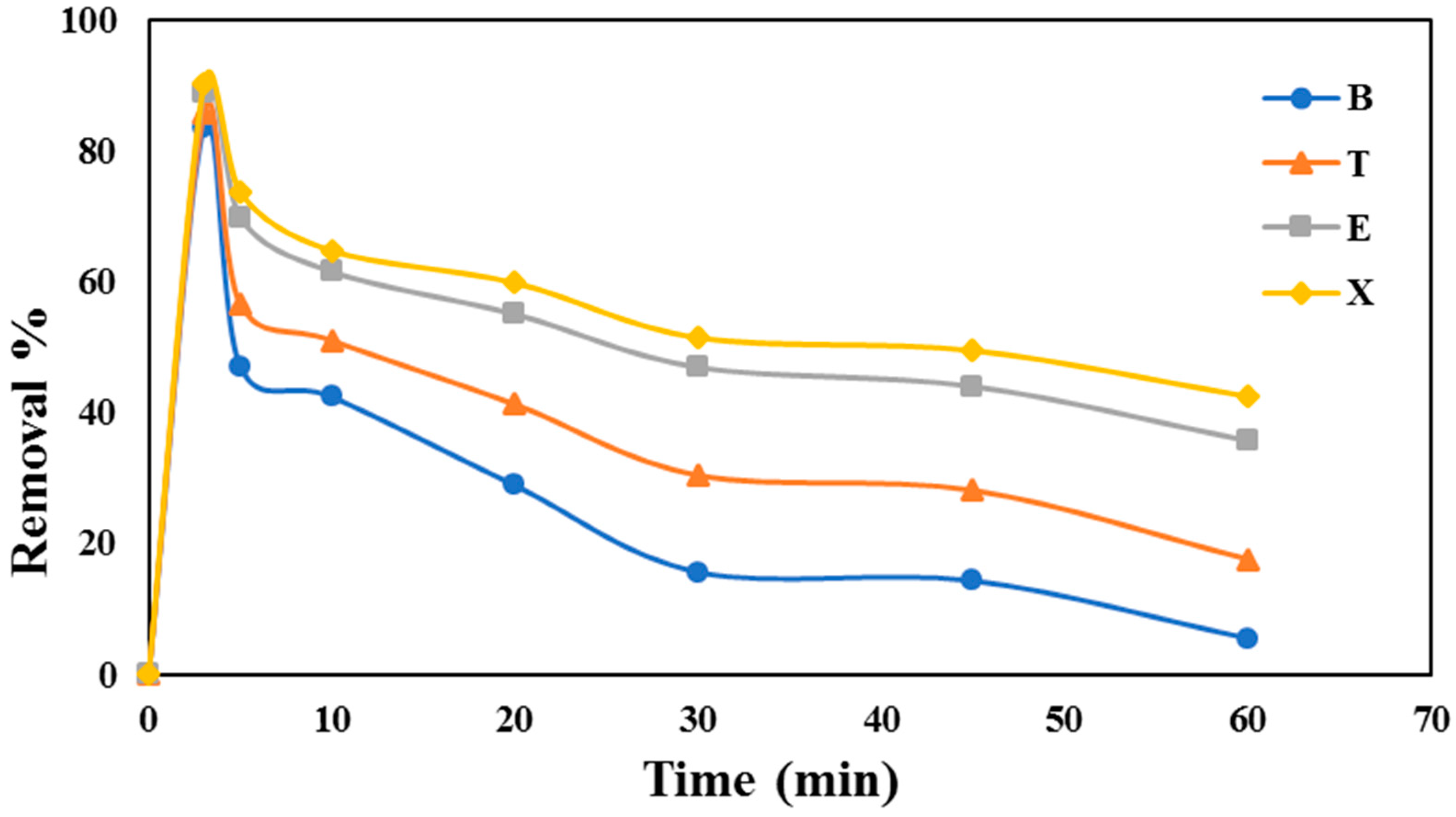
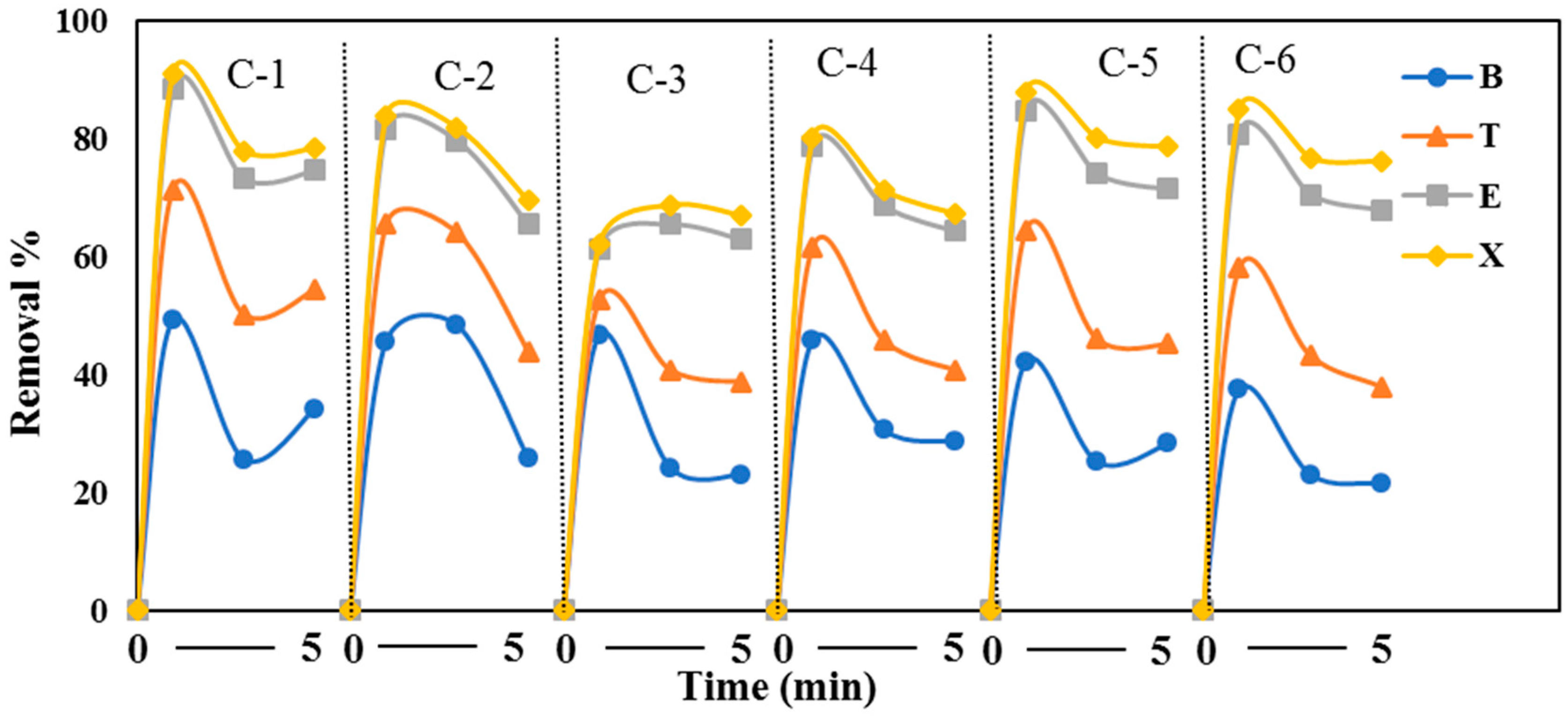
3.5. Effect of UV Light on the Removal of VOCs
3.6. Surface Area Loading Rate (SLR) and Surface Retention Time (SRT)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Acronyms
| ZSM-5 | zeolite socony mobil-5 |
| Fe-ZSM-5 | iron zeolite socony mobil-5 |
| VOCs | volatile organic compounds |
| BTEX | benzene, toluene, ethylbenzene, and xylene |
| SLR | surface loading rate |
| SRT | surface retention time |
References
- Li, J.; He, H.; Hu, C.; Zhao, J. The abatement of major pollutants in air and water by environmental catalysis. Front. Environ. Sci. Eng. 2013, 7, 302–325. [Google Scholar] [CrossRef]
- Qu, F.; Zhu, L.; Yang, K. Adsorption behaviors of volatile organic compounds (VOCs) on porous clay heterostructures (PCH). J. Hazard. Mater. 2009, 170, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Dai, J.; Liu, F.; Bao, J.; Wang, Y.; Yang, Y.; Yang, Y.; Zhao, D. Photocatalytic oxidation of indoor toluene: Process risk analysis and influence of relative humidity, photocatalysts, and VUV irradiation. Sci. Total Environ. 2012, 438, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ang, H.M.; Tade, M.O. Volatile organic compounds in indoor environment and photocatalytic oxidation: State of the art. Environ. Int. 2007, 33, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Choi, N. Fabrication of functional polyurethane/rare earth nanocomposite membranes by electrospinning and its VOCs absorption capacity from air. Nanomaterials 2017, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Weisel, C.P.; Alimokhtari, S.; Sanders, P.F. Indoor air VOC concentrations in suburban and rural New Jersey. Environ. Sci. Technol. 2008, 42, 8231–8238. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.S.; Pennisi, S.V.; Son, K.-C.; Kays, S.J. Screening indoor plants for volatile organic pollutant removal efficiency. HortScience 2009, 44, 1377–1381. [Google Scholar]
- Ikhlaq, A.; Brown, D.R.; Kasprzyk-Hordern, B. Mechanisms of catalytic ozonation on alumina and zeolites in water: Formation of hydroxyl radicals. Appl. Catal. B Environ. 2012, 123–124, 94–106. [Google Scholar] [CrossRef]
- Zhong, L.; Haghighat, F.; Lee, C.-S.; Lakdawala, N. Performance of ultraviolet photocatalytic oxidation for indoor air applications: Systematic experimental evaluation. J. Hazard. Mater. 2013, 261, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Haghighat, F. Photocatalytic air cleaners and materials technologies—Abilities and limitations. Build. Environ. 2015, 91, 191–203. [Google Scholar] [CrossRef]
- Zhong, L.; Brancho, J.J.; Batterman, S.; Bartlett, B.M.; Godwin, C. Experimental and modeling study of visible light responsive photocatalytic oxidation (PCO) materials for toluene degradation. Appl. Catal. B Environ. 2017, 216, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, M.; Hidaka, M.; Anpo, M. Efficient removal of toluene and benzene in gas phase by the TiO2/Y-zeolite hybrid photocatalyst. J. Hazard. Mater. 2012, 237–238, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Gallastegi-Villa, M.; Aranzabal, A.; González-Marcos, J.A.; González-Velasco, J.R. Metal-loaded ZSM5 zeolites for catalytic purification of dioxin/furans and NOx containing exhaust gases from MWI plants: Effect of different metal cations. Appl. Catal. B Environ. 2016, 184, 238–245. [Google Scholar] [CrossRef]
- Huang, Y.; Ho, S.; Lu, Y.; Niu, R.; Xu, L.; Cao, J.; Lee, S. Removal of indoor volatile organic compounds via photocatalytic oxidation: A short review and prospect. Molecules 2016, 21, 56. [Google Scholar] [CrossRef] [PubMed]
- Mamaghani, A.H.; Haghighat, F.; Lee, C.-S. Photocatalytic oxidation technology for indoor environment air purification: The state-of-the-art. Appl. Catal. B Environ. 2017, 203, 247–269. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, K.; Yang, Y.; Liu, H.; Qiao, Z.; Luo, H. Synthesis of mesoporous Al2O3 with large surface area and large pore diameter by improved precipitation method. Microporous Mesoporous Mater. 2014, 193, 47–53. [Google Scholar] [CrossRef]
- Li, R.; Jia, Y.; Bu, N.; Wu, J.; Zhen, Q. Photocatalytic degradation of methyl blue using Fe2O3/TiO2 composite ceramics. J. Alloys Compd. 2015, 643, 88–93. [Google Scholar] [CrossRef]
- Tokumura, M.; Nakajima, R.; Znad, H.T.; Kawase, Y. Chemical absorption process for degradation of VOC gas using heterogeneous gas–liquid photocatalytic oxidation: Toluene degradation by photo-Fenton reaction. Chemosphere 2008, 73, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Özcan, A.; Atılır Özcan, A.; Demirci, Y.; Şener, E. Preparation of Fe2O3 modified kaolin and application in heterogeneous electro-catalytic oxidation of enoxacin. Appl. Catal. B Environ. 2017, 200, 361–371. [Google Scholar] [CrossRef]
- Lv, Q.; Li, G.; Lu, H.; Cai, W.; Huang, H.; Cheng, C. Preparation of magnetic zeolite γ-Fe2O3/TS-1 with core/shell structure and application in photocatalytic degradation. Microporous Mesoporous Mater. 2015, 203, 202–207. [Google Scholar] [CrossRef]
- Aziz, A.; Kim, S.; Kim, K.S. Fe/ZSM-5 zeolites for organic-pollutant removal in the gas phase: Effect of the iron source and loading. J. Environ. Chem. Eng. 2016, 4, 3033–3040. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Cha, D.; Zhang, X.; Basset, J.M. High-surface-area silica nanospheres (KCC-1) with a fibrous morphology. Angew. Chem. Int. Ed. 2010, 49, 9652–9656. [Google Scholar] [CrossRef] [PubMed]
- Tempelman, C.H.L.; De Rodrigues, V.O.; Van Eck, E.R.H.; Magusin, P.C.M.M.; Hensen, E.J.M. Desilication and silylation of Mo/HZSM-5 for methane dehydroaromatization. Microporous Mesoporous Mater. 2015, 203, 259–273. [Google Scholar] [CrossRef]
- Bazyari, A.; Khodadadi, A.A.; Haghighat Mamaghani, A.; Beheshtian, J.; Thompson, L.T.; Mortazavi, Y. Microporous titania–silica nanocomposite catalyst-adsorbent for ultra-deep oxidative desulfurization. Appl. Catal. B Environ. 2016, 180, 65–77. [Google Scholar] [CrossRef]
- Blanch-Raga, N.; Palomares, A.E.; Martínez-Triguero, J.; Valencia, S. Cu and Co modified beta zeolite catalysts for the trichloroethylene oxidation. Appl. Catal. B Environ. 2016, 187, 90–97. [Google Scholar] [CrossRef]
- Chlala, D.; Giraudon, J.M.; Nuns, N.; Lancelot, C.; Vannier, R.-N.; Labaki, M.; Lamonier, J.F. Active Mn species well dispersed on Ca2+ enriched apatite for total oxidation of toluene. Appl. Catal. B Environ. 2016, 184, 87–95. [Google Scholar] [CrossRef]
- Aziz, A.; Park, H.; Kim, S.; Kim, K.S. Phenol and ammonium removal by using Fe-ZSM-5 synthesized by ammonium citrate iron source. Int. J. Environ. Sci. Technol. 2016, 13, 2805–2816. [Google Scholar]
- Aziz, A.; Kim, K.S. Synergistic effect of UV pretreated Fe-ZSM-5 catalysts for heterogeneous catalytic complete oxidation of VOC: A technology development for sustainable use. J. Hazard. Mater. 2017, 340, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.; Kim, S.; Kim, K.S. Heterogeneous oxidation of organics from wastewater: Fe-ZSM-5 prepared by different silicon sources. Desalin. Water Treat. 2017, 58, 385–390. [Google Scholar] [CrossRef]
- Aziz, A.; Kim, K. Investigation of tertiary butyl alcohol as template for the synthesis of ZSM-5 zeolite. J. Porous Mater. 2015, 22, 1401–1406. [Google Scholar] [CrossRef]
- Scott Fogler, H. Elements of Chemical Reaction Engineering, 4th ed.; Prentice Hall PTR: Upper Saddle River, NJ, USA, 2005; ISBN 0-13-047394-4. [Google Scholar]
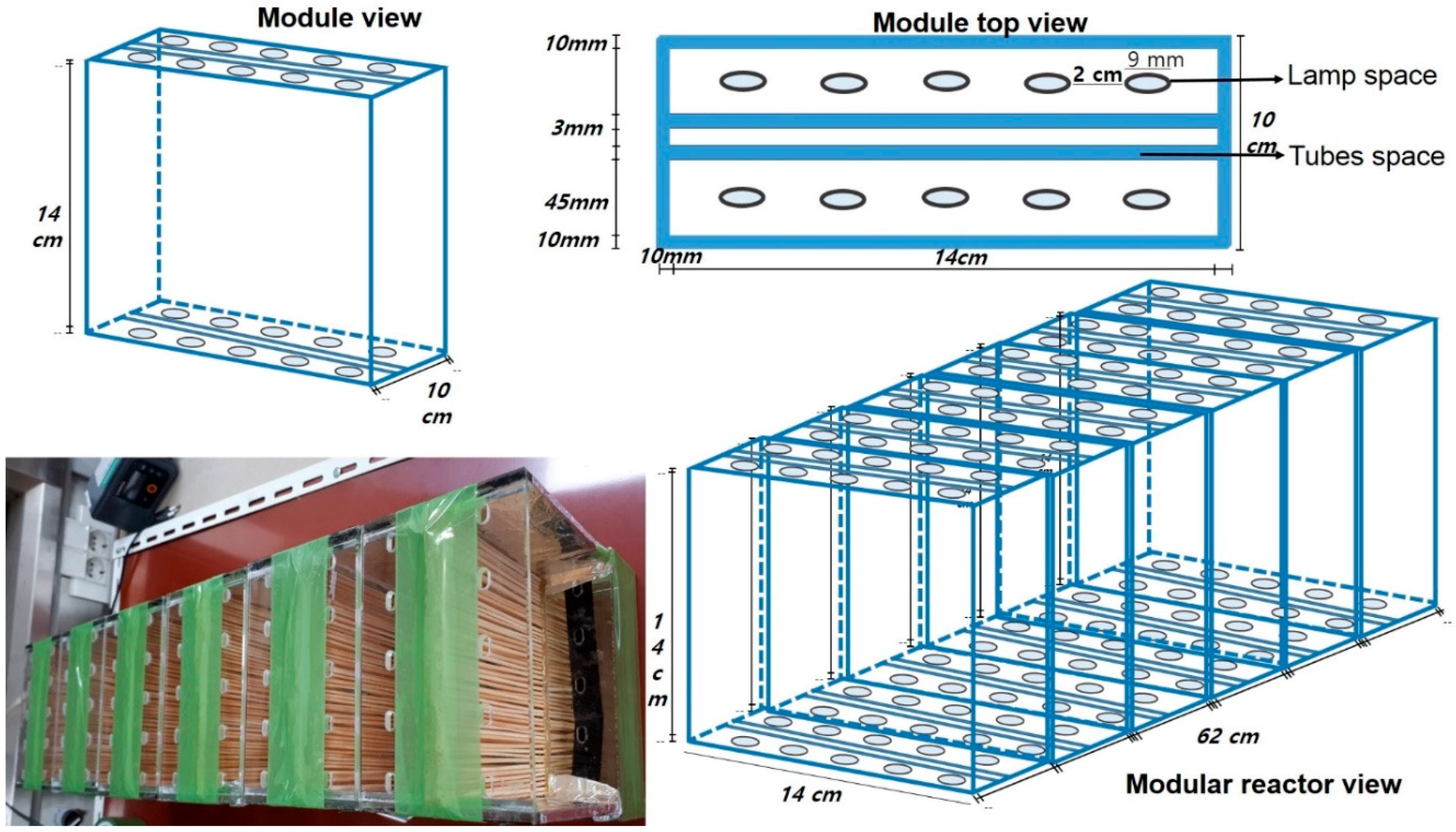


| Reactor Parameters | Value |
|---|---|
| Reactor length | 62 cm |
| Reactor width | 14 cm |
| Reactor height | 14 cm |
| Reactor volume | 12,152 cm3 = 0.0122 m3 = 12.1520 L |
| Number of modules in reactor | 06 |
| Module width | 14 cm |
| Module height | 14 cm |
| No. of tubes in one module (in two rows) | 56 × 2 = 112 |
| Total number of tubes in reactor | 6 × 112 = 672 |
| Tube length (effective) | 14 cm |
| Tube diameter | 0.2000 cm |
| Surface area of one tube () | 8.86 cm2 = 0.0009 m2 |
| Total surface area of all tubes | 5953.92 cm2 = 0.5954 m2 |
| Number of UV lamps in one box (two rows) | 2 × 5 = 10 |
| Length of UV lamp (effective) | 14 cm |
| Total number of lamps in reactor | 60 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aziz, A.; Sajjad, M.; Kim, S.; Saifuddin, M.; Kim, K.S. Development of Sustainable Technology to Mitigate VOCs Pollution from Air Using a Continuous System Composed of Fe-ZSM-5 Coated on Polypropylene Tubes Coupled with UV Irradiation at the Pilot Scale. Appl. Sci. 2018, 8, 1920. https://doi.org/10.3390/app8101920
Aziz A, Sajjad M, Kim S, Saifuddin M, Kim KS. Development of Sustainable Technology to Mitigate VOCs Pollution from Air Using a Continuous System Composed of Fe-ZSM-5 Coated on Polypropylene Tubes Coupled with UV Irradiation at the Pilot Scale. Applied Sciences. 2018; 8(10):1920. https://doi.org/10.3390/app8101920
Chicago/Turabian StyleAziz, Abdul, Muhammad Sajjad, Suho Kim, Md Saifuddin, and Kwang Soo Kim. 2018. "Development of Sustainable Technology to Mitigate VOCs Pollution from Air Using a Continuous System Composed of Fe-ZSM-5 Coated on Polypropylene Tubes Coupled with UV Irradiation at the Pilot Scale" Applied Sciences 8, no. 10: 1920. https://doi.org/10.3390/app8101920
APA StyleAziz, A., Sajjad, M., Kim, S., Saifuddin, M., & Kim, K. S. (2018). Development of Sustainable Technology to Mitigate VOCs Pollution from Air Using a Continuous System Composed of Fe-ZSM-5 Coated on Polypropylene Tubes Coupled with UV Irradiation at the Pilot Scale. Applied Sciences, 8(10), 1920. https://doi.org/10.3390/app8101920




