Triple Planar Heterojunction of SnO2/WO3/BiVO4 with Enhanced Photoelectrochemical Performance under Front Illumination
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis
2.2. Materials Characterization
2.3. Photoelectrochemical Characterization
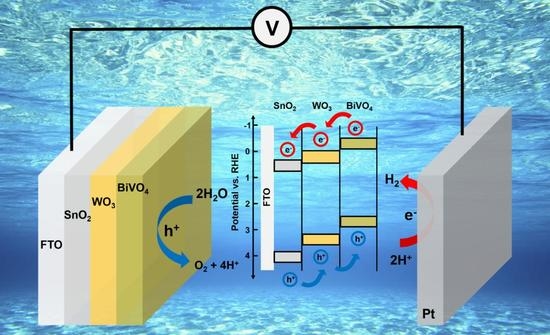
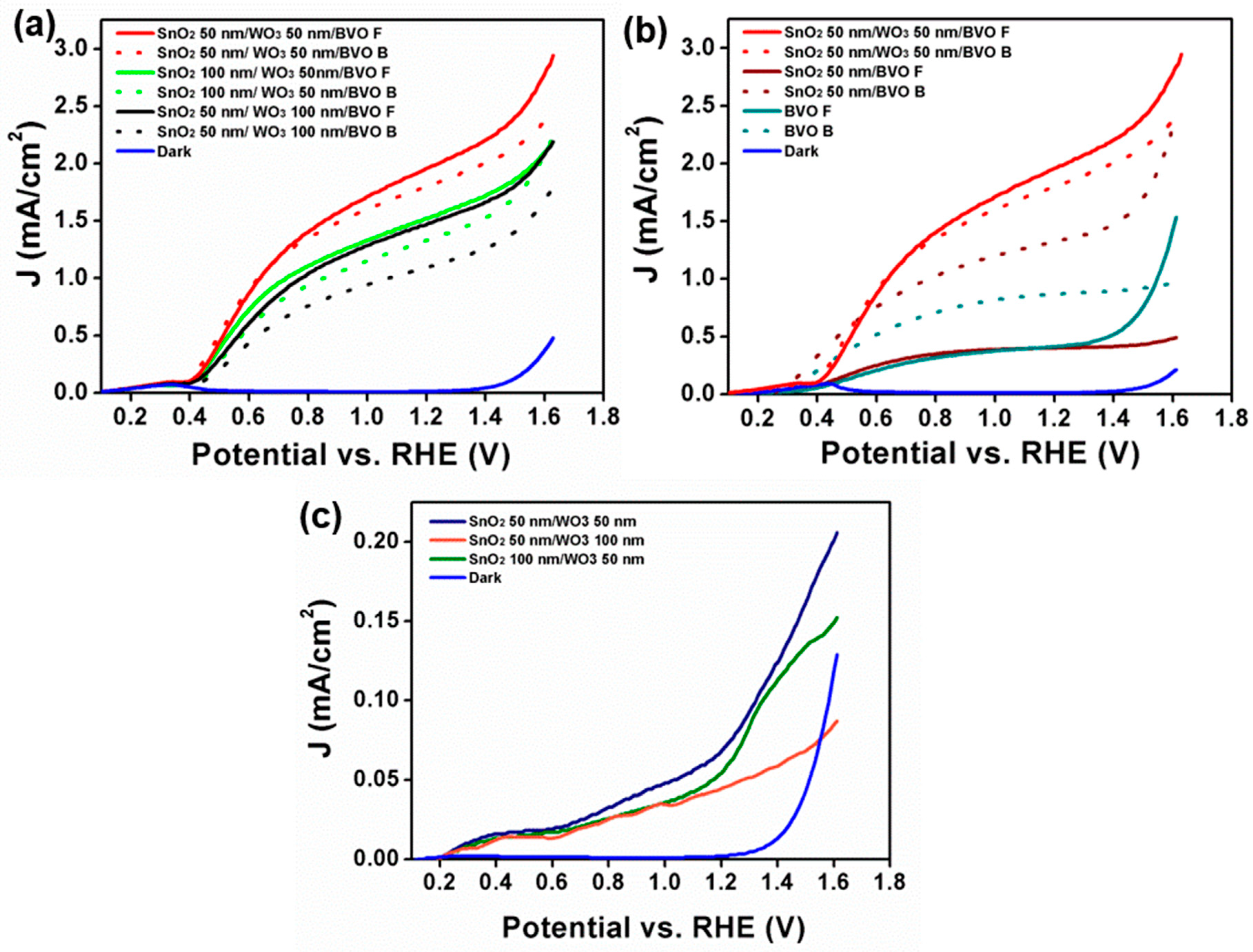
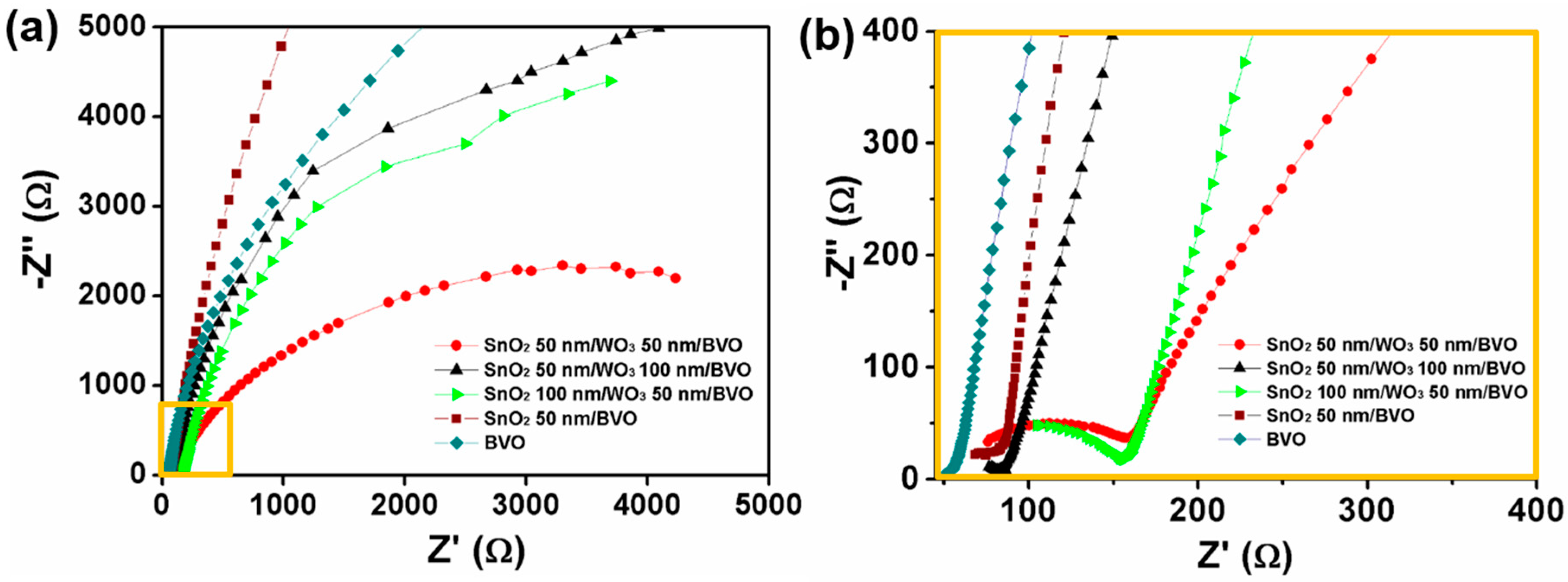
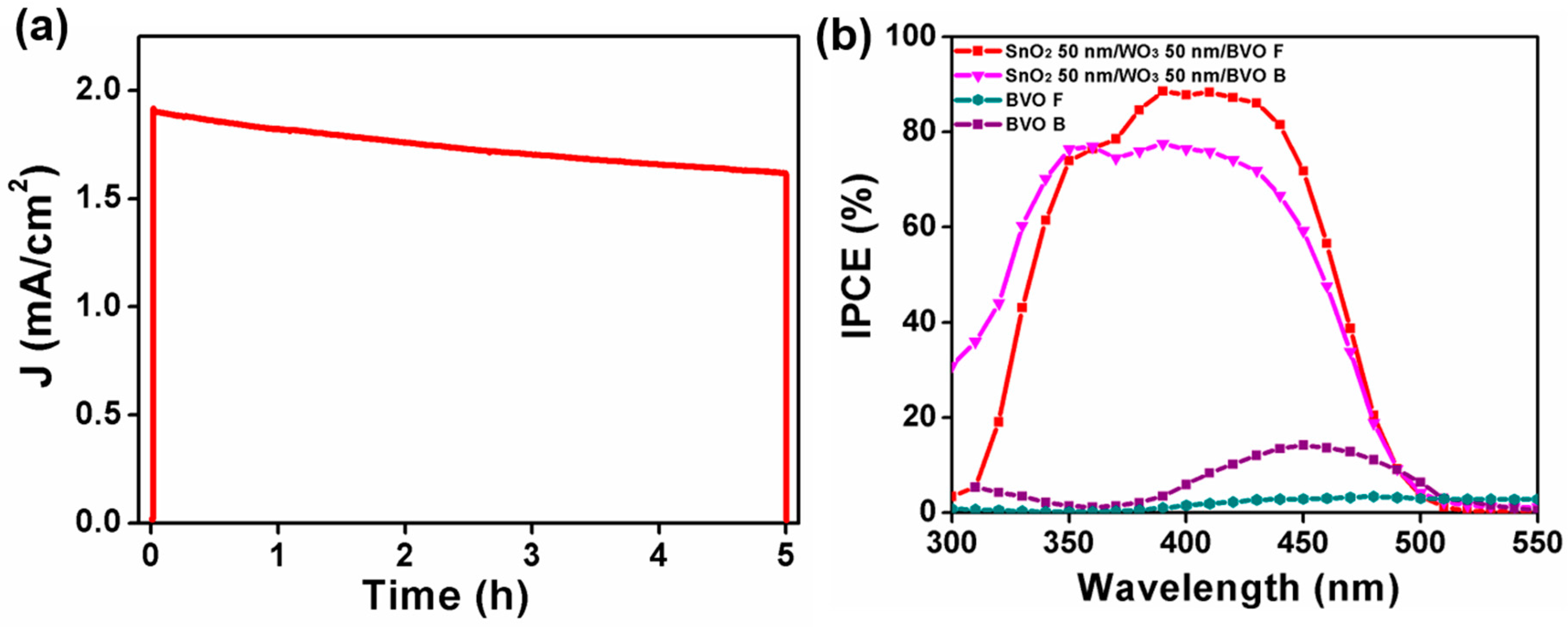
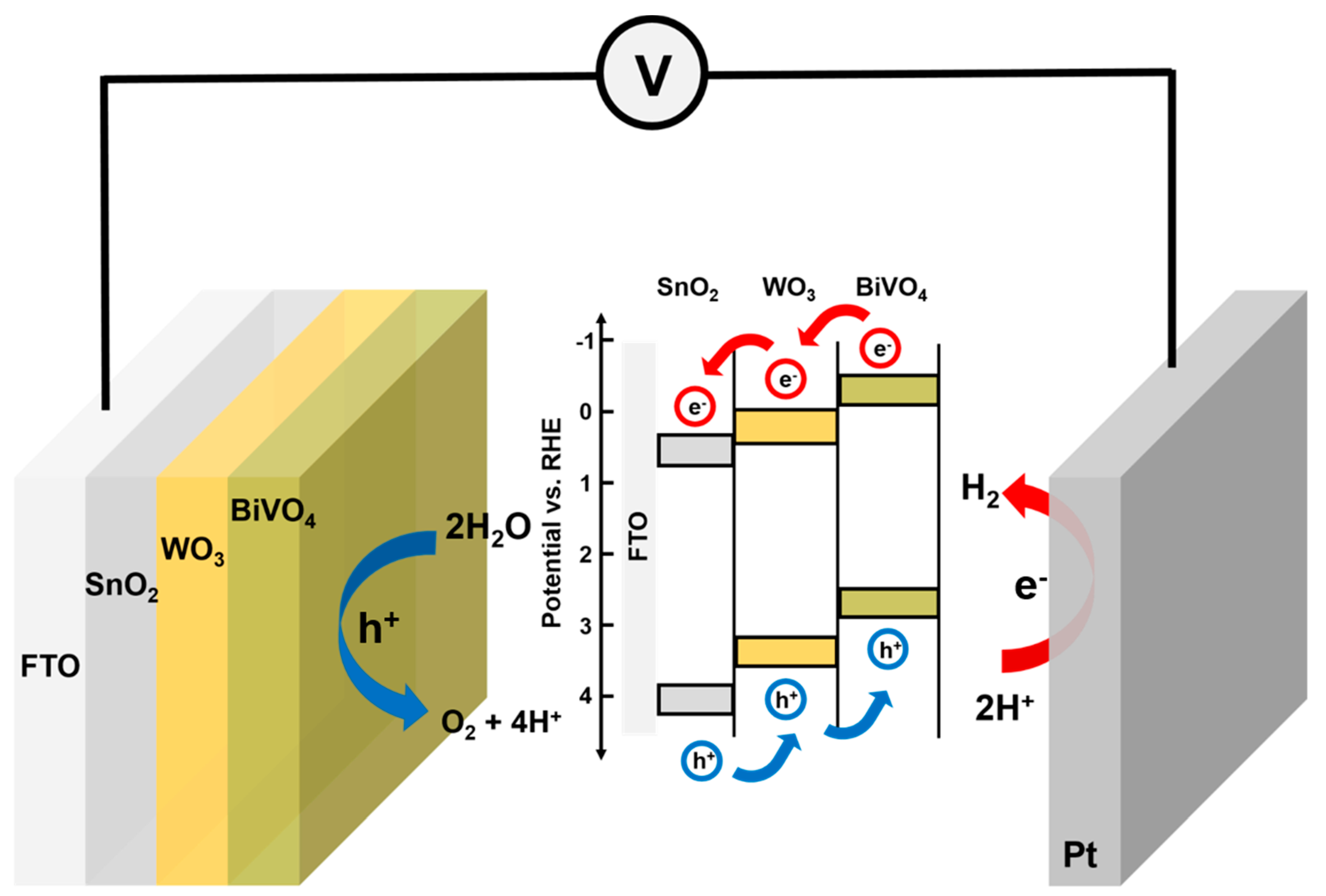
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yamamoto, M.; Tanaka, K. Artificial Molecular Photosynthetic Systems: Towards Efficient Photoelectrochemical Water Oxidation. ChemPlusChem 2016, 81, 1028–1044. [Google Scholar] [CrossRef]
- Xing, J.; Fang, W.Q.; Zhao, H.J.; Yang, H.G. Inorganic photocatalysts for overall water splitting. Chem. Asian J. 2012, 7, 642–657. [Google Scholar] [CrossRef] [PubMed]
- Takanabe, K.; Domen, K. Preparation of Inorganic Photocatalytic Materials for Overall Water Splitting. ChemCatChem 2012, 4, 1485–1497. [Google Scholar] [CrossRef]
- Chen, Z.; Dinh, H.N.; Miller, E. Photoelectrochemical Water Splitting Standards, Experimental Methods and Protocols; Springer: Berlin, Germany, 2013. [Google Scholar]
- Tamirat, A.G.; Rick, J.; Dubale, A.A.; Su, W.-N.; Hwang, B.-J. Using hematite for photoelectrochemical water splitting: A review of current progress and challenges. Nanoscale Horiz. 2016, 1, 243–267. [Google Scholar] [CrossRef]
- Moniz, S.J.A.; Shevlin, S.A.; Martin, D.J.; Guo, Z.-X.; Tang, J. Visible-light driven heterojunction photocatalysts for water splitting-a critical review. Energy Environ. Sci. 2015, 8, 731–759. [Google Scholar] [CrossRef]
- Sivula, K.; van de Krol, R. Semiconducting materials for photoelectrochemical energy conversion. Nat. Rev. Mater. 2016, 15010. [Google Scholar] [CrossRef]
- Bhat, S.S.M.; Jang, H.W. Recent Advances in Bismuth-Based Nanomaterials for Photoelectrochemical Water Splitting. ChemSusChem 2017, 10, 3001–3018. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Andoshe, D.M.; Shim, Y.S.; Moon, C.W.; Sohn, W.; Choi, S.; Kim, T.L.; Lee, M.; Park, H.; Hong, K.; et al. Toward High-Performance Hematite Nanotube Photoanodes: Charge-Transfer Engineering at Heterointerfaces. ACS Appl. Mater. Interfaces 2016, 8, 23793–23800. [Google Scholar] [CrossRef] [PubMed]
- Walsh, A.; Yan, Y.; Huda, M.N.; Al-Jassim, M.M.; Wei, S.-H.H. Band Edge Electronic Structure of BiVO4: Elucidating the Role of the Bi s and V d Orbitals. Chem. Mater. 2009, 21, 547–551. [Google Scholar] [CrossRef]
- Abdi, F.F.; Van De Krol, R. Nature and light dependence of bulk recombination in Co-Pi-catalyzed BiVO4 photoanodes. J. Phys. Chem. C 2012, 116, 9398–9404. [Google Scholar] [CrossRef]
- Abdi, F.F.; Savenije, T.J.; May, M.M.; Dam, B.; Van De Krol, R. The origin of slow carrier transport in BiVO4 thin film photoanodes: A time-resolved microwave conductivity study. J. Phys. Chem. Lett. 2013, 4, 2752–2757. [Google Scholar] [CrossRef]
- Seabold, J.A.; Zhu, K.; Neale, N.R. Efficient solar photoelectrolysis by nanoporous Mo: BiVO4 through controlled electron transport. Phys. Chem. Chem. Phys. 2014, 16, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.W.; Jeon, T.H.; Jang, J.S.; Choi, W.; Park, H. Strategic modification of BiVO4 for improving photoelectrochemical water oxidation performance. J. Phys. Chem. C 2013, 117, 9104–9112. [Google Scholar] [CrossRef]
- Shi, X.; Choi, I.Y.; Zhang, K.; Kwon, J.; Kim, D.Y.; Lee, J.K.; Oh, S.H.; Kim, J.K.; Park, J.H. Efficient photoelectrochemical hydrogen production from bismuth vanadate-decorated tungsten trioxide helix nanostructures. Nat. Commun. 2014, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhong, D.K.; Choi, S.; Gamelin, D.R. Near-complete suppression of surface recombination in solar photoelectrolysis by “Co-Pi” catalyst-modified W: BiVO4. J. Am. Chem. Soc. 2011, 133, 18370–18377. [Google Scholar] [CrossRef] [PubMed]
- Butler, K.T.; Dringoli, B.J.; Zhou, L.; Rao, P.M.; Walsh, A.; Titova, L.V. Ultrafast carrier dynamics in BiVO4 thin film photoanode material: Interplay between free carriers, trapped carriers and low-frequency lattice vibrations. J. Mater. Chem. A 2016, 4, 18516–18523. [Google Scholar] [CrossRef]
- Liu, T.; Pasumarthi, V.; Laporte, C.; Feng, Z.; Li, Q.; Yang, J.; Li, C.; Dupuis, M. Bimodal hole transport in bulk BiVO4 from computation. J. Mater. Chem. A 2018, 6, 3714–3723. [Google Scholar] [CrossRef]
- Bornoz, P.; Abdi, F.F.; Tilley, S.D.; Dam, B.; Van De Krol, R.; Graetzel, M.; Sivula, K. A bismuth vanadate-cuprous oxide tandem cell for overall solar water splitting. J. Phys. Chem. C 2014, 118, 16959–16966. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, D.; Zhang, X.; Chen, Y.; Kong, L.; Chen, P.; Wang, Y.; Wang, C.; Wang, L.; Liu, Y. Enhanced photoelectrochemical performance of nanoporous BiVO4 photoanode by combining surface deposited cobalt-phosphate with hydrogenation treatment. Electrochim. Acta 2016, 195, 51–58. [Google Scholar] [CrossRef]
- Chae, S.Y.; Lee, C.S.; Jung, H.; Joo, O.-S.; Min, B.K.; Kim, J.H.; Hwang, Y.J. Insight into Charge Separation in WO3/BiVO4 Heterojunction for Solar Water Splitting. ACS Appl. Mater. Interfaces 2017, 9, 19780–19790. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Jia, Q.; Nishiyama, H.; Yamada, T.; Kudo, A.; Domen, K. A front-illuminated nanostructured transparent BiVO4 photoanode for >2% efficient water splitting. Adv. Energy Mater. 2016, 6, 2–8. [Google Scholar] [CrossRef]
- Chen, L.; Alarcon-Llado, E.; Hettick, M.; Sharp, I.D.; Lin, Y.; Javey, A.; Ager, J.W. Reactive sputtering of bismuth vanadate photoanodes for solar Water splitting. J. Phys. Chem. C 2013, 117, 21635–21642. [Google Scholar] [CrossRef]
- Liang, Y.; Tsubota, T.; Mooij, L.P.A.; Van De Krol, R. Highly improved quantum efficiencies for thin film BiVO4 photoanodes. J. Phys. Chem. C 2011, 115, 17594–17598. [Google Scholar] [CrossRef]
- Chae, S.Y.; Jung, H.; Jeon, H.S.; Min, B.K.; Hwang, Y.J.; Joo, O.-S. Morphology control of one-dimensional heterojunctions for highly efficient photoanodes used for solar water splitting. J. Mater. Chem. A 2014, 2, 11408. [Google Scholar] [CrossRef]
- Byun, S.; Kim, B.; Jeon, S.; Shin, B. Effects of a SnO2 hole blocking layer in a BiVO4-based photoanode on photoelectrocatalytic water oxidation. J. Mater. Chem. A 2017, 5, 6905–6913. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, Y.; Zhang, J.; Rao, P.M. Photoanode with Enhanced Performance Achieved by Coating BiVO4 onto ZnO-Templated Sb-Doped SnO2 Nanotube Scaffold. ACS Appl. Mater. Interfaces 2017, 9, 11356–11362. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhao, C.; Giri, B.; Allen, P.; Xu, X.; Joshi, H.; Fan, Y.; Titova, L.V.; Rao, P.M. High Light Absorption and Charge Separation Efficiency at Low Applied Voltage from Sb-Doped SnO2/BiVO4 Core/Shell Nanorod-Array Photoanodes. Nano Lett. 2016, 16, 3463–3474. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.S.M.; Suh, J.M.; Choi, S.; Hong, S.-P.; Lee, S.A.; Kim, C.; Moon, C.W.; Lee, M.G.; Jang, H.W. Substantially enhanced front illumination photocurrent in porous SnO2 nanorods/networked BiVO4 heterojunction photoanodes. J. Mater. Chem. A 2018. [Google Scholar] [CrossRef]
- Baek, J.H.; Kim, B.J.; Han, G.S.; Hwang, S.W.; Kim, D.R.; Cho, I.S.; Jung, H.S. BiVO4/WO3/SnO2 double-heterojunction photoanode with enhanced charge separation and visible-transparency for bias-free solar water-splitting with a perovskite solar cell. ACS Appl. Mater. Interfaces 2017, 9, 1479–1487. [Google Scholar] [CrossRef] [PubMed]
- Kamimura, J.; Bogdanoff, P.; Abdi, F.F.; Lähnemann, J.; Van De Krol, R.; Riechert, H.; Geelhaar, L. Photoelectrochemical Properties of GaN Photoanodes with Cobalt Phosphate Catalyst for Solar Water Splitting in Neutral Electrolyte. J. Phys. Chem. C 2017, 121, 12540–12545. [Google Scholar] [CrossRef]
- Lee, D.K.; Choi, K.-S. Enhancing long-term photostability of BiVO4 photoanodes for solar water splitting by tuning electrolyte composition. Nat. Energy 2017, 3, 53–60. [Google Scholar] [CrossRef]
- Grigioni, I.; Corti, A.; Dozzi, M.V.; Selli, E. Photoactivity and Stability of WO3/BiVO4 Photoanodes: Effects of the Contact Electrolyte and of Ni/Fe Oxyhydroxide Protection. J. Phys. Chem. C 2018, 122, 13969–13978. [Google Scholar] [CrossRef]
- Kalanur, S.S.; Yoo, I.H.; Park, J.; Seo, H. Insights into the electronic bands of WO3/BiVO4/TiO2, revealing high solar water splitting efficiency. J. Mater. Chem. A 2017, 5, 1455–1461. [Google Scholar] [CrossRef]
- Grigioni, I.; Stamplecoskie, K.G.; Selli, E.; Kamat, P.V. Dynamics of Photogenerated Charge Carriers in WO3/BiVO4 Heterojunction Photoanodes. J. Phys. Chem. C 2015, 119, 20792–20800. [Google Scholar] [CrossRef]
- Rao, P.M.; Cai, L.; Liu, C.; Cho, I.S.; Lee, C.H.; Weisse, J.M.; Yang, P.; Zheng, X. Simultaneously efficient light absorption and charge separation in WO3/BiVO4 Core/shell nanowire photoanode for photoelectrochemical water oxidation. Nano Lett. 2014, 14, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, L.; Lin, L.; Wygant, B.R.; Liu, Y.; Zhu, Y.; Zheng, Y.; Mullins, C.B.; Zhao, Y.; Zhang, X.; et al. Highly Efficient Photoelectrochemical Water Splitting from Hierarchical WO3/BiVO4 Nanoporous Sphere Arrays. Nano Lett. 2017, 17, 8012–8017. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Tang, R.; Zhang, L.; Yin, L. Au Nanoparticles coupled Three-dimensional Macroporous BiVO4/SnO2 Inverse Opal Heterostructure for Efficient Photoelectrochemical Water Splitting. Electrochim. Acta 2017, 248, 593–602. [Google Scholar] [CrossRef]
- Chen, S.; Yang, J.; Wu, J. Three-Dimensional Undoped Crystalline SnO2 Nanodendrite Arrays Enable Efficient Charge Separation in BiVO4/SnO2 Heterojunction Photoanodes for Photoelectrochemical Water Splitting. ACS Appl. Energy Mater. 2018, 1, 2143–2149. [Google Scholar] [CrossRef]
- Resasco, J.; Zhang, H.; Kornienko, N.; Becknell, N.; Lee, H.; Guo, J.; Briseno, A.L.; Yang, P. TiO2/BiVO4 Nanowire Heterostructure Photoanodes Based on Type II Band Alignment. ACS Cent. Sci. 2016, 2, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.J.; Lee, S.; Jang, J.S.; Lee, J.S. Heterojunction BiVO4/WO3 electrodes for enhanced photoactivity of water oxidation. Energy Environ. Sci. 2011, 4, 1781. [Google Scholar] [CrossRef]
- Chatchai, P.; Murakami, Y.; Kishioka, S.; Nosaka, A.Y.; Nosaka, Y. Efficient photocatalytic activity of water oxidation over WO3/BiVO4 composite under visible light irradiation. Electrochim. Acta 2009, 54, 1147–1152. [Google Scholar] [CrossRef]
- Shi, X.; Herraiz-Cardona, I.; Bertoluzzi, L.; Lopez-Varo, P.; Bisquert, J.; Park, J.H.; Gimenez, S. Understanding the synergistic effect of WO3–BiVO4 heterostructures by impedance spectroscopy. Phys. Chem. Chem. Phys. 2016, 18, 9255–9261. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhat, S.S.M.; Lee, S.A.; Suh, J.M.; Hong, S.-P.; Jang, H.W. Triple Planar Heterojunction of SnO2/WO3/BiVO4 with Enhanced Photoelectrochemical Performance under Front Illumination. Appl. Sci. 2018, 8, 1765. https://doi.org/10.3390/app8101765
Bhat SSM, Lee SA, Suh JM, Hong S-P, Jang HW. Triple Planar Heterojunction of SnO2/WO3/BiVO4 with Enhanced Photoelectrochemical Performance under Front Illumination. Applied Sciences. 2018; 8(10):1765. https://doi.org/10.3390/app8101765
Chicago/Turabian StyleBhat, Swetha S. M., Sol A Lee, Jun Min Suh, Seung-Pyo Hong, and Ho Won Jang. 2018. "Triple Planar Heterojunction of SnO2/WO3/BiVO4 with Enhanced Photoelectrochemical Performance under Front Illumination" Applied Sciences 8, no. 10: 1765. https://doi.org/10.3390/app8101765
APA StyleBhat, S. S. M., Lee, S. A., Suh, J. M., Hong, S.-P., & Jang, H. W. (2018). Triple Planar Heterojunction of SnO2/WO3/BiVO4 with Enhanced Photoelectrochemical Performance under Front Illumination. Applied Sciences, 8(10), 1765. https://doi.org/10.3390/app8101765