Eliminating Light-Induced Degradation in Commercial p-Type Czochralski Silicon Solar Cells
Abstract
1. Introduction
2. Alternative Silicon Materials to Avoid Boron-Oxygen (B-O) Light-Induced Degradation (LID)
2.1. Decreasing the Interstitial Oxygen Concentration
2.2. Decreasing the Boron Doping Concentration
2.3. Additional Non-Boron Related Doping During Crystal Growth
3. Processes to Eliminate B-O LID
3.1. Thermal Processing
3.2. Annealing Incorporating Minority Carrier Injection
4. Progress in the Understanding of Illuminated B-O LID Mitigation Processes
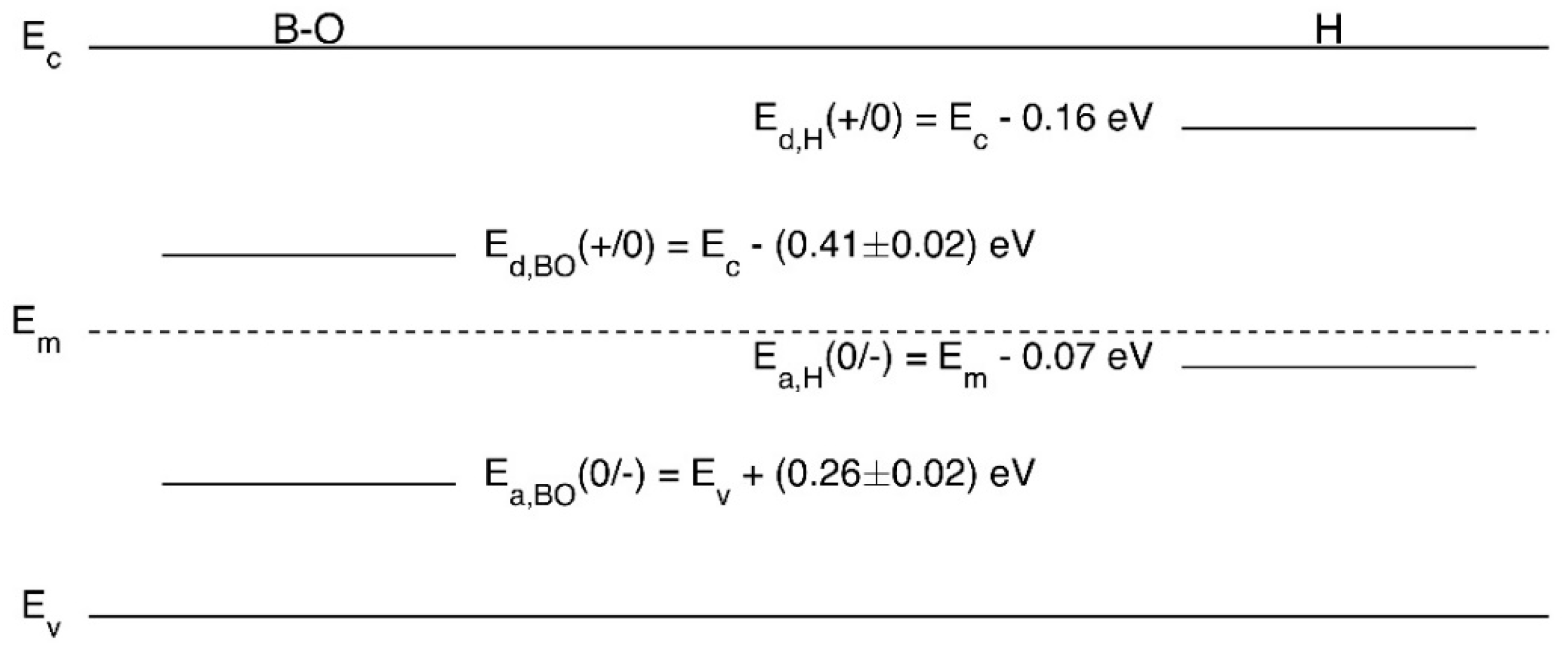
4.1. Role of Hydrogen
4.2. Manipulation of Hydrogen Charge States
4.3. Alternative Theory for Permanent Deactivation and Confusion Related to the Involvement of Hydrogen
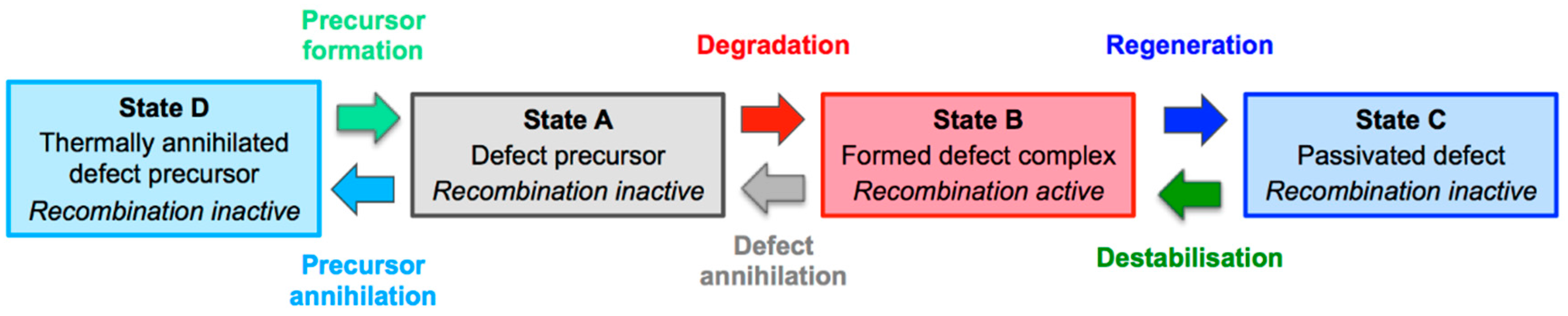
4.4. Role of Defect Formation
4.5. Rapid Laser-Based Processes for LID Elimination
5. Eliminating B-O LID during Cell and Module Fabrication
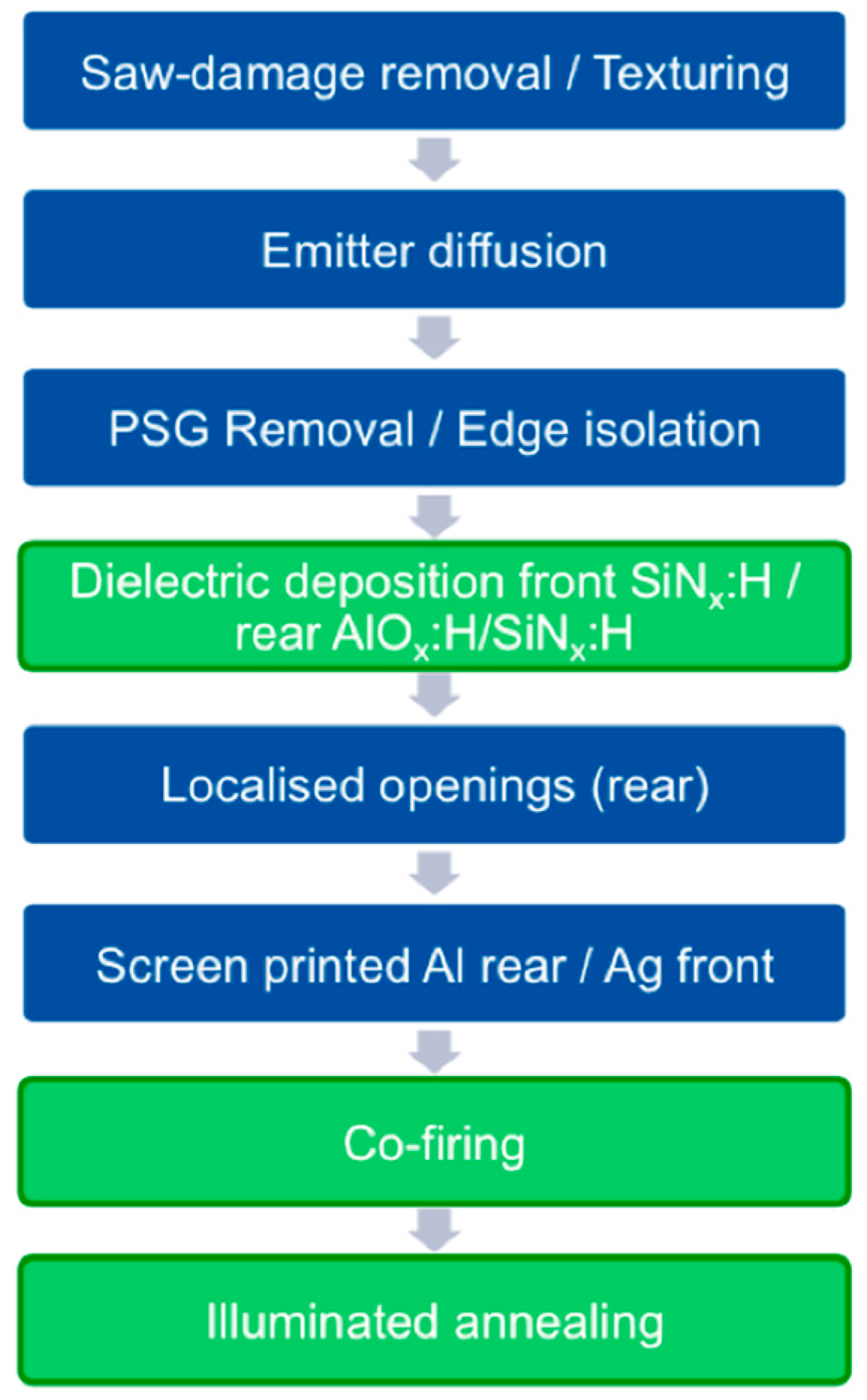
5.1. LID Mitigation during Cell Fabrication
5.2. B-O LID Mitigation during Module Encapsulation
5.3. Natural Recovery from B-O Related LID during Operation in the Field
6. Summary
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Edenhofer, O.; Pichs-Madruga, R.; Sokona, Y.; Minx, J.C.; Farahani, E.; Susanne, K.; Seyboth, K.; Adler, A.; Baum, I.; Brunner, S.; et al. Climate Change 2014: Mitigation of Climate Change; IPCC: Geneva, Switzerland, 2014; ISBN 9781107654815. [Google Scholar]
- Kuhlbrodt, T.; Rahmstorf, S.; Zickfeld, K.; Vikebø, F.B.; Sundby, S.; Hofmann, M.; Link, P.M.; Bondeau, A.; Cramer, W.; Jaeger, C. An integrated assessment of changes in the thermohaline circulation. Clim. Chang. 2009, 96, 489–537. [Google Scholar] [CrossRef]
- Hoffmann, W. PV solar electricity industry: Market growth and perspective. Sol. Energy Mater. Sol. Cells 2006, 90, 3285–3311. [Google Scholar] [CrossRef]
- Zheng, C.; Kammen, D.M. An innovation-focused roadmap for a sustainable global photovoltaic industry. Energy Policy 2014, 67, 159–169. [Google Scholar] [CrossRef]
- International Technology Roadmap for Photovoltaic: 2016 Results, 8th ed.; VDMA: Berlin, Germany, 2017.
- Fischer, H.; Pschunder, W. Investigation of photon and thermal induced changes in silicon solar cells. In Proceedings of the 10th IEEE Photovoltaic Specialists Conference, Palo Alto, CA, USA, 13–15 November 1973; pp. 404–411. [Google Scholar]
- Ramspeck, K.; Zimmermann, S.; Nagel, H.; Metz, A.; Gassenbauer, Y.; Birkmann, B.; Seidl, A. Light induced degradation of rear passivated mc-Si solar cells. In Proceedings of the 27th European Photovoltaic Solar Energy Conference, Frankfurt, Germany, 24–28 September 2012; pp. 861–865. [Google Scholar] [CrossRef]
- Schmidt, J.; Bothe, K. Structure and transformation of the metastable boron- and oxygen-related defect center in crystalline silicon. Phys. Rev. B 2004, 69, 24107. [Google Scholar] [CrossRef]
- Hallam, B.; Abbott, M.; Nærland, T.; Wenham, S. Fast and slow lifetime degradation in boron-doped Czochralski silicon described by a single defect. Phys. Status Solidi Rapid Res. Lett. 2016, 10, 520–524. [Google Scholar] [CrossRef]
- Hallam, B.; Kim, M.; Abbott, M.; Nampalli, N.; Nærland, T.; Stefani, B.; Wenham, S. Recent insights into boron-oxygen related degradation: Evidence of a single defect. Sol. Energy Mater. Sol. Cells 2017, 173, 25–32. [Google Scholar] [CrossRef]
- Voronkov, V.; Falster, R. The nature of boron-oxygen lifetime-degrading centres in silicon. Phys. Status Solidi B 2016, 13, 712–717. [Google Scholar] [CrossRef]
- Niewelt, T.; Sch, J.; Warta, W.; Glunz, S.W.; Schubert, M.C. Degradation of crystalline silicon due to boron–oxygen defects. IEEE J. Photovolt. 2017, 7, 383–398. [Google Scholar] [CrossRef]
- Knobloch, J.; Glunz, S.W.W.; Biro, D.; Warta, W.; Schaffer, E.; Wettling, W. Solar cells with efficiencies above 21% processed from Czochroalski grown silicon. In Proceedings of the 25th IEEE Photovoltaic Specialists Conference, Washington, DC, USA, 13–17 May 1996; pp. 405–408. [Google Scholar]
- Cho, E.; Lai, J.-H.; Ok, Y.; Upadhyaya, A.D.; Rohatgi, A.; Binns, M.J.; Appel, J.; Guo, J.; Fang, H.; Good, E.A. Light-induced degradation free and high efficiency p-type indium-doped PERC solar cells on Czochralski silicon. In Proceedings of the IEEE 42nd Photovoltaic Specialist Conference (PVSC), New Orleans, LA, USA, 14–19 June 2015; pp. 1–4. [Google Scholar]
- Lee, K.; Kim, M.-S.; Lim, J.-K.; Ahn, J.-H.; Hwang, M.-I.; Cho, E.-C. Natural recovery from LID: Regeneration under field conditions? In Proceedings of the 31st European Photovoltaic Solar Energy Conference and Exhibition- EU PVSEC, Hamburg, Germany, 14–18 September 2015; p. 1837. [Google Scholar]
- Hallam, B.; Bilbao, J.; Payne, D.; Chan, C.; Kim, M.; Chen, D.; Gorman, N.; Abbott, M.; Wenham, S. Modelling the long-term behaviour of boron-oxygen defect passivation in the field using Typical Meteorological Year Data (TMY2). In Proceedings of the 32nd European Photovoltaic Solar Energy Conference and Exhibition, München, Germany, 21–24 June 2016; pp. 555–559. [Google Scholar] [CrossRef]
- Hill, J. Global Solar Market Demand Expected to Reach 100 Gigawatts in 2017, Says SolarPower Europe. Available online: https://cleantechnica.com/2017/10/27/global-solar-market-demand-expected-reach-100-gw-2017-solarpower-europe/ (accessed on 6 November 2017).
- Colville, F. Forecast for PV cell production & capex: 2016–2018. In Proceedings of the Oral Presentation at PVCellTech, Kuala Lumpur, Malaysia, 16 March 2016. [Google Scholar]
- PV Insights. Available online: http://pvinsights.com (accessed on 6 November 2017).
- Glunz, S.W.; Rein, S.; Warta, W.; Knobloch, J.; Wettling, W. On the degradation of Cz-silicon solar cells. In Proceedings of the 2nd World Conference on Photovoltaic Solar Energy Conversion, Vienna, Austria, 6–10 July 1998; pp. 1343–1346. [Google Scholar]
- Glunz, S.W.; Rein, S.; Knobloch, J.; Wettling, W.; Abe, T. Comparison of boron-and gallium-doped p-type Czochralski silicon for photovoltaic application. Prog. Photovolt. Res. Appl. 1999, 7, 463–469. [Google Scholar] [CrossRef]
- Rein, S.; Diez, S.; Falster, R.; Glunz, S.W. Quantitative correlation of the metastable defect in Cz-silicon with different impurities. In Proceedings of the 3rd World Conference on Photovoltaic Energy Conversion, Osaka, Japan, 11–18 May 2003; pp. 1048–1052. [Google Scholar]
- Bothe, K.; Sinton, R.; Schmidt, J. Fundamental boron-oxygen-related carrier lifetime limit in mono-and multicrystalline silicon. Prog. Photovolt. Res. Appl. 2005, 13, 287–296. [Google Scholar] [CrossRef]
- Bothe, K.; Schmidt, J. Electronically activated boron-oxygen-related recombination centers in crystalline silicon. J. Appl. Phys. 2006, 99, 013701. [Google Scholar] [CrossRef]
- Schmidt, J.; Aberle, A.G.; Hezel, R. Investigation of carrier lifetime instabilities in Cz-grown silicon. In Proceedings of the 26th IEEE Photovoltaic Specialists Conference, Anaheim, CA, USA, 29 September–3 October 1997; pp. 13–18. [Google Scholar]
- Lim, S.Y.; Rougieux, F.E.; Macdonald, D. Boron-oxygen defect imaging in p-type Czochralski silicon. Appl. Phys. Lett. 2013, 103, 092105. [Google Scholar] [CrossRef]
- Forster, M.; Fourmond, E.; Rougieux, F.E.; Cuevas, A.; Gotoh, R.; Fujiwara, K.; Uda, S.; Lemiti, M. Boron-oxygen defect in Czochralski-silicon co-doped with gallium and boron. Appl. Phys. Lett. 2012, 100, 042110. [Google Scholar] [CrossRef]
- Lim, B.; Rougieux, F.; Macdonald, D.; Bothe, K.; Schmidt, J. Generation and annihilation of boron-oxygen-related recombination centers in compensated p-and n-type silicon. J. Appl. Phys. 2010, 108, 103722. [Google Scholar] [CrossRef]
- Macdonald, D.; Rougieux, F.; Cuevas, A.; Lim, B.; Schmidt, J.; Di Sabatino, M.; Geerligs, L.J. Light-induced boron-oxygen defect generation in compensated p-type Czochralski silicon. J. Appl. Phys. 2009, 105, 093704. [Google Scholar] [CrossRef]
- Geilker, J.; Kwapil, W.; Rein, S. Light-induced degradation in compensated p- and n-type Czochralski silicon wafers. J. Appl. Phys. 2011, 109. [Google Scholar] [CrossRef]
- Sumino, K.; Harada, H.; Yonenaga, I. The origin of the difference in the mechanical strengths of Czochralski-grown silicon and float-zone-grown silicon. Jpn. J. Appl. Phys. 1980, 19, L49. [Google Scholar] [CrossRef]
- Grant, N.E.; Rougieux, F.E.; MacDonald, D.; Bullock, J.; Wan, Y. Grown-in defects limiting the bulk lifetime of p-type float-zone silicon wafers. J. Appl. Phys. 2015, 117, 055711. [Google Scholar] [CrossRef]
- Rougieux, F.E.; Grant, N.E.; Macdonald, D. Thermal deactivation of lifetime-limiting grown-in point defects in n-type Czochralski silicon wafers. Phys. Status Solidi Rapid Res. Lett. 2013, 7, 616–618. [Google Scholar] [CrossRef]
- Niewelt, T.; Selinger, M.; Grant, N.E.; Kwapil, W.; Murphy, J.D.; Schubert, M.C. Light-induced activation and deactivation of bulk defects in boron-doped float-zone silicon. J. Appl. Phys. 2017, 121, 185702. [Google Scholar] [CrossRef]
- Hoshi, K.; Isawa, N.; Suzuki, T.; Ohkubo, Y. Czochralski silicon crystals grown in a transverse magnetic field. J. Electrochem. Soc. 1985, 132, 693–700. [Google Scholar] [CrossRef]
- Organ, A.E.; Riley, N. Oxygen transport in magnetic Czochralski growth of silicon. J. Cryst. Growth 1987, 82, 465–476. [Google Scholar] [CrossRef]
- Zulehner, W. Czochralski growth of silicon. J. Cryst. Growth 1983, 65, 189–213. [Google Scholar] [CrossRef]
- Nakajima, K.; Murai, R.; Morishita, K.; Kutsukake, K.; Usami, N. Growth of multicrystalline Si ingots for solar cells using noncontact crucible method without touching the crucible wall. In Proceedings of the 38th IEEE Photovoltaic Specialists Conference (PVSC), Austin, TX, USA, 3–8 June 2012; pp. 1830–1832. [Google Scholar] [CrossRef]
- Togawa, S.; Izunome, K.; Kawanishi, S.; Chung, S.I.; Terashima, K.; Kimura, S. Oxygen transport from a silica crucible in Czochralski silicon growth. J. Cryst. Growth 1996, 165, 362–371. [Google Scholar] [CrossRef]
- Arafune, K.; Sasaki, T.; Wakabayashi, F.; Terada, Y.; Ohshita, Y.; Yamaguchi, M. Study on defects and impurities in cast-grown polycrystalline silicon substrates for solar cells. Phys. B Condens. Matter 2006, 376–377, 236–239. [Google Scholar] [CrossRef]
- Möller, H.J.; Funke, C.; Rinio, M.; Scholz, S. Multicrystalline silicon for solar cells. Thin Solid Films 2005, 487, 179–187. [Google Scholar] [CrossRef]
- Buonassisi, T.; Istratov, A.A.; Pickett, M.D.; Rakotoniaina, J.-P.; Breitenstein, O.; Marcus, M.A.; Heald, S.M.; Weber, E.R. Transition metals in photovoltaic-grade ingot-cast multicrystalline silicon: Assessing the role of impurities in silicon nitride crucible lining material. J. Cryst. Growth 2006, 287, 402–407. [Google Scholar] [CrossRef]
- Stoddard, N.; Wu, B.; Witting, I.; Wagener, M.C.; Park, Y.; Rozgonyi, G.A.; Clark, R. Casting single crystal silicon: Novel defect profiles from BP Solar’s mono2 TM wafers. Solid State Phenom. 2008, 131, 1–8. [Google Scholar] [CrossRef]
- Guerrero, I.; Parra, V.; Carballo, T.; Black, A.; Miranda, M.; Cancillo, D.; Moralejo, B.; Jiménez, J.; Lelièvre, J.-F.; Cañizo, C. About the origin of low wafer performance and crystal defect generation on seed-cast growth of industrial mono-like silicon ingots. Prog. Photovolt. Res. Appl. 2014, 22, 923–932. [Google Scholar] [CrossRef]
- ITRPV Working Group and Others. International Technology Roadmap for Photovoltaics (ITRPV.net): Results 2015, 7th ed.; ITRPV: Frankfurt, Germany, 2016. [Google Scholar]
- Lan, C.W.; Yang, C.F.; Lan, A.; Yang, M.; Yu, A.; Hsu, H.P.; Hsu, B.; Hsu, C. Engineering silicon crystals for photovoltaics. CrystEngComm 2016, 18, 1474–1485. [Google Scholar] [CrossRef]
- Kersten, F.; Engelhart, P.; Ploigt, H.-C.; Stekolnikov, A.; Lindner, T.; Stenzel, F.; Bartzsch, M.; Szpeth, A.; Petter, K.; Heitmann, J.; et al. A new mc-Si degradation effect called LeTID. In Proceedings of the 42nd IEEE Photovoltaic Specialists Conference, New Orleans, LA, USA, 14–19 June 2015; pp. 1–5. [Google Scholar]
- Fertig, F.; Krauss, K.; Rein, S. Light-induced degradation of PECVD aluminium oxide passivated silicon solar cells. Phys. Status Solidi Rapid Res. Lett. 2015, 9, 41–46. [Google Scholar] [CrossRef]
- Nakayashiki, K.; Hofstetter, J.; Morishige, A.E.; Li, T.-T.A.; Needleman, D.B.; Jensen, M.A.; Buonassisi, T. Engineering solutions and root-cause analysis for light-induced degradation in p-type multicrystalline silicon PERC modules. IEEE J. Photovolt. 2016, 6, 860–868. [Google Scholar] [CrossRef]
- Zuschlag, A.; Skorka, D.; Hahn, G. Degradation and regeneration in mc-Si after different gettering steps. Prog. Photovolt. Res. Appl. 2016. [Google Scholar] [CrossRef]
- Shockley, W.; Read, W.T., Jr. Statistics of the recombinations of holes and electrons. Phys. Rev. 1952, 87, 835. [Google Scholar] [CrossRef]
- Hall, R.N. Electron-hole recombination in germanium. Phys. Rev. 1952, 87, 387. [Google Scholar] [CrossRef]
- Graff, K.; Pieper, H. The properties of iron in silicon. J. Electrochem. Soc. 1981, 128, 669–674. [Google Scholar] [CrossRef]
- Macdonald, D.; Roth, T.; Deenapanray, P.N.K.K.; Bothe, K.; Pohl, P.; Schmidt, J. Formation rates of iron-acceptor pairs in crystalline silicon. J. Appl. Phys. 2005, 98, 083509. [Google Scholar] [CrossRef]
- Garandet, J.P. New determinations of diffusion coefficients for various dopants in liquid silicon. Int. J. Thermophys. 2007, 28, 1285–1303. [Google Scholar] [CrossRef]
- Trumbore, F.A. Solid solubilities of impurity elements in germanium and silicon. Bell Labs Tech. J. 1960, 39, 205–233. [Google Scholar] [CrossRef]
- Rosenits, P.; Roth, T.; Glunz, S.W.; Beljakowa, S. Determining the defect parameters of the deep aluminum-related defect center in silicon. Appl. Phys. Lett. 2007, 91. [Google Scholar] [CrossRef]
- Möller, C.; Lauer, K. Light-induced degradation in indium-doped silicon. Phys. Status Solidi Rapid Res. Lett. 2013, 7, 461–464. [Google Scholar] [CrossRef]
- Richter, A.; Glunz, S.W.; Werner, F.; Schmidt, J.; Cuevas, A. Improved quantitative description of Auger recombination in crystalline silicon. Phys. Rev. B 2012, 86, 165202. [Google Scholar] [CrossRef]
- Schmidt, J.; Lim, B.; Walter, D.; Bothe, K.; Gatz, S.; Dullweber, T.; Altermatt, P.P. Impurity-related limitations of next-generation industrial silicon solar Cells. IEEE J. Photovolt. 2013, 3, 114–118. [Google Scholar] [CrossRef]
- Falster, R.J.; Gambaro, D.; Cornara, M.; Olmo, M.; Pagani, M. Effect of high temperature pre-anneal on oxygen precipitates nucleation kinetics in Si. Solid State Phenom. 1997, 57, 123–128. [Google Scholar] [CrossRef]
- Haunschild, J.; Reis, I.E.; Geilker, J.; Rein, S. Detecting efficiency-limiting defects in Czochralski-grown silicon wafers in solar cell production using photoluminescence imaging. Phys. Status Solidi Rapid Res. Lett. 2011, 5, 199–201. [Google Scholar] [CrossRef]
- Cousins, P.J.; Smith, D.D.; Luan, H.-C.; Manning, J.; Dennis, T.D.; Waldhauer, A.; Wilson, K.E.; Harley, G.; Mulligan, W.P. Generation 3: Improved performance at lower cost. In Proceedings of the 35th IEEE Photovoltaic Specialists Conference (PVSC), Honolulu, HI, USA, 20–25 June 2010; pp. 275–278. [Google Scholar]
- Letty, E.; Veirman, J.; Favre, W.; Lemiti, M. Solar energy materials & solar cells bulk defect formation under light soaking in seed-end n-type Czochralski silicon wafers—Effect on silicon heterojunction solar cells. Sol. Energy Mater. Sol. Cells 2017, 166, 147–156. [Google Scholar] [CrossRef]
- Yu, X.; Wang, P.; Chen, P.; Li, X.; Yang, D. Suppression of boron—Oxygen defects in p-type Czochralski silicon. Appl. Phys. Lett. 2010, 97, 051903. [Google Scholar] [CrossRef]
- Taishi, T.; Huang, X.; Yonenaga, I.; Hoshikawa, K. Dislocation behavior in heavily germanium-doped silicon crystal. Mater. Sci. Semicond. Process. 2002, 5, 409–412. [Google Scholar] [CrossRef]
- Newman, R.C. Defects in silicon. Rep. Prog. Phys. 1982, 45, 1163–1210. [Google Scholar] [CrossRef]
- Vaqueiro-Contreras, M.; Markevich, V.P.; Halsall, M.P.; Peaker, A.R.; Santos, P.; Coutinho, J.; Öberg, S.; Murin, L.I.; Falster, R.; Binns, J.; et al. Powerful recombination centers resulting from reactions of hydrogen with carbon-oxygen defects in n-type Czochralski-grown silicon. Phys. Status Solidi Rapid Res. Lett. 2017, 8, 6–11. [Google Scholar] [CrossRef]
- Wang, Z.; Han, P.; Lu, H.; Qian, H.; Chen, L.; Meng, Q.; Tang, N.; Gao, F.; Jia, Y.; Wu, J.; et al. Advanced PERC and PERL production cells with 20.3% record efficiency for standard commercial p-type silicon wafers. Prog. Photovolt. Res. Appl. 2012, 20, 260–268. [Google Scholar] [CrossRef]
- Weaver, J.F. EGEG: LONGi at 22.7% Efficiency—Year End Launch, 40% of Electricity in US from Glass, More. Available online: https://electrek.co/2017/10/24/egeg-longi-at-22-7-efficiency-year-end-launch-40-of-electricity-in-us-from-glass-more/ (accessed on 6 November 2017).
- Hallam, B.; Hamer, P.; Kim, M.; Nampalli, N.; Gorman, N.; Chen, D.; Chan, C.; Abbott, M.; Wenham, S. Direct Transitions Between States A and C in the Boron-Oxygen Defect System? Fact or Fiction? In Proceedings of the 43rd IEEE Photovoltaic Specialists Conference, Portland, OR, USA, 5–10 June 2016; pp. 2430–2433. [Google Scholar] [CrossRef]
- Walter, D.C.; Falster, R.; Voronkov, V.V.; Schmidt, J. On the equilibrium concentration of boron-oxygen defects in crystalline silicon. Sol. Energy Mater. Sol. Cells 2017, 173, 33–36. [Google Scholar]
- Nampalli, N.; Li, H.; Kim, M.; Stefani, B.; Wenham, S.; Hallam, B.; Abbott, M. Multiple pathways for permanent deactivation of boron-oxygen defects in p-type silicon. Sol. Energy Mater. Sol. Cells 2017, 173, 12–17. [Google Scholar] [CrossRef]
- Glunz, S.W.; Rein, S.; Warta, W.; Knobloch, J.; Wettling, W. Degradation of carrier lifetime in Cz silicon solar cells. Sol. Energy Mater. Sol. Cells 2001, 65, 219–229. [Google Scholar] [CrossRef]
- Glunz, S.W.; Rein, S.; Lee, J.Y.; Warta, W. Minority carrier lifetime degradation in boron-doped Czochralski silicon. J. Appl. Phys. 2001, 90, 2397–2404. [Google Scholar] [CrossRef]
- Nagel, H.; Merkle, A.; Metz, A.; Hezel, R. Permanent reduction of excess-carrier-induced recombination centers in solar grade Czochralski silicon by a short yet effective anneal. In Proceedings of the 16th European Photovoltaic Solar Energy Conference, Glasgow, UK, 1–5 May 2000; pp. 1197–1200. [Google Scholar]
- Saitoh, T.; Wang, X.; Hashigami, H.; Abe, T.; Igarashi, T.; Glunz, S.; Rein, S.; Wettling, W.; Yamasaki, I.; Sawai, H.; et al. Suppression of light degradation of carrier lifetimes in low-resistivity CZ—Si solar cells. Sol. Energy Mater. Sol. Cells 2001, 65, 277–285. [Google Scholar] [CrossRef]
- Bothe, K.; Schmidt, J.; Hezel, R. Effective reduction of the metastable defect concentration in boron-doped Czochralski silicon for solar cells. In Proceedings of the 29th IEEE Photovoltaic Specialists Conference, New Orleans, LA, USA, 19–24 May 2002; pp. 194–197. [Google Scholar]
- Rougieux, F.E.; Lim, B.; Schmidt, J.; Forster, M.; Macdonald, D.; Cuevas, A. Influence of net doping, excess carrier density and annealing on the boron oxygen related defect density in compensated n-type silicon. J. Appl. Phys. 2011, 110, 063708. [Google Scholar] [CrossRef]
- Walter, D.C.; Lim, B.; Bothe, K.; Voronkov, V.V.; Falster, R.; Schmidt, J. Effect of rapid thermal annealing on recombination centres in boron-doped Czochralski-grown silicon. Appl. Phys. Lett. 2014, 104, 042111. [Google Scholar] [CrossRef]
- Unsur, V.; Hussain, B.; Ebong, A. Complete recovery of light induced degradation of Cz silicon solar cells with rapid thermal processing. In Proceedings of the 43rd Photovoltaic Specialists Conference, Portland, OR, USA, 5–10 June 2016; pp. 717–719. [Google Scholar] [CrossRef]
- Kouhlane, Y.; Bouhafs, D.; Khelifati, N.; Belhousse, S.; Menari, H.; Guenda, A.; Khelfane, A. Effect of rapid thermal processing on light-induced degradation of carrier lifetime in Czochralski p-type silicon bare wafers. J. Electron. Mater. 2016, 45, 5621–5625. [Google Scholar] [CrossRef]
- Herguth, A.; Schubert, G.; Käs, M.; Hahn, G. A new approach to prevent the negative impact of the metastable defect in boron doped Cz silicon solar cells. In Proceedings of the 4th IEEE World Conference on Photovoltaic Energy Conversion, Waikoloa, HI, USA, 7–12 May 2006; pp. 940–943. [Google Scholar]
- Herguth, A.; Schubert, G.; Kaes, M.; Hahn, G. Avoiding boron-oxygen related degradation in highly boron doped Cz silicon. In Proceedings of the 21st European Photovoltaic Solar Energy Conference, Dresden, Germany, 4–8 September 2006; pp. 530–537. [Google Scholar]
- Herguth, A.; Hahn, G. Boron-oxygen related defects in Cz-silicon solar cells degradation, regeneration and beyond. In Proceedings of the 24th European Photovoltaic Solar Energy Conference, Hamburg, Germany, 21–25 September 2009; pp. 974–976. [Google Scholar]
- Herguth, A.; Hahn, G. Kinetics of the boron-oxygen related defect in theory and experiment. J. Appl. Phys. 2010, 108, 114509. [Google Scholar] [CrossRef]
- Wilking, S.; Beckh, C.; Ebert, S.; Herguth, A.; Hahn, G. Influence of bound hydrogen states on BO-regeneration kinetics and consequences for high-speed regeneration processes. Sol. Energy Mater. Sol. Cells 2014, 131, 2–8. [Google Scholar] [CrossRef]
- Hallam, B.J.; Abbott, M.; Nampalli, N.; Hamer, P.G.; Wenham, S.R. Implications of accelerated recombination-active defect complex formation for mitigating carrier-induced degradation in silicon. IEEE J. Photovolt. 2015, 6, 92–99. [Google Scholar] [CrossRef]
- Hallam, B.; Abbott, M.; Nampalli, N.; Hamer, P.; Wenham, S. Influence of the formation- and passivation rate of Boron-Oxygen defects for mitigating carrier-induced degradation in silicon within a Hydrogen-Based Model. J. Appl. Phys. 2016, 119, 065701. [Google Scholar] [CrossRef]
- Steckenreiter, V.; Walter, D.C.; Schmidt, J. Kinetics of the permanent deactivation of the boron-oxygen complex in crystalline silicon as a function of illumination intensity. AIP Adv. 2017, 7, 035305. [Google Scholar] [CrossRef]
- Steckenreiter, V.; Walter, D.; Schmidt, J. Two-stage permanent deactivation of the boron-oxygen-related recombination center in crystalline silicon. Energy Procedia 2017, 124, 799–805. [Google Scholar] [CrossRef]
- Hallam, B.; Abbott, M.; Bilbao, J.; Hamer, P.; Gorman, N.; Kim, M.; Chen, D.; Hammerton, K.; Payne, D.; Chan, C.; et al. Modelling kinetics of the boron-oxygen defect system. Energy Procedia 2016, 92, 42–51. [Google Scholar] [CrossRef]
- Wilking, S.; Forster, M.; Herguth, A.; Hahn, G. From simulation to experiment: Understanding BO-regeneration kinetics. Sol. Energy Mater. Sol. Cells 2015, 142, 87–91. [Google Scholar] [CrossRef]
- Nærland, T.U.; Haug, H.; Angelskar, H.; Sondena, R.; Marstein, E.S.; Arnberg, L. Studying light-induced degradation by lifetime decay analysis: Excellent fit to solution of simple second-order rate equation. IEEE J. Photovolt. 2013, 3, 1265–1270. [Google Scholar] [CrossRef]
- Lim, B.; Hermann, S.; Bothe, K.; Schmidt, J.; Brendel, R. Permanent deactivation of the boron-oxygen recombination center in silicon solar cells. In Proceedings of the 23rd European Photovoltaic Solar Energy Conference, Valencia, Spain, 1–5 September 2008; pp. 1018–1022. [Google Scholar]
- Lim, B.; Bothe, K.; Schmidt, J. Deactivation of the boron–oxygen recombination center in silicon by illumination at elevated temperature. Phys. Status Solidi Rapid Res. Lett. 2008, 2, 93–95. [Google Scholar] [CrossRef]
- Wilking, S.; Ebert, S.; Beckh, C.; Herguth, A.; Hahn, G. Of Apples and oranges: Why comparing bo regeneration rates requires injection level correction. In Proceedings of the 32nd European Photovoltaic Solar Energy Conference, München, Germany, 21–24 June 2016; pp. 487–494. [Google Scholar]
- Herguth, A.; Schubert, G.; Käs, M.; Hahn, G. Investigations on the long time behavior of the metastable boron-oxygen complex in crystalline silicon. Prog. Photovolt. Res. Appl. 2008, 16, 135–140. [Google Scholar] [CrossRef]
- Hallam, B. High Efficiency Laser-Doped Silicon Solar Cells with Advanced Hydrogenation. Ph.D. Thesis, University of New South Wales, Sydney, Australia, 2014. [Google Scholar]
- Hamer, P. Hydrogen Charge States and Dopant Interactions in Crystalline Silicon Solar Cells. Ph.D. Thesis, University of New South Wales, Sydney, Australia, 2014. [Google Scholar]
- Münzer, K. Hydrogenated silicon nitride for regeneration of light induced degradation. In Proceedings of the 24th European Photovoltaic Solar Energy Conference, Hamburg, Germany, 21–25 September 2009; pp. 1558–1561. [Google Scholar]
- Wilking, S.; Herguth, A.; Hahn, G. Influence of hydrogen on the regeneration of boron-oxygen related defects in crystalline silicon. J. Appl. Phys. 2013, 113, 194503. [Google Scholar] [CrossRef]
- Wilking, S.; Ebert, S.; Herguth, A.; Hahn, G. Influence of hydrogen effusion from hydrogenated silicon nitride layers on the regeneration of boron-oxygen related defects in crystalline silicon. J. Appl. Phys. 2013, 114, 194512. [Google Scholar] [CrossRef]
- Walter, D.C.; Lim, B.; Bothe, K.; Falster, R.; Voronkov, V.V.; Schmidt, J. Lifetimes exceeding 1 ms in 1-Ω cm boron-doped Cz-silicon. Sol. Energy Mater. Sol. Cells 2014, 131, 51–57. [Google Scholar] [CrossRef]
- Wilking, S.; Engelhardt, J.; Ebert, S.; Beckh, C.; Herguth, A.; Hahn, G. High speed regeneration of BO-defects: Improving long-term solar cell performance within seconds. In Proceedings of the 29th European Photovoltaic Solar Energy Conference and Exhibition, Hamburg, Germany, 4–18 September 2014; pp. 366–372. [Google Scholar]
- Krugel, G.; Wolke, W.; Geilker, J.; Rein, S.; Preu, R. Impact of hydrogen concentration on the regeneration of light induced degradation. Energy Procedia 2011, 8, 47–51. [Google Scholar] [CrossRef]
- Dubois, S.; Tanay, F.; Veirman, J.; Enjalbert, N.; Stendera, J.; Butté, S.; Pochet, P.; Caliste, D.; Mao, Y.; Timerkaeva, D.; et al. The BOLID project: Suppression of the boron-oxygen related light-induced-degradation. In Proceedings of the 27th European Photovoltaic Solar Energy Conference and Exhibition, Frankfurt, Germany, 24–28 September 2012; pp. 749–754. [Google Scholar]
- Wilking, S. Das Wasserstoff-Modell der Bor-Sauerstoff-Regeneration. Ph.D. Thesis, University of Konstanz, Konstanz, Germany, 2017. [Google Scholar]
- Herring, C.; Johnson, N.M.; de Walle, C.G. Energy levels of isolated interstitial hydrogen in silicon. Phys. Rev. B 2001, 64, 125209. [Google Scholar] [CrossRef]
- Mathiot, D. Modeling of hydrogen diffusion in n-and p-type silicon. Phys. Rev. B 1989, 40, 5867–5870. [Google Scholar] [CrossRef]
- Hallam, B.J.; Hamer, P.G.; Wenham, S.R.; Abbott, M.D.; Sugianto, A.; Wenham, A.M.; Chan, C.E.; Xu, G.; Kraiem, J.; Degoulange, J.; et al. Advanced bulk defect passivation for silicon solar cells. IEEE J. Photovolt. 2014, 4, 88–95. [Google Scholar] [CrossRef]
- Rizk, R.; de Mierry, P.; Ballutaud, D.; Aucouturier, M.; Mathiot, D. Hydrogen diffusion and passivation processes in p- and n-type crystalline silicon. Phys. Rev. B 1991, 44, 6141–6151. [Google Scholar] [CrossRef]
- Bonde Nielsen, K.; Nielsen, B.; Hansen, J.; Andersen, E.; Andersen, J. Bond-centered hydrogen in silicon studied by in situ deep-level transient spectroscopy. Phys. Rev. B 1999, 60, 1716–1728. [Google Scholar] [CrossRef]
- Estreicher, S.K.; Docaj, A.; Bebek, M.B.; Backlund, D.J.; Stavola, M. Hydrogen in C-rich Si and the diffusion of vacancy-H complexes. Phys. Status Solidi A 2012, 209, 1872–1879. [Google Scholar] [CrossRef]
- Hamer, P.; Hallam, B.; Wenham, S.; Abbott, M. Manipulation of hydrogen charge states for passivation of p-type wafers in photovoltaics. IEEE J. Photovolt. 2014, 4, 1252–1260. [Google Scholar] [CrossRef]
- Hallam, B.J.; Wenham, S.R.; Hamer, P.G.; Abbott, M.D.; Sugianto, A.; Chan, C.E.; Wenham, A.M.; Eadie, M.G.; Xu, G. Hydrogen passivation of B-O defects in Czochralski silicon. Energy Procedia 2013, 38, 561–570. [Google Scholar] [CrossRef]
- Wilking, S.; Herguth, A.; Hahn, G. Influence of hydrogenated passivation layers on the regeneration of boron-oxygen related defects. Energy Procedia 2013, 38, 642–648. [Google Scholar] [CrossRef]
- Sun, C.; Rougieux, F.E.; Macdonald, D. A unified approach to modelling the charge state of monatomic hydrogen and other defects in crystalline silicon. J. Appl. Phys. 2015, 117, 045702. [Google Scholar] [CrossRef]
- Gläser, M.; Lausch, D. Towards a quantitative model for BO regeneration by means of charge state control of hydrogen. Energy Procedia 2015, 77, 592–598. [Google Scholar] [CrossRef]
- Niewelt, T.; Schön, J.; Broisch, J.; Warta, W.; Schubert, M. Electrical characterization of the slow boron oxygen defect component in Czochralski silicon. Phys. Status Solidi Rapid Res. Lett. 2015, 9, 692–696. [Google Scholar] [CrossRef]
- Lim, B.; Bothe, K.; Schmidt, J. Accelerated deactivation of the boron-oxygen-related recombination centre in crystalline silicon. Semicond. Sci. Technol. 2011, 26, 095009. [Google Scholar] [CrossRef]
- Nampalli, N.; Hallam, B.; Chan, C.; Abbott, M.; Wenham, S. Evidence for the role of hydrogen in the stabilization of minority carrier lifetime in boron-doped Czochralski silicon. Appl. Phys. Lett. 2015, 106, 173501. [Google Scholar] [CrossRef]
- Nampalli, N.; Hallam, B.J.; Chan, C.E.; Abbott, M.D.; Wenham, S.R. Influence of hydrogen on the mechanism of permanent passivation of boron-oxygen defects in p-type Czochralski silicon. IEEE J. Photovolt. 2015, 5, 1580–1585. [Google Scholar] [CrossRef]
- Walter, D.C.; Schmidt, J. Impact of hydrogen on the permanent deactivation of the boron-oxygen-related recombination center in crystalline silicon. Sol. Energy Mater. Sol. Cells 2016, 158, 91–97. [Google Scholar] [CrossRef]
- Seager, C.H.; Anderson, R.A.; Panitz, J.K.G. The diffusion of hydrogen in silicon and mechanisms for unintentional hydrogenation during ion beam processing. J. Mater. Res. 1987, 2, 96–106. [Google Scholar] [CrossRef]
- Sopori, B.; Zhang, Y.; Ravindra, N.M. Silicon device processing in H-ambients: H-diffusion mechanisms and influence on electronic properties. J. Electron. Mater. 2001, 30, 1616–1627. [Google Scholar] [CrossRef]
- Van Wieringen, A.; Warmoltz, N. On the permeation of hydrogen and helium in single crystal silicon and germanium at elevated temperatures. Physica 1956, 22, 849–865. [Google Scholar] [CrossRef]
- Macdonald, D.; Liu, A.; Cuevas, A.; Lim, B.; Schmidt, J. The impact of dopant compensation on the boron-oxygen defect in p-and n-type crystalline silicon. Phys. Status Solidi A 2011, 208, 559–563. [Google Scholar] [CrossRef]
- Hashigami, H.; Itakura, Y.; Saitoh, T. Effect of illumination conditions on Czochralski-grown silicon solar cell degradation. J. Appl. Phys. 2003, 93, 4240–4245. [Google Scholar] [CrossRef]
- Nærland, T.U.; Angelskår, H.; Marstein, E.S. Direct monitoring of minority carrier density during light induced degradation in Czochralski silicon by photoluminescence imaging. J. Appl. Phys. 2013, 113, 193707. [Google Scholar] [CrossRef]
- Hamer, P.; Hallam, B.; Abbott, M.; Wenham, S. Accelerated formation of the boron-oxygen complex in p-type Czochralski silicon. Phys. Status Solidi Rapid Res. Lett. 2015, 9, 297–300. [Google Scholar] [CrossRef]
- Schön, J.; Niewelt, T.; Broisch, J.; Warta, W.; Schubert, M. Characterization and modelling of the boron-oxygen defect activation in compensated n-type silicon. J. Appl. Phys. 2015, 118, 245702. [Google Scholar] [CrossRef]
- Hamer, P.; Nampalli, N.; Hameiri, Z.; Kim, M.; Chen, D.; Gorman, N.; Hallam, B.; Abbott, M.; Wenham, S. Boron-Oxygen defect formation rates and activity at elevated temperatures. Energy Procedia 2016, 92, 791–800. [Google Scholar] [CrossRef]
- Wilson, M.; Edelman, P.; Savtchouk, A.; D’Amico, J.; Findlay, A.; Lagowski, J. Accelerated light-induced degradation (ALID) for monitoring of defects in PV silicon wafers and solar cells. J. Electron. Mater. 2010, 39, 642–647. [Google Scholar] [CrossRef]
- Nampalli, N. Characterisation and Passivation of Boron-Oxygen Defects in P-Type Czochralski Silicon. Ph.D. Thesis, University of New South Wales, Sydney, Australia, 2017. [Google Scholar]
- Song, L.; Wenham, A.; Wang, S.; Hamer, P.; Shakil, A.; Hallam, B.; Mai, L.; Abbott, M.; Hawkes, E.; Chong, C.; et al. Laser enhanced hydrogen passivation of silicon wafers. Int. J. Photoenergy 2015, 501, 193892. [Google Scholar] [CrossRef]
- Hallam, B.J.; Hamer, P.G.; Wang, S.; Song, L.; Nampalli, N.; Abbott, M.D.; Chan, C.E.; Lu, D.; Wenham, A.M.; Mai, L.; et al. Advanced hydrogenation of dislocation clusters and boron-oxygen defects in silicon solar cells. Energy Procedia 2015, 77, 799–809. [Google Scholar] [CrossRef]
- Hamer, P.; Hallam, B.; Abbott, M.; Chan, C.; Nampalli, N.; Wenham, S. Investigations on accelerated processes for the boron-oxygen defect in p-type Czochralski silicon. Sol. Energy Mater. Sol. Cells 2016, 145, 440–446. [Google Scholar] [CrossRef]
- Hallam, B.; Chen, D.; Kim, M.; Stefani, B.; Hoex, B.; Abbott, M.; Wenham, S. The role of hydrogenation and gettering in enhancing the efficiency of next generation Si solar cells: An industrial perspective. Phys. Status Solidi A 2017, 1700305. [Google Scholar] [CrossRef]
- Sheoran, M.; Upadhyaya, A.; Rohatgi, A. Bulk lifetime and efficiency enhancement due to gettering and hydrogenation of defects during cast multicrystalline silicon solar cell fabrication. Solid. State Electron. 2008, 52, 612–617. [Google Scholar] [CrossRef]
- Sheoran, M.; Kim, D.S.; Rohatgi, A.; Dekkers, H.F.W.; Beaucarne, G.; Young, M.; Asher, S. Hydrogen diffusion in silicon from plasma-enhanced chemical vapor deposited silicon nitride film at high temperature. Appl. Phys. Lett. 2008, 92, 172107. [Google Scholar] [CrossRef]
- Kleekajai, S.; Jiang, F.; Stavola, M.; Yelundur, V.; Nakayashiki, K.; Rohatgi, A.; Hahn, G.; Seren, S.; Kalejs, J. Concentration and penetration depth of H introduced into crystalline Si by hydrogenation methods used to fabricate solar cells. J. Appl. Phys. 2006, 100, 093517. [Google Scholar] [CrossRef]
- Kleekajai, S.; Wen, L.; Peng, C.; Stavola, M.; Yelundur, V.; Nakayashiki, K.; Rohatgi, A.; Kalejs, J. Infrared study of the concentration of H introduced into Si by the postdeposition annealing of a SiNx coating. J. Appl. Phys. 2009, 106, 123510. [Google Scholar] [CrossRef]
- Fischbeck, G. Keep a LID on it. PV-Magazine, 16 November 2015; 44–47. [Google Scholar]
- Hallam, B.; Chan, C.; Payne, D.N.R.; Lausch, D.; Gläser, M.; Abbott, M.; Wenham, S. Techniques for mitigating light-induced degradation (LID) in commercial silicon solar cells. Photovolt. Int. 2016, 33, 37–46. [Google Scholar]
- Pernau, T.; Romer, O.; Scheiffele, W.; Reichart, A.; Jooß, W. Rather high speed regeneration of BO-defects: Regeneration experiments with large cell batches. In Proceedings of the 31st European Photovoltaic Solar Energy Conference, Hamburg, Germany, 14–18 September 2015; pp. 918–920. [Google Scholar]
- Hallam, B.J.; Chan, C.E.; Chen, R.; Wang, S.; Ji, J.; Mai, L.; Abbott, M.D.; Payne, D.N.R.; Kim, M.; Chen, D.; et al. Rapid mitigation of carrier-induced degradation in commercial silicon solar cells. Jpn. J. Appl. Phys. 2017, 56, 08MB13. [Google Scholar] [CrossRef]
- Herguth, A.; Hahn, G. Towards a high throughput solution for boron-oxygen related regeneration. In Proceedings of the 28th European Photovoltaic Solar Energy Conference, Paris, France, 30 September–4 October 2013; pp. 1507–1511. [Google Scholar] [CrossRef]
- Fertig, F.; Broisch, J.; Biro, D.; Rein, S. Stability of the regeneration of the boron-oxygen complex in silicon solar cells during module certification. Sol. Energy Mater. Sol. Cells 2014, 121, 157–162. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hallam, B.; Herguth, A.; Hamer, P.; Nampalli, N.; Wilking, S.; Abbott, M.; Wenham, S.; Hahn, G. Eliminating Light-Induced Degradation in Commercial p-Type Czochralski Silicon Solar Cells. Appl. Sci. 2018, 8, 10. https://doi.org/10.3390/app8010010
Hallam B, Herguth A, Hamer P, Nampalli N, Wilking S, Abbott M, Wenham S, Hahn G. Eliminating Light-Induced Degradation in Commercial p-Type Czochralski Silicon Solar Cells. Applied Sciences. 2018; 8(1):10. https://doi.org/10.3390/app8010010
Chicago/Turabian StyleHallam, Brett, Axel Herguth, Phillip Hamer, Nitin Nampalli, Svenja Wilking, Malcolm Abbott, Stuart Wenham, and Giso Hahn. 2018. "Eliminating Light-Induced Degradation in Commercial p-Type Czochralski Silicon Solar Cells" Applied Sciences 8, no. 1: 10. https://doi.org/10.3390/app8010010
APA StyleHallam, B., Herguth, A., Hamer, P., Nampalli, N., Wilking, S., Abbott, M., Wenham, S., & Hahn, G. (2018). Eliminating Light-Induced Degradation in Commercial p-Type Czochralski Silicon Solar Cells. Applied Sciences, 8(1), 10. https://doi.org/10.3390/app8010010




